Abstract
Silk fibroin has applications in different medical fields such as tissue engineering, regenerative medicine, drug delivery and medical devices. Advances in silk chemistry and biomaterial designs have yielded exciting tools for generating new silk-based materials and technologies. Selective chemistries can enhance or tune the features of silk, such as mechanics, biodegradability, processability and biological interactions, to address challenges in medically relevant materials (hydrogels, films, sponges and fibres). This Review details the design and utility of silk biomaterials for different applications, with particular focus on chemistry. This Review consists of three segments: silk protein fundamentals, silk chemistries and functionalization mechanisms. This is followed by a description of different crosslinking chemistries facilitating network formation, including the formation of composite biomaterials. Utility in the fields of tissue engineering, drug delivery, 3D printing, cell coatings, microfluidics and biosensors are highlighted. Looking to the future, we discuss silk biomaterial design strategies to continue to improve medical outcomes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bucciarelli, A. & Motta, A. Use of Bombyx mori silk fibroin in tissue engineering: from cocoons to medical devices, challenges, and future perspectives. Biomater. Adv. 139, 212982 (2022).
Li, C. et al. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 5, 61–81 (2020).
Li, C. et al. Fiber‐based biopolymer processing as a route toward sustainability. Adv. Mater. 34, 2105196 (2022).
Marelli, B. Biomaterials for boosting food security. Science 376, 146–147 (2022).
Post, M. J. et al. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 1, 403–415 (2020).
Vepari, C. & Kaplan, D. L. Silk as a biomaterial. Prog. Polym. Sci. 32, 991–1007 (2007).
Kundu, S. C., Dash, B. C., Dash, R. & Kaplan, D. L. Natural protective glue protein, sericin bioengineered by silkworms: potential for biomedical and biotechnological applications. Prog. Polym. Sci. 33, 998–1012 (2008).
Omenetto, F. G. & Kaplan, D. L. New opportunities for an ancient material. Science 329, 528–531 (2010).
Department Of Health And Human Services. Code of Federal Regulations Title 21: Natural nonabsorbable silk surgical suture. FDA https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=878.5030 (2023).
Melke, J., Midha, S., Ghosh, S., Ito, K. & Hofmann, S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 31, 1–16 (2016).
Holland, C., Numata, K., Rnjak‐Kovacina, J. & Seib, F. P. The biomedical use of silk: past, present, future. Adv. Healthc. Mater. 8, 1800465 (2019).
Janani, G. et al. Insight into silk-based biomaterials: from physicochemical attributes to recent biomedical applications. ACS Appl. Bio Mater. 2, 5460–5491 (2019).
Fine, N. A. et al. SERI surgical scaffold, prospective clinical trial of a silk-derived biological scaffold in two-stage breast reconstruction: 1-year data. Plast. Reconstr. Surg. 135, 339–351 (2015).
Gulka, C. P. et al. A novel silk‐based vocal fold augmentation material: 6‐month evaluation in a canine model. Laryngoscope 129, 1856–1862 (2019).
Brown, J. E. et al. Injectable silk protein microparticle-based fillers: a novel material for potential use in glottic insufficiency. J. Voice 33, 773–780 (2019).
Levin, B., Rajkhowa, R., Redmond, S. L. & Atlas, M. D. Grafts in myringoplasty: utilizing a silk fibroin scaffold as a novel device. Expert. Rev. Med. Device 6, 653–664 (2009).
Koh, L.-D. et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 46, 86–110 (2015).
Guo, C., Li, C. & Kaplan, D. L. Enzymatic degradation of Bombyx mori silk materials: a review. Biomacromolecules 21, 1678–1686 (2020).
Leal‐Egaña, A. & Scheibel, T. Silk‐based materials for biomedical applications. Biotechnol. Appl. Biochem. 55, 155–167 (2010).
Rockwood, D. N. et al. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 6, 1612 (2011).
Wu, J., Sahoo, J. K., Li, Y., Xu, Q. & Kaplan, D. L. Challenges in delivering therapeutic peptides and proteins: a silk-based solution. J. Control. Release 345, 176–189 (2022).
Falcucci, T. et al. Degradable silk‐based subcutaneous oxygen sensors. Adv. Funct. Mater. 32, 2202020 (2022). This study demonstrates a degradable oxygen-sensing platform based on silk fibroin.
Kasoju, N. & Bora, U. Silk fibroin in tissue engineering. Adv. Healthc. Mater. 1, 393–412 (2012).
Zhang, W. et al. Silk fibroin biomaterial shows safe and effective wound healing in animal models and a randomized controlled clinical trial. Adv. Healthc. Mater. 6, 1700121 (2017).
Farokhi, M., Mottaghitalab, F., Fatahi, Y., Khademhosseini, A. & Kaplan, D. L. Overview of silk fibroin use in wound dressings. Trends Biotechnol. 36, 907–922 (2018).
Wenk, E., Merkle, H. P. & Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control. Release 150, 128–141 (2011).
Agostinacchio, F., Mu, X., Dirè, S., Motta, A. & Kaplan, D. L. In situ 3D printing: opportunities with silk inks. Trends Biotechnol. 39, 719–730 (2021).
Chakraborty, J., Mu, X., Pramanick, A., Kaplan, D. L. & Ghosh, S. Recent advances in bioprinting using silk protein-based bioinks. Biomaterials 287, 121672 (2022).
Chawla, S., Midha, S., Sharma, A. & Ghosh, S. Silk‐based bioinks for 3D bioprinting. Adv. Healthc. Mater. 7, 1701204 (2018).
Hasturk, O. et al. Cytoprotection of human progenitor and stem cells through encapsulation in alginate templated, dual crosslinked silk and silk–gelatin composite hydrogel microbeads. Adv. Healthc. Mater. 11, 2200293 (2022).
Murphy, A. R. & Kaplan, D. L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 19, 6443–6450 (2009).
Ha, S.-W., Gracz, H. S., Tonelli, A. E. & Hudson, S. M. Structural study of irregular amino acid sequences in the heavy chain of Bombyx mori silk fibroin. Biomacromolecules 6, 2563–2569 (2005).
Wang, Q., Chen, Q., Yang, Y. & Shao, Z. Effect of various dissolution systems on the molecular weight of regenerated silk fibroin. Biomacromolecules 14, 285–289 (2013). This study summarizes the effect of various degumming and dissolution processes on silk chain degradation and MW.
Wang, F., Cao, T.-T. & Zhang, Y.-Q. Effect of silk protein surfactant on silk degumming and its properties. Mater. Sci. Eng. C 55, 131–136 (2015).
Zhang, Y. A comparative study of silk degumming methods. Acta Sericol. Sin. 28, 75–79 (2002).
Yuksek, M., Kocak, D., Beyit, A. & Merdan, N. Effect of degumming performed with different type natural soaps and through ultrasonic method on the properties of silk fiber. Adv. Environ. Biol. 6, 801–808 (2012).
Ha, S.-W., Park, Y. H. & Hudson, S. M. Dissolution of Bombyx mori silk fibroin in the calcium nitrate tetrahydrate−methanol system and aspects of wet spinning of fibroin solution. Biomacromolecules 4, 488–496 (2003).
Um, I. C., Kweon, H. Y., Lee, K. G. & Park, Y. H. The role of formic acid in solution stability and crystallization of silk protein polymer. Int. J. Biol. Macromol. 33, 203–213 (2003).
Phillips, D. M. et al. Dissolution and regeneration of Bombyx mori silk fibroin using ionic liquids. J. Am. Chem. Soc. 126, 14350–14351 (2004).
Yao, J., Masuda, H., Zhao, C. & Asakura, T. Artificial spinning and characterization of silk fiber from Bombyx mori silk fibroin in hexafluoroacetone hydrate. Macromolecules 35, 6–9 (2002).
Ha, S.-W., Tonelli, A. E. & Hudson, S. M. Structural studies of Bombyx mori silk fibroin during regeneration from solutions and wet fiber spinning. Biomacromolecules 6, 1722–1731 (2005).
Sahoo, J. K. et al. Silk degumming time controls horseradish peroxidase-catalyzed hydrogel properties. Biomater. Sci. 8, 4176–4185 (2020).
Hasturk, O., Sahoo, J. K. & Kaplan, D. L. Synthesis and characterization of silk ionomers for layer-by-layer electrostatic deposition on individual mammalian cells. Biomacromolecules 21, 2829–2843 (2020).
Sahoo, J. K. et al. Sugar functionalization of silks with pathway‐controlled substitution and properties. Adv. Biol. 5, 2100388 (2021).
Chen, J., Venkatesan, H. & Hu, J. Chemically modified silk proteins. Adv. Eng. Mater. 20, 1700961 (2018).
Serban, M. A. & Kaplan, D. L. pH-sensitive ionomeric particles obtained via chemical conjugation of silk with poly (amino acid)s. Biomacromolecules 11, 3406–3412 (2010).
Heichel, D. L. & Burke, K. A. Enhancing the carboxylation efficiency of silk fibroin through the disruption of noncovalent interactions. Bioconjug. Chem. 31, 1307–1312 (2020).
Murphy, A. R., John, P. S. & Kaplan, D. L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 29, 2829–2838 (2008). This study reports diazonium coupling on silk chains as a modification strategy to alter different material properties.
Gotoh, Y., Tsukada, M., Minoura, N. & Imai, Y. Synthesis of poly (ethylene glycol)-silk fibroin conjugates and surface interaction between L-929 cells and the conjugates. Biomaterials 18, 267–271 (1997).
Gotoh, Y., Niimi, S., Hayakawa, T. & Miyashita, T. Preparation of lactose–silk fibroin conjugates and their application as a scaffold for hepatocyte attachment. Biomaterials 25, 1131–1140 (2004).
Gotoh, Y., Minoura, N. & Miyashita, T. Preparation and characterization of conjugates of silk fibroin and chitooligosaccharides. Colloid Polym. Sci. 280, 562–568 (2002).
Gotoh, Y., Tsukada, M. & Minoura, N. Chemical modification of silk fibroin with cyanuric chloride-activated poly (ethylene glycol): analyses of reaction site by proton NMR spectroscopy and conformation of the conjugates. Bioconjug. Chem. 4, 554–559 (1993).
Gotoh, Y., Tsukada, M., Aiba, S.-I. & Minoura, N. Chemical modification of silk fibroin with N-acetyl-chito-oligosaccharides. Int. J. Biol. Macromol. 18, 19–26 (1996).
Deng, H. Nitrite anions induce nitrosative deamination of peptides and proteins. Rapid Commun. Mass. Spectrom. 20, 3634–3638 (2006).
Tsioris, K. et al. Functionalized‐silk‐based active optofluidic devices. Adv. Funct. Mater. 20, 1083–1089 (2010).
Tamada, Y. Sulfation of silk fibroin by chlorosulfonic acid and the anticoagulant activity. Biomaterials 25, 377–383 (2004).
Liu, X., Xu, W., Zhang, C., Liu, H. & Fang, J. Homogeneous sulfation of silk fibroin in an ionic liquid. Mater. Lett. 143, 302–304 (2015).
Subia, B., Chandra, S., Talukdar, S. & Kundu, S. C. Folate conjugated silk fibroin nanocarriers for targeted drug delivery. Integr. Biol. 6, 203–214 (2014).
Santi, S. et al. A bio-inspired multifunctionalized silk fibroin. ACS Biomater. Sci. Eng. 7, 507–516 (2021).
Vidal, G. et al. Enhanced cellular adhesion on titanium by silk functionalized with titanium binding and RGD peptides. Acta Biomater. 9, 4935–4943 (2013).
Sofia, S., McCarthy, M. B., Gronowicz, G. & Kaplan, D. L. Functionalized silk‐based biomaterials for bone formation. J. Biomed. Mater. Res. 54, 139–148 (2001).
Wang, X. & Kaplan, D. L. Functionalization of silk fibroin with NeutrAvidin and biotin. Macromol. Biosci. 11, 100–110 (2011).
Vepari, C. P. & Kaplan, D. L. Covalently immobilized enzyme gradients within three‐dimensional porous scaffolds. Biotechnol. Bioeng. 93, 1130–1137 (2006).
Fountain, J. N. et al. Towards non‐stick silk: tuning the hydrophobicity of silk fibroin protein. ChemBioChem 23, e202200429 (2022).
Hoyle, C. E. & Bowman, C. N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 49, 1540–1573 (2010).
Battigelli, A., Almeida, B. & Shukla, A. Recent advances in bioorthogonal click chemistry for biomedical applications. Bioconjug. Chem. 33, 263–271 (2022).
Xi, W., Scott, T. F., Kloxin, C. J. & Bowman, C. N. Click chemistry in materials science. Adv. Funct. Mater. 24, 2572–2590 (2014).
Zhao, H. et al. Decoration of silk fibroin by click chemistry for biomedical application. J. Struct. Biol. 186, 420–430 (2014).
Das, S., Pati, D., Tiwari, N., Nisal, A. & Sen Gupta, S. Synthesis of silk fibroin–glycopolypeptide conjugates and their recognition with lectin. Biomacromolecules 13, 3695–3702 (2012).
Raynal, L. et al. Facile and versatile solid state surface modification of silk fibroin membranes using click chemistry. J. Mater. Chem. B 6, 8037–8042 (2018).
Sampaio, S., Miranda, T. M., Santos, J. G. & Soares, G. M. Preparation of silk fibroin–poly (ethylene glycol) conjugate films through click chemistry. Polym. Int. 60, 1737–1744 (2011).
Kaur, J., Saxena, M. & Rishi, N. An overview of recent advances in biomedical applications of click chemistry. Bioconjug. Chem. 32, 1455–1471 (2021).
Zhang, X. et al. Surface modification of Bombyx mori silk fibroin film via thiol–ene click chemistry. Processes 8, 498 (2020).
Zhang, X. et al. Chemical modification of Bombyx mori silk fibers with vinyl groups for thiol–ene click chemistry. BMC Chem. 13, 1–7 (2019).
Zhang, Y. S. & Khademhosseini, A. Advances in engineering hydrogels. Science 356, eaaf3627 (2017).
Kim, U.-J. et al. Structure and properties of silk hydrogels. Biomacromolecules 5, 786–792 (2004).
Wang, X., Kluge, J. A., Leisk, G. G. & Kaplan, D. L. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials 29, 1054–1064 (2008).
Gorenkova, N., Osama, I., Seib, F. P. & Carswell, H. V. In vivo evaluation of engineered self-assembling silk fibroin hydrogels after intracerebral injection in a rat stroke model. ACS Biomater. Sci. Eng. 5, 859–869 (2018).
Yucel, T., Cebe, P. & Kaplan, D. L. Vortex-induced injectable silk fibroin hydrogels. Biophys. J. 97, 2044–2050 (2009).
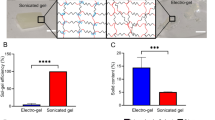
Leisk, G. G., Lo, T. J., Yucel, T., Lu, Q. & Kaplan, D. L. Electrogelation for protein adhesives. Adv. Mater. 22, 711–715 (2010). This study features a report on electric-field-assisted silk-based reversible hydrogel adhesives.
Wu, X. et al. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin. Acta Biomater. 8, 2185–2192 (2012).
Nagarkar, S. et al. Some mechanistic insights into the gelation of regenerated silk fibroin sol. Ind. Eng. Chem. Res. 48, 8014–8023 (2009).
Matsumoto, A. et al. Mechanisms of silk fibroin sol−gel transitions. J. Phys. Chem. B 110, 21630–21638 (2006).
Kim, H. et al. Effect of silk fibroin molecular weight on physical property of silk hydrogel. Polymer 90, 26–33 (2016). This study demonstrates the effect of silk MW on the physically crosslinked silk hydrogel properties.
Lu, Q. et al. Silk fibroin electrogelation mechanisms. Acta Biomater. 7, 2394–2400 (2011).
Farokhi, M. et al. Crosslinking strategies for silk fibroin hydrogels: promising biomedical materials. Biomed. Mater. 16, 022004 (2021).
Kaewprasit, K., Kobayashi, T. & Damrongsakkul, S. Thai silk fibroin gelation process enhancing by monohydric and polyhydric alcohols. Int. J. Biol. Macromol. 118, 1726–1735 (2018). This study explores the role of different alcohols in silk fibroin gelation.
Partlow, B. P., Applegate, M. B., Omenetto, F. G. & Kaplan, D. L. Dityrosine cross-linking in designing biomaterials. ACS Biomater. Sci. Eng. 2, 2108–2121 (2016).
Raven, D. J., Earland, C. & Little, M. Occurrence of dityrosine in Tussah silk fibroin and keratin. Biochim. Biophys. Acta Prot. Struct. 251, 96–99 (1971).
Andersen, S. O. The cross-links in resilin identified as dityrosine and trityrosine. Biochim. Biophys. Acta Gen. Subj. 93, 213–215 (1964).
Partlow, B. P. et al. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 24, 4615–4624 (2014). This study demonstrates tunable, elastomeric, enzymatically crosslinked hydrogels based on silk.
Tabatabai, A. P., Partlow, B. P., Raia, N. R., Kaplan, D. L. & Blair, D. L. Silk molecular weight influences the kinetics of enzymatically cross-linked silk hydrogel formation. Langmuir 34, 15383–15387 (2018).
McGill, M., Coburn, J. M., Partlow, B. P., Mu, X. & Kaplan, D. L. Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design. Acta Biomater. 63, 76–84 (2017).
Hasturk, O., Jordan, K. E., Choi, J. & Kaplan, D. L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 232, 119720 (2020). This study demonstrates improvement of different biomaterial properties of silk hydrogels by altering formulation and chemical modification.
Raia, N. R., Jia, D., Ghezzi, C. E., Muthukumar, M. & Kaplan, D. L. Characterization of silk-hyaluronic acid composite hydrogels towards vitreous humor substitutes. Biomaterials 233, 119729 (2020). This study explores silk–hyaluronic acid composite hydrogels as potential vitreous substitutes.
Raia, N. R. et al. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 131, 58–67 (2017). Silk and hyaluronic acid are enzymatically crosslinked to form a composite hydrogel system and their biomaterial properties are explored.
Choi, J., McGill, M., Raia, N. R., Hasturk, O. & Kaplan, D. L. Silk hydrogels crosslinked by the fenton reaction. Adv. Healthc. Mater. 8, 1900644 (2019). This study establishes a report of the Fenton reaction to facilitate silk hydrogel formation.
Choi, J., Hasturk, O., Mu, X., Sahoo, J. K. & Kaplan, D. L. Silk hydrogels with controllable formation of dityrosine, 3,4-dihydroxyphenylalanine, and 3,4-dihydroxyphenylalanine–Fe3+ complexes through chitosan particle-assisted Fenton reactions. Biomacromolecules 22, 773–787 (2021).
Whittaker, J. L., Choudhury, N. R., Dutta, N. K. & Zannettino, A. Facile and rapid ruthenium mediated photo-crosslinking of Bombyx mori silk fibroin. J. Mater. Chem. B 2, 6259–6270 (2014).
Cui, X. et al. Rapid photocrosslinking of silk hydrogels with high cell density and enhanced shape fidelity. Adv. Healthc. Mater. 9, 1901667 (2020).
Applegate, M. B. et al. Photocrosslinking of silk fibroin using riboflavin for ocular prostheses. Adv. Mater. 28, 2417–2420 (2016).
Piluso, S. et al. Rapid and cytocompatible cell-laden silk hydrogel formation via riboflavin-mediated crosslinking. J. Mater. Chem. B 8, 9566–9575 (2020).
Karimi, F. et al. Surface biofunctionalization of silk biomaterials using dityrosine cross-linking. ACS Appl. Mater. Interfaces 14, 31551–31566 (2022).
Ryu, S. et al. Dual mode gelation behavior of silk fibroin microgel embedded poly(ethylene glycol) hydrogels. J. Mater. Chem. B 4, 4574–4584 (2016).
Chen, S. et al. Construction of injectable silk fibroin/polydopamine hydrogel for treatment of spinal cord injury. Chem. Eng. J. 399, 125795 (2020).
Kharkar, P. M., Rehmann, M. S., Skeens, K. M., Maverakis, E. & Kloxin, A. M. Thiol–ene click hydrogels for therapeutic delivery. ACS Biomater. Sci. Eng. 2, 165–179 (2016).
Huang, X., Zhang, M., Ming, J., Ning, X. & Bai, S. High-strength and high-toughness silk fibroin hydrogels: a strategy using dynamic host–guest interactions. ACS Appl. Bio Mater. 3, 7103–7112 (2020).
Yu, R., Yang, Y., He, J., Li, M. & Guo, B. Novel supramolecular self-healing silk fibroin-based hydrogel via host–guest interaction as wound dressing to enhance wound healing. Chem. Eng. J. 417, 128278 (2021). Host–guest molecular recognition was used to facilitate silk hydrogel formation with self-healing and injectable properties.
Liu, L., Han, Y. & Lv, S. Design of self-healing and electrically conductive silk fibroin-based hydrogels. ACS Appl. Mater. Interfaces 11, 20394–20403 (2019).
Lu, Q., Han, Y., Ma, Y. & Lv, S. Design of silk fibroin-based supramolecular hydrogels through host–guest interactions: influence of the crosslinking type. Colloids Surf. A Physicochem. Eng. Asp. 652, 129898 (2022).
Bai, L. et al. Surface modification and properties of Bombyx mori silk fibroin films by antimicrobial peptide. Appl. Surf. Sci. 254, 2988–2995 (2008).
Gil, E. S. et al. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 31, 8953–8963 (2010).
Karageorgiou, V. et al. Bone morphogenetic protein‐2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J. Biomed. Mater. Res. Part A 71, 528–537 (2004).
Wu, J. et al. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials 35, 3744–3755 (2014).
Chen, J. et al. Human bone marrow stromal cell and ligament fibroblast responses on RGD‐modified silk fibers. J. Biomed. Mater. Res. Part A 67, 559–570 (2003).
Chang, G., Kim, H.-J., Kaplan, D., Vunjak-Novakovic, G. & Kandel, R. Porous silk scaffolds can be used for tissue engineering annulus fibrosus. Eur. Spine J. 16, 1848–1857 (2007).
Kim, J. W., Ki, C. S., Park, Y. H., Kim, H. J. & Um, I. C. Effect of RGDS and KRSR peptides immobilized on silk fibroin nanofibrous mats for cell adhesion and proliferation. Macromol. Res. 18, 442–448 (2010).
Sun, J., Zhang, Y., Li, B., Gu, Y. & Chen, L. Controlled release of BMP-2 from a collagen-mimetic peptide-modified silk fibroin–nanohydroxyapatite scaffold for bone regeneration. J. Mater. Chem. B 5, 8770–8779 (2017).
McGill, M., Grant, J. M. & Kaplan, D. L. Enzyme-mediated conjugation of peptides to silk fibroin for facile hydrogel functionalization. Ann. Biomed. Eng. 48, 1905–1915 (2020).
Tsui, J. H. et al. Conductive silk–polypyrrole composite scaffolds with bioinspired nanotopographic cues for cardiac tissue engineering. J. Mater. Chem. B 6, 7185–7196 (2018).
Sun, W. et al. Viability and neuronal differentiation of neural stem cells encapsulated in silk fibroin hydrogel functionalized with an IKVAV peptide. J. Tissue Eng. Regen. Med. 11, 1532–1541 (2017).
Canabady-Rochelle, L. L. et al. Bioinspired silicification of silica-binding peptide–silk protein chimeras: comparison of chemically and genetically produced proteins. Biomacromolecules 13, 683–690 (2012).
Reeves, A. R., Spiller, K. L., Freytes, D. O., Vunjak-Novakovic, G. & Kaplan, D. L. Controlled release of cytokines using silk-biomaterials for macrophage polarization. Biomaterials 73, 272–283 (2015).
Brown, J. E. et al. Shape memory silk protein sponges for minimally invasive tissue regeneration. Adv. Healthc. Mater. 6, 1600762 (2017).
Qian, Y. et al. Surface modification of nanofibrous matrices via layer-by-layer functionalized silk assembly for mitigating the foreign body reaction. Biomaterials 164, 22–37 (2018).
Calabrese, R. & Kaplan, D. L. Silk ionomers for encapsulation and differentiation of human MSCs. Biomaterials 33, 7375–7385 (2012).
Pallotta, I. et al. Characteristics of platelet gels combined with silk. Biomaterials 35, 3678–3687 (2014).
Johnston, E. R., Miyagi, Y., Chuah, J.-A., Numata, K. & Serban, M. A. Interplay between silk fibroin’s structure and its adhesive properties. ACS Biomater. Sci. Eng. 4, 2815–2824 (2018).
Heichel, D. L. & Burke, K. A. Dual-mode cross-linking enhances adhesion of silk fibroin hydrogels to intestinal tissue. ACS Biomater. Sci. Eng. 5, 3246–3259 (2019).
Serban, M. A., Panilaitis, B. & Kaplan, D. L. Silk fibroin and polyethylene glycol‐based biocompatible tissue adhesives. J. Biomed. Mater. Res. Part A 98, 567–575 (2011).
Burke, K. A., Roberts, D. C. & Kaplan, D. L. Silk fibroin aqueous-based adhesives inspired by mussel adhesive proteins. Biomacromolecules 17, 237–245 (2016). Catechol-modified silks are developed and used as adhesives.
Yin, Z. et al. Controllable performance of a dopamine-modified silk fibroin-based bio-adhesive by doping metal ions. Biomed. Mater. 16, 045025 (2021).
Gao, X. et al. A medical adhesive used in a wet environment by blending tannic acid and silk fibroin. Biomater. Sci. 8, 2694–2701 (2020).
Zheng, H., Lin, N., He, Y. & Zuo, B. Self-healing, self-adhesive silk fibroin conductive hydrogel as a flexible strain sensor. ACS Appl. Mater. Interfaces 13, 40013–40031 (2021).
Seo, J. W., Kim, H., Kim, K., Choi, S. Q. & Lee, H. J. Calcium‐modified silk as a biocompatible and strong adhesive for epidermal electronics. Adv. Funct. Mater. 28, 1800802 (2018). This study demonstrates calcium-modified silk as a strong, conductive, reusable and biocompatible adhesive.
Zheng, Z. et al. 3D bioprinting of self‐standing silk‐based bioink. Adv. Healthc. Mater. 7, 1701026 (2018).
Ghosh, S., Parker, S. T., Wang, X., Kaplan, D. L. & Lewis, J. A. Direct‐write assembly of microperiodic silk fibroin scaffolds for tissue engineering applications. Adv. Funct. Mater. 18, 1883–1889 (2008).
Rodriguez, M. J. et al. 3D freeform printing of silk fibroin. Acta Biomater. 71, 379–387 (2018).
Mu, X. et al. 3D printing of monolithic proteinaceous cantilevers using regenerated silk fibroin. Molecules 27, 2148 (2022).
Mu, X. et al. 3D printing of silk protein structures by aqueous solvent‐directed molecular assembly. Macromol. Biosci. 20, 1900191 (2020). This study developes 3D-prining techniques on the basis of silk fibroin.
Sommer, M. R., Schaffner, M., Carnelli, D. & Studart, A. R. 3D printing of hierarchical silk fibroin structures. ACS Appl. Mater. Interfaces 8, 34677–34685 (2016).
Du, X. et al. 3D printing of mesoporous bioactive glass/silk fibroin composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 103, 109731 (2019).
Fitzpatrick, V. et al. Functionalized 3D-printed silk-hydroxyapatite scaffolds for enhanced bone regeneration with innervation and vascularization. Biomaterials 276, 120995 (2021).
Zhou, M. et al. Copper peptide-incorporated 3D-printed silk-based scaffolds promote vascularized bone regeneration. Chem. Eng. J. 422, 130147 (2021).
Jose, R. R., Brown, J. E., Polido, K. E., Omenetto, F. G. & Kaplan, D. L. Polyol–silk bioink formulations as two-part room-temperature curable materials for 3D printing. ACS Biomater. Sci. Eng. 1, 780–788 (2015).
Rodriguez, M. J. et al. Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials 117, 105–115 (2017).
Liu, C. et al. 3D printing silk–gelatin–propanediol scaffold with enhanced osteogenesis properties through p-Smad1/5/8 activated Runx2 pathway. J. Biomater. Sci. Polym. Ed. 32, 1515–1529 (2021).
Karamat-Ullah, N. et al. 3D printing of antibacterial, biocompatible, and biomimetic hybrid aerogel-based scaffolds with hierarchical porosities via integrating antibacterial peptide-modified silk fibroin with silica nanostructure. ACS Biomater. Sci. Eng. 7, 4545–4556 (2021).
Zhang, X. et al. Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering. Mater. Sci. Eng. C 118, 111388 (2021).
Floren, M., Migliaresi, C. & Motta, A. Processing techniques and applications of silk hydrogels in bioengineering. J. Funct. Biomater. 7, 26 (2016).
Compaan, A. M., Christensen, K. & Huang, Y. Inkjet bioprinting of 3D silk fibroin cellular constructs using sacrificial alginate. ACS Biomater. Sci. Eng. 3, 1519–1526 (2017).
Heichel, D. L., Tumbic, J. A., Boch, M. E., Ma, A. W. & Burke, K. A. Silk fibroin reactive inks for 3D printing crypt-like structures. Biomed. Mater. 15, 055037 (2020).
Trucco, D. et al. Modeling and fabrication of silk fibroin–gelatin-based constructs using extrusion-based three-dimensional bioprinting. ACS Biomater. Sci. Eng. 7, 3306–3320 (2021).
Dickerson, M. B. et al. 3D printing of regenerated silk fibroin and antibody-containing microstructures via multiphoton lithography. ACS Biomater. Sci. Eng. 3, 2064–2075 (2017).
Kim, S. H. et al. 3D bioprinted silk fibroin hydrogels for tissue engineering. Nat. Protoc. 16, 5484–5532 (2021).
Kim, S. H. et al. Precisely printable and biocompatible silk fibroin bioink for digital light processing 3D printing. Nat. Commun. 9, 1620 (2018). This study explores methacrylated silk fibroin as bioink for digital light processing 3D bioprinting for tissue engineering applications.
Hong, H. et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 232, 119679 (2020).
Rajput, M., Mondal, P., Yadav, P. & Chatterjee, K. Light-based 3D bioprinting of bone tissue scaffolds with tunable mechanical properties and architecture from photocurable silk fibroin. Int. J. Biol. Macromol. 202, 644–656 (2022).
Ye, C. et al. Robust and responsive silk ionomer microcapsules. Biomacromolecules 12, 4319–4325 (2011).
Shchepelina, O., Drachuk, I., Gupta, M. K., Lin, J. & Tsukruk, V. V. Silk‐on‐silk layer‐by‐layer microcapsules. Adv. Mater. 23, 4655–4660 (2011).
Ye, C. et al. Permeability and micromechanical properties of silk ionomer microcapsules. Langmuir 28, 12235–12244 (2012).
Chiang, M.-Y. et al. 4D spatiotemporal modulation of biomolecules distribution in anisotropic corrugated microwrinkles via electrically manipulated microcapsules within hierarchical hydrogel for spinal cord regeneration. Biomaterials 271, 120762 (2021).
Coburn, J. M., Na, E. & Kaplan, D. L. Modulation of vincristine and doxorubicin binding and release from silk films. J. Control. Rel. 220, 229–238 (2015).
Wenk, E. et al. The use of sulfonated silk fibroin derivatives to control binding, delivery and potency of FGF-2 in tissue regeneration. Biomaterials 31, 1403–1413 (2010).
Atterberry, P. N. et al. Sustained delivery of chemokine CXCL12 from chemically modified silk hydrogels. Biomacromolecules 16, 1582–1589 (2015).
Murab, S. et al. Matrix-embedded cytokines to simulate osteoarthritis-like cartilage microenvironments. Tissue Eng. Part A 19, 1733–1753 (2013).
Mao, B. et al. Cyclic cRGDfk peptide and chlorin e6 functionalized silk fibroin nanoparticles for targeted drug delivery and photodynamic therapy. Biomaterials 161, 306–320 (2018).
Hou, W. et al. Decorating bacteria with a therapeutic nanocoating for synergistically enhanced biotherapy. Small 17, 2101810 (2021).
Drachuk, I. et al. Silk macromolecules with amino acid–poly(ethylene glycol) grafts for controlling layer-by-layer encapsulation and aggregation of recombinant bacterial cells. ACS Nano 9, 1219–1235 (2015).
Hasturk, O. et al. Silk nanocoatings of mammalian cells for cytoprotection against mechanical stress. MRS Bull. 46, 795–806 (2021).
Bettinger, C. J. et al. Silk fibroin microfluidic devices. Adv. Mater. 19, 2847–2850 (2007). This study features fabrication of microfluidic devices on the basis of silk by aqueous moulding techniques.
Zhou, M. et al. Constructing silk fibroin-based three-dimensional microfluidic devices via a tape mask-assisted multiple-step etching technique. ACS Appl. Bio Mater. 4, 8039–8048 (2021).
Zhao, S. et al. Bio-functionalized silk hydrogel microfluidic systems. Biomaterials 93, 60–70 (2016). This study develops a biofunctionalized microfluidic system on the basis of silk hydrogel by the multilayer fabrication method.
Zhu, B. et al. Silk fibroin for flexible electronic devices. Adv. Mater. 28, 4250–4265 (2016).
Wang, C., Xia, K., Zhang, Y. & Kaplan, D. L. Silk-based advanced materials for soft electronics. Acc. Chem. Res. 52, 2916–2927 (2019).
Matzeu, G. et al. Large‐scale patterning of reactive surfaces for wearable and environmentally deployable sensors. Adv. Mater. 32, 2001258 (2020).
Xu, M., Jiang, Y., Pradhan, S. & Yadavalli, V. K. Use of silk proteins to form organic, flexible, degradable biosensors for metabolite monitoring. Front. Mater. 6, 331 (2019).
He, F. et al. Stretchable, biocompatible, and multifunctional silk fibroin-based hydrogels toward wearable strain/pressure sensors and triboelectric nanogenerators. ACS Appl. Mater. Interfaces 12, 6442–6450 (2020).
Hu, Y. et al. Flexible sensor based on Fe3O4‐COOH@ Ti3C2Tx MXene rapid‐gelating hydrogel for human motion monitoring. Adv. Mater. Interfaces 9, 2200487 (2022).
Wen, D.-L. et al. Silk fibroin-based wearable all-fiber multifunctional sensor for smart clothing. Adv. Fiber Mater. 4, 873–884 (2022).
Wang, C. et al. Carbonized silk fabric for ultrastretchable, highly sensitive, and wearable strain sensors. Adv. Mater. 28, 6640–6648 (2016).
Wang, Q., Jian, M., Wang, C. & Zhang, Y. Carbonized silk nanofiber membrane for transparent and sensitive electronic skin. Adv. Funct. Mater. 27, 1605657 (2017).
Wang, Q. et al. Self‐healable multifunctional electronic tattoos based on silk and graphene. Adv. Funct. Mater. 29, 1808695 (2019).
Cui, Y. et al. A stretchable and transparent electrode based on PEGylated silk fibroin for in vivo dual‐modal neural‐vascular activity probing. Adv. Mater. 33, 2100221 (2021).
Choi, J. et al. Instantaneous formation of silk protein aerosols and fibers with a portable spray device under ambient conditions. Adv. Mater. Technol. https://doi.org/10.1002/admt.202201392 (2023).
Liu, C. et al. Toward large-scale fabrication of triboelectric nanogenerator (TENG) with silk-fibroin patches film via spray-coating process. Nano Energy 41, 359–366 (2017).
Wang, C. et al. Silk-derived highly active oxygen electrocatalysts for flexible and rechargeable Zn–air batteries. Chem. Mater. 31, 1023–1029 (2019).
DeBari, M. K., King, C. I. III, Altgold, T. A. & Abbott, R. D. Silk fibroin as a green material. ACS Biomater. Sci. Eng. 7, 3530–3544 (2021).
Cao, Y. & Wang, B. Biodegradation of silk biomaterials. Int. J. Mol. Sci. 10, 1514–1524 (2009).
Huang, W. et al. Design of multistimuli responsive hydrogels using integrated modeling and genetically engineered silk–elastin‐like proteins. Adv. Funct. Mater. 26, 4113–4123 (2016).
Huang, W., Rollett, A. & Kaplan, D. L. Silk–elastin-like protein biomaterials for the controlled delivery of therapeutics. Expert. Opin. Drug Deliv. 12, 779–791 (2015).
Acknowledgements
The authors gratefully acknowledge NIH, the National Science Foundation, the Air Force Office of Scientific Research and the Army Research Office for funding that led to some of the research cited here. The authors thank Y.-T. L. Dingle of Pipette and Stylus LLC for her assistance with some of the figure preparations.
Author information
Authors and Affiliations
Contributions
J.K.S. and D.L.K. conceived the topic and designed the structure and content of the Review. J.K.S. wrote the first draft with contributions from O.H. and T.F. All authors contributed to the discussion, reviewing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Philipp Seib, Saphia Matthew, Yingying Zhang and Keiji Numata for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- 3D printing
-
Three-dimensional deposition, construction and solidification of biomaterials with a predefined geometry and composition.
- β-Sheet
-
Secondary structure of proteins formed by hydrogen bonds between silk chains.
- Biocompatibility
-
One of the most important properties while designing a biomaterial for biomedical application in which a material is compatible with the host microenvironment without inducing any inflammatory or cytotoxic effect.
- Bioinks
-
Materials used in the 3D bioprinting process for deposition and fabrication of 3D structures.
- Carbodiimide coupling
-
Chemical conjugation reaction between a carboxylic acid and amine residue that leads to the formation of an amide bond.
- Crosslinking
-
Specific bond formation that initiates and facilitates network formation and gelation.
- Hydrogels
-
Crosslinked polymeric networks, formed by entanglement of polymer chains in aqueous environment.
- Sacrificial networks
-
Weak, reversible networks present within a stronger network and broken before the stronger, backbone network.
- Silk fibroin
-
Structural, fibrous protein polymer, extracted from the cocoons of Bombyx mori silkworm.
- Stiffness
-
Measured by elastic modulus, stiffness is the ability of hydrogel to resist deformation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahoo, J.K., Hasturk, O., Falcucci, T. et al. Silk chemistry and biomedical material designs. Nat Rev Chem 7, 302–318 (2023). https://doi.org/10.1038/s41570-023-00486-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00486-x
This article is cited by
-
Silk fibroin hydrogel adhesive enables sealed-tight reconstruction of meniscus tears
Nature Communications (2024)