Abstract
Glioma is the most common primary cancer of the central nervous system, and around 50% of patients present with the most aggressive form of the disease, glioblastoma. Conventional therapies, including surgery, radiotherapy, and pharmacotherapy (typically chemotherapy with temozolomide), have not resulted in major improvements in the survival outcomes of patients with glioblastoma. Reasons for this lack of progress include invasive tumour growth in an essential organ, which limits the utility of local therapy, as well as the protection of tumour cells by the blood–brain barrier, their intrinsic resistance to the induction of cell death, and lack of dependence on single, targetable oncogenic pathways, all of which impose challenges for systemic therapy. Furthermore, the unique immune environment of the central nervous system needs to be considered when pursuing immune-based therapeutic approaches for glioblastoma. Nevertheless, a range of different immunotherapies are currently being actively investigated in patients with this disease, spurred on by advances in immuno-oncology for other tumour types. Herein, we examine the current state of immunotherapy for gliomas, notably glioblastoma, the implications for combining the current standard-of-care treatment modalities with immunotherapies, potential biomarkers of response, and future directions for glioblastoma immuno-oncology.
Key points
-
The current standard of care for patients with glioblastoma includes surgery, temozolomide chemotherapy, radiotherapy, and corticosteroids, all of which have immunosuppressive effects; we must be cognizant of this complexity when developing immunotherapies.
-
Evidence for immunostimulatory effects of these treatments in the clinic, including abscopal effects, induction of immunogenic cell death, and depletion of regulatory T cells by temozolomide, remains limited.
-
Vaccination has been considered one of the most promising approaches to improving the outcomes of patients with glioblastoma, although negative results from several phase II and phase III trials challenge the current concept of vaccination as a single-modality immunotherapy.
-
Oncolytic viruses might exert pro-inflammatory responses that could potentially be exploited in future combined modality immunotherapy studies, whereas the future of chimeric antigen receptor (CAR) T cell therapy for glioblastoma depends on the identification of stably expressed and sufficiently tumour-specific antigens.
-
Immune-checkpoint inhibitors have promising therapeutic activity in preclinical glioblastoma models, whereas the results emerging from clinical trials in patients with recurrent glioblastoma are disappointing; larger studies are underway in the frontline treatment setting.
-
Future immune-based strategies are focused on combinations of different immune-checkpoint inhibitors with diverse treatment modalities that reverse local immunosuppression in the microenvironment, converting a ‘cold’ tumour into a ‘hot’ tumour.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lathia, J. D., Mack, S. C., Mulkearns-Hubert, E. E., Valentim, C. L. L. & Rich, J. N. Cancer stem cells in glioblastoma. Genes Dev. 29, 1203–1217 (2015).
Hambardzumyan, D., Amankulor, N. M., Helmy, K. Y., Becher, O. J. & Holland, E. C. Modeling adult gliomas using RCAS/t-va technology. Transl Oncol. 2, IN6 (2009).
Weller, M. et al. Glioma. Nat. Rev. Dis. Primers 1, 15017 (2015).
Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131, 803–820 (2016).
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro. Oncol. 17 (Suppl. 4), iv1–iv62 (2015).
Reifenberger, G., Wirsching, H.-G., Knobbe-Thomsen, C. B. & Weller, M. Advances in the molecular genetics of gliomas — implications for classification and therapy. Nat. Rev. Clin. Oncol. 14, 434 (2017).
Weller, M. et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 18, e315–e329 (2017).
Kreth, F.-W. et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann. Oncol. 24, 3117–3123 (2013).
Beiko, J. et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro. Oncol. 16, 81–91 (2014).
Walker, M. D. et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: a cooperative clinical trial. J. Neurosurg. 49, 333–343 (1978).
Walker, M. D. et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N. Engl. J. Med. 303, 1323–1329 (1980).
Roa, W. et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J. Clin. Oncol. 22, 1583–1588 (2004).
Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005).
Perry, J. R. et al. Short-course radiation plus temozolomide in elderly patients with glioblastoma. N. Engl. J. Med. 376, 1027–1037 (2017).
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003 (2005).
Chinot, O. L. et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 709–722 (2014).
Gilbert, M. R., Sulman, E. P. & Mehta, M. P. Bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 370, 2048–2049 (2014).
Stupp, R. et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet. Oncol. 15, 1100–1108 (2014).
Stupp, R. et al. Effect of tumor-treating fields plus maintenance temozolomide versus maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318, 2306–2316 (2017).
Stupp, R. et al. NovoTTF-100 A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur. J. Cancer 48, 2192–2202 (2012).
Weller, M. et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin. Cancer Res. 21, 2057–2064 (2015).
Han, K. et al. Progression-free survival as a surrogate endpoint for overall survival in glioblastoma: a literature-based meta-analysis from 91 trials. Neuro. Oncol. 16, 696–706 (2014).
Weller, M., Cloughesy, T., Perry, J. R. & Wick, W. Standards of care for treatment of recurrent glioblastoma — are we there yet? Neuro. Oncol. 15, 4–27 (2013).
Gramatzki, D. et al. Glioblastoma in the Canton of Zurich, Switzerland revisited: 2005 to 2009. Cancer 122, 2206–2215 (2016).
Wick, W. et al. Lomustine and bevacizumab in progressive glioblastoma. N. Engl. J. Med. 377, 1954–1963 (2017).
Billingham, R. E., Brent, L. & Medawar, P. B. Actively acquired tolerance of foreign cells. Nature 172, 603–606 (1953).
Billingham, R. E., Brent, L., Medawar, P. B. & Sparrow, E. M. Quantitative studies on tissue transplantation immunity. I. The survival times of skin homografts exchanged between members of different inbred strains of mice. Proc. R. Soc. B Biol. Sci. 143, 43–58 (1954).
Medawar, P. B. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 29, 58–69 (1948).
Woodroofe, M. N., Bellamy, A. S., Feldmann, M., Davison, A. N. & Cuzner, M. L. Immunocytochemical characterisation of the immune reaction in the central nervous system in multiple sclerosis. Possible role for microglia in lesion growth. J. Neurol. Sci. 74, 135–152 (1986).
Schiffer, D., Mellai, M., Bovio, E. & Annovazzi, L. The neuropathological basis to the functional role of microglia/macrophages in gliomas. Neurol. Sci. 38, 1571–1577 (2017).
Waksman, B. H. & Adams, R. D. Allergic neuritis: an experimental disease of rabbits induced by the injection of peripheral nervous tissue and adjuvants. J. Exp. Med. 102, 213–236 (1955).
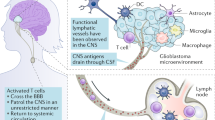
Louveau, A. et al. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015).
Canessa, A., Del Bono, V., Miletich, F. & Pistoia, V. Serum cytokines in toxoplasmosis: increased levels of interferon-gamma in immunocompetent patients with lymphadenopathy but not in AIDS patients with encephalitis. J. Infect. Dis. 165, 1168–1170 (1992).
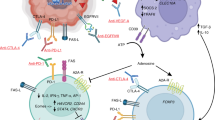
Nduom, E. K., Weller, M. & Heimberger, A. B. Immunosuppressive mechanisms in glioblastoma. Neuro. Oncol. 17 (Suppl. 7), vii9–vii14 (2015).
Schweitzer, T., Vince, G. H., Herbold, C., Roosen, K. & Tonn, J.-C. Extraneural metastases of primary brain tumors. J. Neurooncol. 53, 107–114 (2001).
Westphal, M. & Lamszus, K. Circulating biomarkers for gliomas. Nat. Rev. Neurol. 11, 556 (2015).
Müller, C. et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 6, 247ra101 (2014).
Roszman, T., Elliott, L. & Brooks, W. Modulation of T-cell function by gliomas. Immunol. Today 12, 370–374 (1991).
Bloch, O. et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin. Cancer Res. 19, 3165–3175 (2013).
Chae, M. et al. Increasing glioma-associated monocytes leads to increased intratumoral and systemic myeloid-derived suppressor cells in a murine model. Neuro. Oncol. 17, 978–991 (2015).
Li, B. et al. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 17, 174 (2016).
Grossman, S. A. et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin. Cancer Res. 17, 5473–5480 (2011).
Jackson, C. M. et al. Systemic tolerance mediated by melanoma brain tumors is reversible by radiotherapy and vaccination. Clin. Cancer Res. 22, 1161–1172 (2016).
Chongsathidkiet, P. et al. Downregulation of sphingosine-1-phosphate receptor type 1 mediates T-cell sequestration in bone marrow amidst glioblastoma. J. Neurosurg. 126, 1442 (2017).
Wainwright, D. A. et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 20, 5290–5301 (2014).
Heimberger, A. B. et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro. Oncol. 10, 98–103 (2008).
Bodmer, S. et al. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J. Immunol. 143, 3222–3229 (1989).
Huettner, C., Czub, S., Kerkau, S., Roggendorf, W. & Tonn, J.-C. Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 17, 3217–3224 (1997).
Huettner, C., Paulus, W. & Roggendorf, W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am. J. Pathol. 146, 317 (1995).
Lauro, G. M., Di Lorenzo, N., Grossi, M., Maleci, A. & Guidetti, B. Prostaglandin E 2 as an immunomodulating factor released in vitro by human glioma cells. Acta Neuropathol. 69, 278–282 (1986).
Wischhusen, J., Friese, M. A., Mittelbronn, M., Meyermann, R. & Weller, M. HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J. Neuropathol. Exp. Neurol. 64, 523–528 (2005).
Wiendl, H. et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J. Immunol. 168, 4772–4780 (2002).
Didenko, V. V., Ngo, H. N., Minchew, C. & Baskin, D. S. Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. J. Neurosurg. 96, 580–584 (2002).
Parsa, A. T. et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat. Med. 13, 84 (2007).
Parney, I. F., Waldron, J. S. & Parsa, A. T. Flow cytometry and in vitro analysis of human glioma–associated macrophages. J. Neurosurg. 110, 572–582 (2009).
Dunn, G. P., Dunn, I. F. & Curry, W. T. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. Arch. 7, 12 (2007).
Komohara, Y., Ohnishi, K., Kuratsu, J. & Takeya, M. Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J. Pathol. 216, 15–24 (2008).
Greter, M. et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat. Med. 11, 328 (2005).
Preusser, M., Lim, M., Hafler, D. A., Reardon, D. A. & Sampson, J. H. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat. Rev. Neurol. 11, 504–514 (2015).
Weller, M. et al. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int. J. Cancer 134, 2437–2447 (2014).
van den Bent, M. J. et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro. Oncol. 17, 935–941 (2015).
Felsberg, J. et al. Epidermal growth factor receptor variant III (EGFRvIII) positivity in EGFR-amplified glioblastomas: prognostic role and comparison between primary and recurrent tumors. Clin. Cancer Res. 23, 6846–6855 (2017).
Schuster, J. et al. A phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro. Oncol. 17, 854–861 (2015).
Sampson, J. H. et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 28, 4722–4729 (2010).
Sampson, J. H. et al. Greater chemotherapy-induced lymphopenia enhances tumor-specific immune responses that eliminate EGFRvIII-expressing tumor cells in patients with glioblastoma. Neuro. Oncol. 13, 324–333 (2011).
Weller, M. et al. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): results of a randomized, double-blind, international phase 3 trial. Lancet Oncol. 18, 1373–1385 (2017).
Reardon, D. A. et al. ReACT: Overall survival from a randomized phase II study of rindopepimut (CDX-110) plus bevacizumab in relapsed glioblastoma. J. Clin. Oncol. 33, 2009 (2015).
Khan, K. A. & Kerbel, R. S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. https://doi.org/10.1038/nrclinonc.2018.9 (2018).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. https://doi.org/10.1038/nrclinonc.2018.29 (2018).
Schumacher, T. et al. A vaccine targeting mutant IDH1 induces antitumour immunity. Nature 512, 324 (2014).
Prins, R. M. et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J. Immunol. 176, 157–164 (2006).
Tchirkov, A. et al. Clinical implications of quantitative real-time RT–PCR analysis of hTERT gene expression in human gliomas. Br. J. Cancer 88, 516 (2003).
Killela, P. J. et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl Acad. Sci. USA 110, 6021–6026 (2013).
Suso, E. M. I. et al. hTERT mRNA dendritic cell vaccination: complete response in a pancreatic cancer patient associated with response against several hTERT epitopes. Cancer Immunol. Immunother. 60, 809–818 (2011).
Rampling, R. et al. A Cancer Research UK first time in human phase I trial of IMA950 (novel multipeptide therapeutic vaccine) in patients with newly diagnosed glioblastoma. Clin. Cancer Res. 22, 4776–4785 (2016).
Phuphanich, S. et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol. Immunother. 62, 125–135 (2013).
Wen, P., Reardon, D. A., Phuphanich, S. & Aiken, R. A randomized, double-blind, placebo-controlled phase 2 trial of dendritic cell (DC) vaccination with ICT-107 in newly diagnosed glioblastoma (GBM) patients [abstract]. J. Clin. Oncol. 32 (Suppl), 2005 (2014).
Polyzoidis, S. & Ashkan, K. DCVax®-L — developed by Northwest Biotherapeutics. Hum. Vaccin. Immunother. 10, 3139–3145 (2014).
Liau, L. M. et al. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J. Neurosurg. 90, 1115–1124 (1999).
Liau, L. M. et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin. Cancer Res. 11, 5515–5525 (2005).
Lichty, B. D., Breitbach, C. J., Stojdl, D. F. & Bell, J. C. Going viral with cancer immunotherapy. Nat. Rev. Cancer 14, 559 (2014).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675 (2001).
Martuza, R. L., Malick, A., Markert, J. M., Ruffner, K. L. & Coen, D. M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 252, 854–856 (1991).
Lawler, S. E., Speranza, M.-C., Cho, C.-F. & Chiocca, E. A. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 3, 841–849 (2017).
Foreman, P. M., Friedman, G. K., Cassady, K. A. & Markert, J. M. Oncolytic virotherapy for the treatment of malignant glioma. Neurotherapeutics 14, 333–344 (2017).
Desjardins, A. et al. Patient survival on the dose escalation phase of the Oncolytic Polio/Rhinovirus Recombinant (PVSRIPO) against WHO grade IV malignant glioma (MG) clinical trial compared to historical controls [abstract]. J. Clin. Oncol. 34 (Suppl), 2061 (2016).
Perez, O. D. et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol. Ther. 20, 1689–1698 (2012).
Cloughesy, T. F. et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl Med. 8, 341ra75 (2016).
Sonabend, A. M., Ulasov, I. V., Han, Y. & Lesniak, M. S. Oncolytic adenoviral therapy for glioblastoma multiforme. Neurosurg. Focus 20, E19 (2006).
Lamfers, M. L. M. et al. Potential of the conditionally replicative adenovirus Ad5-Δ24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 62, 5736–5742 (2002).
Chiocca, E. A. et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol. Ther. 10, 958–966 (2004).
Bischoff, J. R. et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274, 373–376 (1996).
Coffin, R. S. From virotherapy to oncolytic immunotherapy: where are we now? Curr. Opin. Virol. 13, 93–100 (2015).
Wheeler, L. A. et al. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro. Oncol. 18, 1137–1145 (2016).
Chiocca, E. A. et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J. Clin. Oncol. 29, 3611–3619 (2011).
Ji, N. et al. Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget 7, 4369–4378 (2016).
Phuong, L. K. et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 63, 2462–2469 (2003).
Russell, S. J., Peng, K.-W. & Bell, J. C. Oncolytic virotherapy. Nat. Biotechnol. 30, 658–670 (2012).
Wollmann, G., Ozduman, K. & van den Pol, A. N. Oncolytic virus therapy of glioblastoma multiforme–concepts and candidates. Cancer J. 18, 69 (2012).
Luke, J. J., Flaherty, K. T., Ribas, A. & Long, G. V. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 14, 463 (2017).
Topalian, S. L., Taube, J. M., Anders, R. A. & Pardoll, D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275 (2016).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Lipson, E. J. et al. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin. Oncol. 42, 587–600 (2015).
Berghoff, A. S. et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro. Oncol. 17, 1064–1075 (2015).
Nduom, E. K. et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro. Oncol. 18, 195–205 (2016).
Fecci, P. E. et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+T cell compartment without affecting regulatory T-cell function. Clin. Cancer Res. 13, 2158–2167 (2007).
Reardon, D. A. et al. Glioblastoma eradication following immune checkpoint blockade in an orthotopic, immunocompetent model. Cancer Immunol. Res. 4, 124–135 (2016).
Zeng, J. et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 86, 343–349 (2013).
Weller, M. et al. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat. Rev. Neurol. 13, 363–374 (2017).
Sampson, J. H. et al. Preliminary safety and activity of nivolumab and its combination with ipilimumab in recurrent glioblastoma (GBM): CHECKMATE-143 [abstract]. J. Clin. Oncol. 33 (Suppl.), 3010 (2015).
Reardon, D. A. et al. Randomized phase 3 study evaluating the efficacy and safety of nivolumab versus bevacizumab in patients with recurrent glioblastoma: CheckMate 143 [abstract]. Neuro. Oncol. 19 (Suppl. 3), OS10.3 (2017).
Omuro, A. et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase 1 cohorts of CheckMate 143. Neuro Oncol. https://doi.org/10.1093/neuonc/nox208 (2017).
Lim, M. et al. Nivolumab (nivo) in combination with radiotherapy (RT) ± temozolomide (TMZ): updated safety results from CheckMate 143 in pts with methylated or unmethylated newly diagnosed glioblastoma (GBM) [abstract]. Ann. Oncol. 28 (Suppl. 5), 3250 (2017).
Roth, P., Valavanis, A. & Weller, M. Long-term control and partial remission after initial pseudoprogression of glioblastoma by anti-PD-1 treatment with nivolumab. Neuro. Oncol. 19, 454–456 (2017).
Bouffet, E. et al. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J. Clin. Oncol. 34, 2206–2211 (2016).
Johanns, T. M. et al. Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade immunotherapy. Cancer Discov. 6, 1230–1236 (2016).
[No authors listed.] FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. U.S. Food & Drug Administration https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm (2017).
Maxwell, J. A. et al. Mismatch repair deficiency does not mediate clinical resistance to temozolomide in malignant glioma. Clin. Cancer Res. 14, 4859–4868 (2008).
Jena, B., Dotti, G. & Cooper, L. J. N. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 116, 1035–1044 (2010).
Morgan, R. A. et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Human Gene Therapy 23, 1043–1053 (2012).
Finney, H. M. Akbar, A. N. & Lawson, A. D. G. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCRζ chain. J. Immunol. 172, 104–113 (2004).
Brown, C. E. et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 375, 2561–2569 (2016).
Brown, C. E. et al. Bioactivity and safety of IL13Rα2-redirected chimeric antigen receptor CD8 + T cells in patients with recurrent glioblastoma. Clin. Cancer Res. 21, 4062–4072 (2015).
Neelapu, S. S. et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat. Rev. Clin. Oncol. 15, 47 (2018).
Brown, C. E. et al. Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol. Ther. 26, 31–44 (2018).
O’Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl Med. 9, eaaa0984 (2017).
Dai, H., Wang, Y., Lu, X. & Han, W. Chimeric antigen receptors modified T-cells for cancer therapy. J. Natl. Cancer Inst. 108, djv439 (2016).
Fesnak, A. D., June, C. H. & Levine, B. L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer 16, 566 (2016).
Morales-Kastresana, A., Labiano, S., Quetglas, J. I. & Melero, I. Better performance of CARs deprived of the PD-1 brake. Clin. Cancer Res. 19, 5546–5548 (2013).
Ninomiya, S. et al. Tumor indoleamine 2, 3-dioxygenase (IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood 125, 3905–3916 (2015).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264 (2013).
Mathios, D. et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Sci. Transl Med. 8, 370ra180 (2016).
Wild, A. T. et al. Lymphocyte-sparing effect of stereotactic body radiation therapy in patients with unresectable pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 94, 571–579 (2016).
Yovino, S., Kleinberg, L., Grossman, S. A., Narayanan, M. & Ford, E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 31, 140–144 (2013).
Horvat, T. Z. et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 33, 3193–3198 (2015).
Pitter, K. L. et al. Corticosteroids compromise survival in glioblastoma. Brain 139, 1458–1471 (2016).
Hygino da Cruz, L. C., Rodriguez, I., Domingues, R. C., Gasparetto, E. L. & Sorensen, A. G. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am. J. Neuroradiol. 32, 1978–1985 (2011).
Ryken, T. C. et al. The role of imaging in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J. Neurooncol. 118, 435–460 (2014).
Chiou, V. L. & Burotto, M. Pseudoprogression and immune-related response in solid tumors. J. Clin. Oncol. 33, 3541–3543 (2015).
Hodi, F. S. et al. Evaluation of immune-related response criteria and RECISTv 1.1 in patients with advanced melanoma treated with pembrolizumab. J. Clin. Oncol. 34, 1510–1517 (2016).
Okada, H. et al. Immunotherapy Response Assessment in Neuro-Oncology (iRANO): a report of the RANO Working Group. Lancet Oncol. 16, 534–542 (2015).
Everson, R. G. et al. Cytokine responsiveness of CD8 + T cells is a reproducible biomarker for the clinical efficacy of dendritic cell vaccination in glioblastoma patients. J. Immunother. Cancer 2, 10 (2014).
Zhai, L. et al. The kynurenine to tryptophan ratio as a prognostic tool for glioblastoma patients enrolling in immunotherapy. J. Clin. Neurosci. 22, 1964–1968 (2015).
Patel, S. P. & Kurzrock, R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 14, 847–856 (2015).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Cescon, D. W., Haibe-Kains, B. & Mak, T. W. APOBEC3B expression in breast cancer reflects cellular proliferation, while a deletion polymorphism is associated with immune activation. Proc. Natl Acad. Sci. USA 112, 2841–2846 (2015).
Wu, A. & Lim, M. Issues to consider in designing immunotherapy clinical trials for glioblastoma management. J. Cancer Ther. 7, 573 (2016).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Bettegowda, C. et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl Med. 6, 224ra24 (2014).
Figueroa, J. M. & Carter, B. S. Detection of glioblastoma in biofluids. J. Neurosurg. https://doi.org/10.3171/2017.3.JNS162280 (2017).
Wang, Y. et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc. Natl Acad. Sci. USA 112, 9704–9709 (2015).
Figueroa, J. M. et al. Detection of wtEGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro. Oncol. https://doi.org/10.1093/neuonc/nox085 (2017).
Huang, T. Y. et al. Detection of Histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol. Commun. 5, 28 (2017).
Pentsova, E. I. et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J. Clin. Oncol. 34, 2404–2415 (2016).
De Mattos-Arruda, L. et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 6, 8839 (2015).
Locasale, J. W. et al. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol. Cell. Proteom. 11, M111.014688 (2012).
Hao, C. et al. Cytokine and cytokine receptor mRNA expression in human glioblastomas: evidence of Th1, Th2 and Th3 cytokine dysregulation. Acta Neuropathol. 103, 171–178 (2002).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492 (2011).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Sade-Feldman, M. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8, 1136 (2017).
Gao, J. et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167, 397–404 (2016).
Yeung, J. T. et al. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin. Cancer Res. 19, 1816–1826 (2013).
Ferguson, S. D., Srinivasan, V. M. & Heimberger, A. B. The role of STAT3 in tumor-mediated immune suppression. J. Neurooncol. 123, 385–394 (2015).
Eil, R. et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 537, 539–543 (2016).
Koyama, S. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7, 10501 (2016).
Kim, J. E. et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin. Cancer Res. 23, 124–136 (2017).
Wu, A. et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro. Oncol. 12, 1113–1125 (2010).
Heimberger, A. B. et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin. Cancer Res. 14, 5166–5172 (2008).
Stevens, A., Klöter, I. & Roggendorf, W. Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer 61, 738–743 (1988).
Quail, D. F. et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 352, aad3018 (2016).
Patel, M. A. et al. Agonist anti-GITR monoclonal antibody and stereotactic radiation induce immune-mediated survival advantage in murine intracranial glioma. J. Immunother. Cancer 4, 28 (2016).
Mathios, D. et al. Therapeutic administration of IL-15 superagonist complex ALT-803 leads to long-term survival and durable antitumor immune response in a murine glioblastoma model. Int. J. Cancer 138, 187–194 (2016).
Belcaid, Z. et al. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLOS ONE 9, e101764 (2014).
Sharabi, A. B. et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol. Res. 3, 345–355 (2015).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012).
Chen, J. Y., Hovey, E., Rosenthal, M., Livingstone, A. & Simes, J. Neuro-oncology practices in Australia: a Cooperative Group for Neuro-Oncology patterns of care study. Asia. Pac. J. Clin. Oncol. 10, 162–167 (2014).
Fadul, C. E. et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J. Immunother. 34, 382–389 (2011).
Inogés, S. et al. A phase II trial of autologous dendritic cell vaccination and radiochemotherapy following fluorescence-guided surgery in newly diagnosed glioblastoma patients. J. Transl Med. 15, 104 (2017).
Wheeler, C. J. et al. Vaccination elicits correlated immune and clinical responses in glioblastoma multiforme patients. Cancer Res. 68, 5955–5964 (2008).
Jouanneau, E. et al. Intrinsically de-sialylated CD103+CD8 T cells mediate beneficial anti-glioma immune responses. Cancer Immunol. Immunother. 63, 911–924 (2014).
Bloch, O. et al. Autologous heat shock protein peptide vaccination for newly diagnosed glioblastoma: impact of peripheral PD-L1 expression on response to therapy. Clin. Cancer Res. 23, 3575–3584 (2017).
Vik-Mo, E. O. et al. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol. Immunother. 62, 1499–1509 (2013).
Dutoit, V. et al. IMA950 multipeptide vaccine adjuvanted with poly-ICLC in combination with standard therapy in newly diagnosed HLA-A2 glioblastoma patients [abstract]. Ann. Oncol. 28 (Suppl. 11), 11PD (2017).
Salacz, M. E., Camarata, P. J., Ots, M., Mcintire, J. & Lovick, D. TVI-Brain-1 — a phase I study to test the safety of a combination of autologous cancer cell vaccination, adoptive transfer of cancer antigen-specific effector T cells and low-dose interleukin 2 during treatment of patients with recurrent grade III/IV glioma. Neuro. Oncol. 14, vi43–vi49 (2012).
Bloch, O. et al. Heat-shock protein peptide complex-96 vaccination for recurrent glioblastoma: a phase II, single-arm trial. Neuro. Oncol. 16, 274–279 (2014).
Sloan, A. E. et al. Adoptive immunotherapy in patients with recurrent malignant glioma: preliminary results of using autologous whole-tumor vaccine plus granulocyte-macrophage colony–stimulating factor and adoptive transfer of anti-CD3–activated lymphocytes. Neurosurg. Focus 9, e9 (2000).
Sampson, J. H. et al. A pilot study of IL-2Rα blockade during lymphopenia depletes regulatory T-cells and correlates with enhanced immunity in patients with glioblastoma. PLoS ONE 7, e31046 (2012).
Vlahovic, G. et al. Feasibility and safety study of GBM stem cell tumor amplified RNA immunotherapy in recurrent glioblastoma. Neuro. Oncol. 15, iii68–iii74 (2013).
Fenstermaker, R. A. et al. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol. Immunother. 65, 1339–1352 (2016).
Olin, M. R. et al. Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induces a CD4+IL17+response. J. Immunother. Cancer 2, 4 (2014).
Prins, R. M. et al. Comparison of glioma-associated antigen peptide-loaded versus autologous tumor lysate-loaded dendritic cell vaccination in malignant glioma patients. J. Immunother. 36, 152–157 (2013).
Fu, S. et al. Initial phase 1 study of WT2725 dosing emulsion in patients with advanced malignancies. J. Clin. Oncol. 35, 2066 (2017).
Geletneky, K. et al. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol. Ther. 25, 2620–2634 (2017).
Alonso, M. M. et al. Oncolytic virus DNX-2401 with a short course of temozolomide for glioblastoma at first recurrence: clinical data and prognostic biomarkers [abstract]. Cancer Res. 77 (Suppl.), CT027 (2017).
Markert, J. M. et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 7, 867–874 (2000).
Kicielinski, K. P. et al. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol. Ther. 22, 1056–1062 (2014).
Markert, J. M. et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. 22, 1048–1055 (2014).
Dillman, R. O. et al. Intralesional lymphokine-activated killer cells as adjuvant therapy for primary glioblastoma. J. Immunother. 32, 914–919 (2009).
Plautz, G. E. et al. T cell adoptive immunotherapy of newly diagnosed gliomas. Clin. Cancer Res. 6, 2209 (2000).
Thaci, B. et al. Significance of interleukin-13 receptor alpha 2-targeted glioblastoma therapy. Neuro. Oncol. 16, 1304–1312 (2014).
Reap, E. et al. Dendritic cells enhance polyfunctionality of adoptively transferred T cells which target cytomegalovirus in glioblastoma. Cancer Res. 78, 256–264 (2017).
Acknowledgements
The authors thank A. Wu of the Johns Hopkins University School of Medicine for her help in formatting the manuscript.
Competing interests
M.L. has received research funding from Accuray, Agenus, Altor, Arbor, BMS, Celldex, and Immunocellular, and has been a consultant for Agenus, Baxter, BMS, Boston Biomedical, Oncorus, Regeneron, SQZ Biotechnologies, Stryker, and Tocagen. M.W. has received research grants from Acceleron, Actelion, Bayer, Merck (EMD), MSD, Novocure, OGD2, PIQUR, and Roche, and has received honoraria for lectures, advisory board participation, or consulting from AbbVie, BMS, Celldex, Merck (EMD), MSD, Novocure, Pfizer, Roche, Teva, and Tocagen. Y.X. and C.B. declare no competing interests.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for the article, discussions of content, and writing, reviewing, and editing the manuscript.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov database: https://clinicaltrials.gov/
Supplementary information
Rights and permissions
About this article
Cite this article
Lim, M., Xia, Y., Bettegowda, C. et al. Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15, 422–442 (2018). https://doi.org/10.1038/s41571-018-0003-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-018-0003-5
This article is cited by
-
Systematic review and pooled analysis of the impact of treatment-induced lymphopenia on survival of glioblastoma patients
Radiation Oncology (2024)
-
ZDHHC5-mediated S-palmitoylation of FAK promotes its membrane localization and epithelial-mesenchymal transition in glioma
Cell Communication and Signaling (2024)
-
IGFBP3 induces PD-L1 expression to promote glioblastoma immune evasion
Cancer Cell International (2024)
-
Tumor associated microglia/macrophages utilize GPNMB to promote tumor growth and alter immune cell infiltration in glioma
Acta Neuropathologica Communications (2024)
-
BTLA biology in cancer: from bench discoveries to clinical potentials
Biomarker Research (2024)