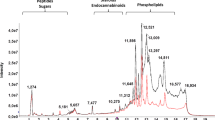
Abstract
The chirality of small metabolic molecules is important in controlling physiological processes and indicating the health status of humans. Abnormal enantiomeric ratios of chiral molecules in biofluids and tissues occur in many diseases, including cancers and kidney and brain diseases. Thus, chiral small molecules are promising biomarkers for disease diagnosis, prognosis, adverse drug-effect monitoring, pharmacodynamic studies and personalized medicine. However, it remains difficult to achieve cost-effective and reliable analysis of small chiral molecules in clinical procedures, in part owing to their large variety and low concentration. In this Review, we describe current and emerging techniques that detect and quantify small-molecule enantiomers and their biological importance.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Etzioni, R. et al. The case for early detection. Nat. Rev. Cancer 3, 243–252 (2003).
Hanash, S. M., Pitteri, S. J. & Faca, V. M. Mining the plasma proteome for cancer biomarkers. Nature 452, 571–579 (2008).
Blennow, K., Hampel, H., Weiner, M. & Zetterberg, H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat. Rev. Neurol. 6, 131–144 (2010).
Rinschen, M. M., Ivanisevic, J., Giera, M. & Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 20, 353–367 (2019).
Rochfort, S. Metabolomics reviewed: a new “omics” platform technology for systems biology and implications for natural products research. J. Nat. Prod. 68, 1813–1820 (2005).
Gomez-Casati, D. F., Zanor, M. I. & Busi, M. V. Metabolomics in plants and humans: applications in the prevention and diagnosis of diseases. Biomed. Res. Int. 2013, 792527 (2013).
Quack, M. Structure and dynamics of chiral molecules. Angew. Chem. Int. Ed. 28, 571–586 (1989).
Evans, P. R. An introduction to stereochemical restraints. Acta Crystallogr. D 63, 58–61 (2007).
Blackmond, D. G. Autocatalytic models for the origin of biological homochirality. Chem. Rev. 120, 4831–4847 (2019).
Plasson, R., Kondepudi, D. K., Bersini, H., Commeyras, A. & Asakura, K. Emergence of homochirality in far‐from‐equilibrium systems: mechanisms and role in prebiotic chemistry. Chirality 19, 589–600 (2007).
Shen, Q., Wang, L., Zhou, H., Yu, L.-S. & Zeng, S. Stereoselective binding of chiral drugs to plasma proteins. Acta Pharmacol. Sin. 34, 998–1006 (2013).
Müller, T. & Kohler, H.-P. Chirality of pollutants — effects on metabolism and fate. Appl. Microbiol. Biotechnol. 64, 300–316 (2004).
Armstrong, D. W., Zukowski, J., Ercal, N. & Gasper, M. Stereochemistry of pipecolic acid found in the urine and plasma of subjects with peroxisomal deficiencies. J. Pharm. Biomed. Anal. 11, 881–886 (1993).
Armstrong, D. W., Gasper, M., Lee, S. H., Zukowski, J. & Ercal, N. d-Amino acid levels in human physiological fluids. Chirality 5, 375–378 (1993).
Utembe, W. Chirality, a neglected physico-chemical property of nanomaterials? A mini-review on the occurrence and importance of chirality on their toxicity. Toxicol. Lett. 311, 58–65 (2019).
Abdulbagi, M., Wang, L., Siddig, O., Di, B. & Li, B. d-Amino acids and d-amino acid-containing peptides: potential disease biomarkers and therapeutic targets? Biomolecules 11, 1716 (2021).
Kranendijk, M., Struys, E. A., Salomons, G. S., Van der Knaap, M. S. & Jakobs, C. Progress in understanding 2-hydroxyglutaric acidurias. J. Inherit. Metab. Dis. 35, 571–587 (2012).
Talasniemi, J. P., Pennanen, S., Savolainen, H., Niskanen, L. & Liesivuori, J. Analytical investigation: assay of d-lactate in diabetic plasma and urine. Clin. Biochem. 41, 1099–1103 (2008).
Hashimoto, A., Oka, T. & Nishikawa, T. Extracellular concentration of endogenous free d-serine in the rat brain as revealed by in vivo microdialysis. Neuroscience 66, 635–643 (1995).
Hashimoto, A. et al. The presence of free d-serine in rat brain. FEBS Lett. 296, 33–36 (1992).
Mothet, J.-P. et al. d-Serine is an endogenous ligand for the glycine site of the N-methyl-d-aspartate receptor. Proc. Natl Acad. Sci. USA 97, 4926–4931 (2000).
Le Bail, M. et al. Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc. Natl Acad. Sci. USA 112, E204–E213 (2015).
Mothet, J. P., Le Bail, M. & Billard, J. M. Time and space profiling of NMDA receptor co‐agonist functions. J. Neurochem. 135, 210–225 (2015).
Dallérac, G. et al. Dopaminergic neuromodulation of prefrontal cortex activity requires the NMDA receptor coagonist d-serine. Proc. Natl Acad. Sci. USA 118, e2023750118 (2021).
Wolosker, H., Blackshaw, S. & Snyder, S. H. Serine racemase: a glial enzyme synthesizing d-serine to regulate glutamate-N-methyl-d-aspartate neurotransmission. Proc. Natl Acad. Sci. USA 96, 13409–13414 (1999).
Sason, H. et al. Asc-1 transporter regulation of synaptic activity via the tonic release of d-serine in the forebrain. Cereb. Cortex 27, 1573–1587 (2017).
Ivanov, A. D. & Mothet, J.-P. The plastic d-serine signaling pathway: sliding from neurons to glia and vice-versa. Neurosci. Lett. 689, 21–25 (2019).
Foltyn, V. N. et al. Serine racemase modulates intracellular d-serine levels through an α,β-elimination activity. J. Biol. Chem. 280, 1754–1763 (2005).
Schell, M. J., Molliver, M. E. & Snyder, S. H. d-Serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc. Natl Acad. Sci. USA 92, 3948–3952 (1995).
Perez, E. J. et al. Enhanced astrocytic d-serine underlies synaptic damage after traumatic brain injury. J. Clin. Invest. 127, 3114–3125 (2017).
Tapanes, S. A. et al. Inhibition of glial d‐serine release rescues synaptic damage after brain injury. Glia 70, 1133–1152 (2022).
Mustafa, A. K. et al. Serine racemase deletion protects against cerebral ischemia and excitotoxicity. J. Neurosci. Res. 30, 1413–1416 (2010).
Bendikov, I. et al. A CSF and postmortem brain study of d-serine metabolic parameters in schizophrenia. Schizophr. Res. 90, 41–51 (2007).
Wolosker, H. & Balu, D. T. d-Serine as the gatekeeper of NMDA receptor activity: implications for the pharmacologic management of anxiety disorders. Transl. Psychiatry 10, 1–10 (2020).
Klatte, K. et al. Impaired d-serine-mediated cotransmission mediates cognitive dysfunction in epilepsy. J. Neurosci. Res. 33, 13066–13080 (2013).
Beesley, S. et al. d-Serine mitigates cell loss associated with temporal lobe epilepsy. Nat. Commun. 11, 4966 (2020).
Mitchell, J. et al. Familial amyotrophic lateral sclerosis is associated with a mutation in d-amino acid oxidase. Proc. Natl Acad. Sci USA 107, 7556–7561 (2010).
Sasabe, J. et al. d-Amino acid oxidase controls motoneuron degeneration through d-serine. Proc. Natl Acad. Sci. USA 109, 627–632 (2012).
Spasova, K. & Fähling, M. d-Serine — A Useful Biomarker for Renal Injury? (Wiley, 2020).
Hamase, K., Morikawa, A. & Zaitsu, K. d-Amino acids in mammals and their diagnostic value. J. Chromatogr. B 781, 73–91 (2002).
Johnson, C. H., Ivanisevic, J. & Siuzdak, G. Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 17, 451–459 (2016).
Schrimpe-Rutledge, A. C., Codreanu, S. G., Sherrod, S. D. & McLean, J. A. Untargeted metabolomics strategies — challenges and emerging directions. J. Am. Soc. Mass Spectrom. 27, 1897–1905 (2016).
Armstrong, D. Analysis of d-amino acids: relevance in human disease. LC GC N. Am. 40, 356–360 (2022).
Murtas, G. & Pollegioni, L. d-Amino acids as novel blood-based biomarkers. Curr. Med. Chem. 29, 4202–4215 (2022).
Paoletti, P., Bellone, C. & Zhou, Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 14, 383–400 (2013).
Hansen, K. B. et al. Structure, function, and allosteric modulation of NMDA receptors. J. Gen. Physiol. 150, 1081–1105 (2018).
Fuchs, S. A., Berger, R., Klomp, L. W. & De Koning, T. J. d-Amino acids in the central nervous system in health and disease. Mol. Genet. Metab. 85, 168–180 (2005).
Wong, J. M. et al. Postsynaptic serine racemase regulates NMDA receptor function. J. Neurosci. 40, 9564–9575 (2020).
Balu, D. T. et al. Serine racemase and d-serine in the amygdala are dynamically involved in fear learning. Biol. Psychiatry 83, 273–283 (2018).
Balu, D. T. et al. Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc. Natl Acad. Sci. USA 110, E2400–E2409 (2013).
Basu, A. C. et al. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry 14, 719–727 (2009).
Shleper, M., Kartvelishvily, E. & Wolosker, H. d-Serine is the dominant endogenous coagonist for NMDA receptor neurotoxicity in organotypic hippocampal slices. J. Neurosci. 25, 9413–9417 (2005).
Katsuki, H., Nonaka, M., Shirakawa, H., Kume, T. & Akaike, A. Endogenous d-serine is involved in induction of neuronal death by N-methyl-d-aspartate and simulated ischemia in rat cerebrocortical slices. J. Pharmacol. Exp. Ther. 311, 836–844 (2004).
Piubelli, L., Murtas, G., Rabattoni, V. & Pollegioni, L. The role of d-amino acids in Alzheimer’s disease. J. Alzheimers Dis. 80, 475–492 (2021).
Ghatak, S., Talantova, M., McKercher, S. R. & Lipton, S. A. Novel therapeutic approach for excitatory/inhibitory imbalance in neurodevelopmental and neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 61, 701–721 (2021).
Hashimoto, K. et al. Decreased serum levels of d-serine in patients with schizophrenia: evidence in support of the N-methyl-d-aspartate receptor hypofunction hypothesis of schizophrenia. Arch. Gen. Psychiatry 60, 572–576 (2003).
Mothet, J. et al. A critical role for the glial-derived neuromodulator d-serine in the age-related deficits of cellular mechanisms of learning and memory. Aging Cell 5, 267–274 (2006).
Turpin, F. et al. Reduced serine racemase expression contributes to age-related deficits in hippocampal cognitive function. Neurobiol. Aging 32, 1495–1504 (2011).
Orzylowski, M., Fujiwara, E., Mousseau, D. D. & Baker, G. B. An overview of the involvement of d-serine in cognitive impairment in normal aging and dementia. Front. Psychiatry 12, 754032 (2021).
Van der Crabben, S. et al. An update on serine deficiency disorders. J. Inherit. Metab. Dis. 36, 613–619 (2013).
Kantrowitz, J. T. et al. Neurophysiological mechanisms of cortical plasticity impairments in schizophrenia and modulation by the NMDA receptor agonist d-serine. Brain 139, 3281–3295 (2016).
Kantrowitz, J. T. et al. d-Serine for the treatment of negative symptoms in individuals at clinical high risk of schizophrenia: a pilot, double-blind, placebo-controlled, randomised parallel group mechanistic proof-of-concept trial. Lancet Psychiatry 2, 403–412 (2015).
Kantrowitz, J. T. et al. Improvement in mismatch negativity generation during d-serine treatment in schizophrenia: correlation with symptoms. Schizophr. Res. 191, 70–79 (2018).
Bado, P. et al. Effects of low-dose d-serine on recognition and working memory in mice. Psychopharmacology 218, 461–470 (2011).
Panizzutti, R. et al. Association between increased serum d-serine and cognitive gains induced by intensive cognitive training in schizophrenia. Schizophr. Res. 207, 63–69 (2019).
Tsai, G., Yang, P., Chung, L.-C., Lange, N. & Coyle, J. T. d-Serine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 44, 1081–1089 (1998).
Heresco-Levy, U. et al. Pilot controlled trial of d-serine for the treatment of post-traumatic stress disorder. Int. J. Neuropsychopharmacol. 12, 1275–1282 (2009).
Moaddel, R. et al. d-Serine plasma concentration is a potential biomarker of (R,S)-ketamine antidepressant response in subjects with treatment-resistant depression. Psychopharmacology 232, 399–409 (2015).
Nava-Gómez, L. et al. Aging-associated cognitive decline is reversed by d-serine supplementation. eNeuro https://doi.org/10.1523/ENEURO.0176-22.2022 (2022).
Madeira, C. et al. d-Serine levels in Alzheimer’s disease: implications for novel biomarker development. Transl. Psychiatry 5, e561 (2015).
Biemans, E. A. et al. CSF d-serine concentrations are similar in Alzheimer’s disease, other dementias, and elderly controls. Neurobiol. Aging 42, 213–216 (2016).
Piubelli, L. et al. Serum d-serine levels are altered in early phases of Alzheimer’s disease: towards a precocious biomarker. Transl. Psychiatry 11, 77 (2021).
Ito, T. et al. Serine racemase is involved in d-aspartate biosynthesis. J. Biochem. 160, 345–353 (2016).
Wolosker, H., D’Aniello, A. & Snyder, S. d-Aspartate disposition in neuronal and endocrine tissues: ontogeny, biosynthesis and release. Neuroscience 100, 183–189 (2000).
Schell, M. J., Cooper, O. B. & Snyder, S. H. d-Aspartate localizations imply neuronal and neuroendocrine roles. Proc. Natl Acad. Sci. USA 94, 2013–2018 (1997).
Ota, N., Shi, T. & Sweedler, J. V. d-Aspartate acts as a signaling molecule in nervous and neuroendocrine systems. Amino Acids 43, 1873–1886 (2012).
De Rosa, A. et al. Prenatal expression of d-aspartate oxidase causes early cerebral d-aspartate depletion and influences brain morphology and cognitive functions at adulthood. Amino Acids 52, 597–617 (2020).
Nuzzo, T. et al. Decreased free d-aspartate levels are linked to enhanced d-aspartate oxidase activity in the dorsolateral prefrontal cortex of schizophrenia patients. NPJ Schizophr. 3, 16 (2017).
Errico, F. et al. Decreased levels of d-aspartate and NMDA in the prefrontal cortex and striatum of patients with schizophrenia. J. Psychiatr. Res. 47, 1432–1437 (2013).
Coyle, J. T. Schizophrenia: basic and clinical. Neurodegener. Dis. 15, 255–280 (2017).
Nuzzo, T. et al. The levels of the NMDA receptor co-agonist d-serine are reduced in the substantia nigra of MPTP-lesioned macaques and in the cerebrospinal fluid of Parkinson’s disease patients. Sci. Rep. 9, 8898 (2019).
Morikawa, A. et al. Determination of free d-aspartic acid, d-serine and d-alanine in the brain of mutant mice lacking d-amino-acid oxidase activity. J. Chromatogr. B 757, 119–125 (2001).
Tsai, G. E., Yang, P., Chang, Y. C. & Chong, M. Y. d-Alanine added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry 59, 230–234 (2006).
Fisher, G. H. et al. Free d-aspartate and d-alanine in normal and Alzheimer brain. Brain Res. Bull. 26, 983–985 (1991).
Xing, Y., Li, X., Guo, X. & Cui, Y. Simultaneous determination of 18 d-amino acids in rat plasma by an ultrahigh-performance liquid chromatography–tandem mass spectrometry method: application to explore the potential relationship between Alzheimer’s disease and d-amino acid level alterations. Anal. Bioanal. Chem. 408, 141–150 (2016).
Semenza, E. R. et al. d-Cysteine is an endogenous regulator of neural progenitor cell dynamics in the mammalian brain. Proc. Natl Acad. Sci. USA 118, e2110610118 (2021).
Hoffmann, G. F. et al. Neurological manifestations of organic acid disorders. Eur. J. Pediatr. 153, S94–S100 (1994).
Achouri, Y. et al. Identification of a dehydrogenase acting on d-2-hydroxyglutarate. Biochem. J. 381, 35–42 (2004).
Junqueira, D. et al. In vitro effects of d-2-hydroxyglutaric acid on glutamate binding, uptake and release in cerebral cortex of rats. J. Neurol. Sci. 217, 189–194 (2004).
Kolker, S. et al. NMDA receptor activation and respiratory chain complex V inhibition contribute to neurodegeneration in d-2-hydroxyglutaric aciduria. Eur. J. Neurosci. 16, 21–28 (2002).
Muntau, A. C. et al. Combined d-2-and l-2-hydroxyglutaric aciduria with neonatal onset encephalopathy: a third biochemical variant of 2-hydroxyglutaric aciduria? Neuropediatrics 31, 137–140 (2000).
Stevens, L. A., Coresh, J., Greene, T. & Levey, A. S. Assessing kidney function — measured and estimated glomerular filtration rate. N. Engl. J. Med. 354, 2473–2483 (2006).
Kimura, T., Hesaka, A. & Isaka, Y. d-Amino acids and kidney diseases. Clin. Exp. Nephrol. 24, 404–410 (2020).
Silbernagl, S., Völker, K. & Dantzler, W. H. d-Serine is reabsorbed in rat renal pars recta. Am. J. Physiol. 276, F857–F863 (1999).
Okushima, H. et al. Intra-body dynamics of d-serine reflects the origin of kidney diseases. Clin. Exp. Nephrol. 25, 893–901 (2021).
Iwakawa, H., Makabe, S., Ito, T., Yoshimura, T. & Watanabe, H. Urinary d-serine level as a predictive biomarker for deterioration of renal function in patients with atherosclerotic risk factors. Biomarkers 24, 159–165 (2019).
Kawamura, M. et al. Measurement of glomerular filtration rate using endogenous d-serine clearance in living kidney transplant donors and recipients. EClinicalMedicine 43, 101223 (2022).
Hesaka, A. et al. d-Serine reflects kidney function and diseases. Sci. Rep. 9, 5104 (2019).
Sasabe, J. et al. Ischemic acute kidney injury perturbs homeostasis of serine enantiomers in the body fluid in mice: early detection of renal dysfunction using the ratio of serine enantiomers. PLoS ONE 9, e86504 (2014).
Young, G., Kendall, S. & Brownjohn, A. d-Amino acids in chronic renal failure and the effects of dialysis and urinary losses. Amino Acids 6, 283–293 (1994).
Min, J. Z. et al. Determination of dl-amino acids, derivatized with R(−)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazole, in nail of diabetic patients by UPLC–ESI–TOF-MS. J. Chromatogr. B 879, 3220–3228 (2011).
Lorenzo, M. P. et al. Optimization and validation of a chiral GC-MS method for the determination of free d-amino acids ratio in human urine: application to a gestational diabetes mellitus study. J. Pharm. Biomed. Anal. 107, 480–487 (2015).
Canfora, E. E., Meex, R. C. R., Venema, K. & Blaak, E. E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15, 261–273 (2019).
Cheng, Q.-Y. et al. Sensitive determination of onco-metabolites of d-and l-2-hydroxyglutarate enantiomers by chiral derivatization combined with liquid chromatography/mass spectrometry analysis. Sci. Rep. 5, 1–11 (2015).
Lu, J. et al. Closing the anion gap: contribution of d-lactate to diabetic ketoacidosis. Clin. Chim. Acta 412, 286–291 (2011).
Ma, S. et al. d-2-Hydroxyglutarate is essential for maintaining oncogenic property of mutant IDH-containing cancer cells but dispensable for cell growth. Oncotarget 6, 8606–8620 (2015).
Struys, E. A., Gibson, K. M. & Jakobs, C. Novel insights into l-2-hydroxyglutaric aciduria: mass isotopomer studies reveal 2-oxoglutaric acid as the metabolic precursor of l-2-hydroxyglutaric acid. J. Inherit. Metab. Dis. 30, 690–693 (2007).
Ye, D., Guan, K. L. & Xiong, Y. Metabolism, activity, and targeting of d- and l-2-hydroxyglutarates. Trends Cancer 4, 151–165 (2018).
Baek, J. & Pennathur, S. Urinary 2-hydroxyglutarate enantiomers are markedly elevated in a murine model of type 2 diabetic kidney disease. Metabolites 11, 469 (2021).
Bastings, J., van Eijk, H. M., Olde Damink, S. W. & Rensen, S. S. d-Amino acids in health and disease: a focus on cancer. Nutrients 11, 2205 (2019).
Nagata, Y. et al. High concentrations of d-amino acids in human gastric juice. Amino Acids 32, 137–140 (2007).
Han, M. et al. Development and validation of a rapid, selective, and sensitive LC–MS/MS method for simultaneous determination of d- and l-amino acids in human serum: application to the study of hepatocellular carcinoma. Anal. Bioanal. Chem. 410, 2517–2531 (2018).
Du, S. et al. Altered profiles and metabolism of l- and d-amino acids in cultured human breast cancer cells vs. non-tumorigenic human breast epithelial cells. J. Pharm. Biomed. Anal. 164, 421–429 (2019).
Ohshima, K. et al. Serine racemase enhances growth of colorectal cancer by producing pyruvate from serine. Nat. Metab. 2, 81–96 (2020).
Cui, Z. et al. Integrated bioinformatics analysis of serine racemase as an independent prognostic biomarker in endometrial cancer. Front. Genet. 13, 906291 (2022).
Broer, A., Rahimi, F. & Broer, S. Deletion of amino acid transporter ASCT2 (SLC1A5) reveals an essential role for transporters SNAT1 (SLC38A1) and SNAT2 (SLC38A2) to sustain glutaminolysis in cancer cells. J. Biol. Chem. 291, 13194–13205 (2016).
Bodner, O. et al. d-Serine signaling and NMDAR-mediated synaptic plasticity are regulated by system A-type of glutamine/d-serine dual transporters. J. Neurosci. 40, 6489–6502 (2020).
Kaplan, E. et al. ASCT1 (Slc1a4) transporter is a physiologic regulator of brain d-serine and neurodevelopment. Proc. Natl Acad. Sci. USA 115, 9628–9633 (2018).
Shibuya, N. et al. A novel pathway for the production of hydrogen sulfide from d-cysteine in mammalian cells. Nat. Commun. 4, 1366 (2013).
Hellmich, M. R. & Szabo, C. Hydrogen Sulfide and Cancer (Springer, 2015).
El Sayed, S. M. et al. d-Amino acid oxidase-induced oxidative stress, 3-bromopyruvate and citrate inhibit angiogenesis, exhibiting potent anticancer effects. J. Bioenerg. Biomembr. 44, 513–523 (2012).
Pollegioni, L., Sacchi, S. & Murtas, G. Human d-amino acid oxidase: structure, function, and regulation. Front. Mol. Biosci. 5, 107 (2018).
North, W. G., Gao, G., Memoli, V. A., Pang, R. H. & Lynch, L. Breast cancer expresses functional NMDA receptors. Breast Cancer Res. Treat. 122, 307–314 (2010).
Deutsch, S. I., Tang, A. H., Burket, J. A. & Benson, A. D. NMDA receptors on the surface of cancer cells: target for chemotherapy? Biomed. Pharmacother. 68, 493–496 (2014).
Hogan-Cann, A. D. & Anderson, C. M. Physiological roles of non-neuronal NMDA receptors. Trends Pharmacol. Sci. 37, 750–767 (2016).
Du, S. et al. Roles of N-methyl-d-aspartate receptors and d-amino acids in cancer cell viability. Mol. Biol. Rep. 47, 6749–6758 (2020).
Szabo, C. & Hellmich, M. R. Endogenously produced hydrogen sulfide supports tumor cell growth and proliferation. Cell Cycle 12, 2915–2916 (2013).
Szabo, C. et al. Tumor-derived hydrogen sulfide, produced by cystathionine-β-synthase, stimulates bioenergetics, cell proliferation, and angiogenesis in colon cancer. Proc. Natl Acad. Sci. USA 110, 12474–12479 (2013).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Intlekofer, A. M. et al. Hypoxia induces production of l-2-hydroxyglutarate. Cell Metab. 22, 304–311 (2015).
Yang, H., Ye, D., Guan, K. L. & Xiong, Y. IDH1 and IDH2 mutations in tumorigenesis: mechanistic insights and clinical perspectives. Clin. Cancer Res 18, 5562–5571 (2012).
Wang, P. et al. Oncometabolite d-2-hydroxyglutarate inhibits ALKBH DNA repair enzymes and sensitizes IDH mutant cells to alkylating agents. Cell Rep. 13, 2353–2361 (2015).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011).
Brückner, H. & Schieber, A. Determination of amino acid enantiomers in human urine and blood serum by gas chromatography–mass spectrometry. Biomed. Chromatogr. 15, 166–172 (2001).
Wolfender, J.-L., Marti, G., Thomas, A. & Bertrand, S. Current approaches and challenges for the metabolite profiling of complex natural extracts. J. Chromatogr. A 1382, 136–164 (2015).
Mochizuki, T. et al. Towards the chiral metabolomics: liquid chromatography–mass spectrometry based dl-amino acid analysis after labeling with a new chiral reagent, (S)-2, 5-dioxopyrrolidin-1-yl-1-(4,6-dimethoxy-1, 3, 5-triazin-2-yl) pyrrolidine-2-carboxylate, and the application to saliva of healthy volunteers. Anal. Chim. Acta 875, 73–82 (2015).
Szökő, É., Vincze, I. & Tábi, T. Chiral separations for d-amino acid analysis in biological samples. J. Pharm. Biomed. Anal. 130, 100–109 (2016).
Cavazzini, A., Pasti, L., Massi, A., Marchetti, N. & Dondi, F. Recent applications in chiral high performance liquid chromatography: a review. Anal. Chim. Acta 706, 205–222 (2011).
Grossman, P. D. & Colburn, J. C. Capillary Electrophoresis: Theory and Practice (Academic, 2012).
Ramautar, R., Berger, R., van der Greef, J. & Hankemeier, T. Human metabolomics: strategies to understand biology. Curr. Opin. Chem. Biol. 17, 841–846 (2013).
Scalbert, A. et al. Mass-spectrometry-based metabolomics: limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics 5, 435–458 (2009).
Lloyd, D. K. & Goodall, D. M. Polarimetric detection in high-performance liquid chromatography. Chirality 1, 251–264 (1989).
Purvinis, G., Cameron, B. D. & Altrogge, D. M. Noninvasive polarimetric-based glucose monitoring: an in vivo study. J. Diabetes Sci. Technol. 5, 380–387 (2011).
Borovkova, M. et al. Evaluating β-amyloidosis progression in Alzheimer’s disease with Mueller polarimetry. Biomed. Opt. Express 11, 4509–4519 (2020).
Greenfield, N. J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1, 2876–2890 (2006).
Keller, B. O., Sui, J., Young, A. B. & Whittal, R. M. Interferences and contaminants encountered in modern mass spectrometry. Anal. Chim. Acta 627, 71–81 (2008).
Wu, L. & Han, D. K. Overcoming the dynamic range problem in mass spectrometry-based shotgun proteomics. Expert Rev. Proteom. 3, 611–619 (2006).
Dettmer, K., Aronov, P. A. & Hammock, B. D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 26, 51–78 (2007).
Vuckovic, D. Current trends and challenges in sample preparation for global metabolomics using liquid chromatography–mass spectrometry. Anal. Bioanal. Chem. 403, 1523–1548 (2012).
Doherty, C. M. & Forbes, R. B. Diagnostic lumbar puncture. Ulst. Med. J. 83, 93 (2014).
Lewis, S. M. & Tatsumi, N. in Dacie Lewis Pr. Hematol. 10th edn Ch. 1 (Elsevier, 2006).
Gübitz, G. & Schmid, M. G. Chiral separation by chromatographic and electromigration techniques: a review. Biopharm. Drug Dispos. 22, 291–336 (2001).
Ang, J. E. et al. Identification of human plasma metabolites exhibiting time-of-day variation using an untargeted liquid chromatography–mass spectrometry metabolomic approach. Chronobiol. Int. 29, 868–881 (2012).
Kim, K. et al. Mealtime, temporal, and daily variability of the human urinary and plasma metabolomes in a tightly controlled environment. PLoS ONE 9, e86223 (2014).
Lin, C.-H., Yang, H.-T. & Lane, H.-Y. d-Glutamate, d-serine, and d-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 185, 172760 (2019).
Armstrong, D. W., Kullman, J. P., Chen, X. & Rowe, M. Composition and chirality of amino acids in aerosol/dust from laboratory and residential enclosures. Chirality 13, 153–158 (2001).
Yin, P., Lehmann, R. & Xu, G. Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal. Bioanal. Chem. 407, 4879–4892 (2015).
Khadka, M. et al. The effect of anticoagulants, temperature, and time on the human plasma metabolome and lipidome from healthy donors as determined by liquid chromatography–mass spectrometry. Biomolecules 9, 200 (2019).
Pedersen-Bjergaard, S. & Rasmussen, K. E. Liquid–liquid–liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal. Chem. 71, 2650–2656 (1999).
Nagata, Y., Akino, T. & Ohno, K. Microdetermination of serum d-amino acids. Anal. Chem. 150, 238–242 (1985).
Brückner, H. & Hausch, M. Gas chromatographic characterization of free d-amino acids in the blood serum of patients with renal disorders and of healthy volunteers. J. Chromatogr. B 614, 7–17 (1993).
Lesche, D. et al. Does centrifugation matter? Centrifugal force and spinning time alter the plasma metabolome. Metabolomics 12, 159 (2016).
Jobard, E. et al. A systematic evaluation of blood serum and plasma pre-analytics for metabolomics cohort studies. Int. J. Mol. Sci. 17, 2035 (2016).
Heireman, L. et al. Causes, consequences and management of sample hemolysis in the clinical laboratory. Clin. Biochem. 50, 1317–1322 (2017).
Jiang, L., He, L. & Fountoulakis, M. Comparison of protein precipitation methods for sample preparation prior to proteomic analysis. J. Chromatogr. A 1023, 317–320 (2004).
Pätzold, R., Schieber, A. & Brückner, H. Gas chromatographic quantification of free d‐amino acids in higher vertebrates. Biomed. Chromatogr. 19, 466–473 (2005).
Patti, G. J. Separation strategies for untargeted metabolomics. J. Sep. Sci. 34, 3460–3469 (2011).
Yu, Z. et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 6, e21230 (2011).
Teahan, O. et al. Impact of analytical bias in metabonomic studies of human blood serum and plasma. Anal. Chem. 78, 4307–4318 (2006).
Asakura, S. & Konno, R. Origin of d-serine present in urine of mutant mice lacking d-amino-acid oxidase activity. Amino Acids 12, 213–223 (1997).
Kato, S., Kito, Y., Hemmi, H. & Yoshimura, T. Simultaneous determination of d-amino acids by the coupling method of d-amino acid oxidase with high-performance liquid chromatography. J. Chromatogr. B 879, 3190–3195 (2011).
Nagata, Y. et al. The presence of high concentrations of free d-amino acids in human saliva. Life Sci. 78, 1677–1681 (2006).
Römisch-Margl, W. et al. Procedure for tissue sample preparation and metabolite extraction for high-throughput targeted metabolomics. Metabolomics 8, 133–142 (2012).
González-Domínguez, R., González-Domínguez, Á., Sayago, A. & Fernández-Recamales, Á. Recommendations and best practices for standardizing the pre-analytical processing of blood and urine samples in metabolomics. Metabolites 10, 229 (2020).
Stevens, V. L., Hoover, E., Wang, Y. & Zanetti, K. A. Pre-analytical factors that affect metabolite stability in human urine, plasma, and serum: a review. Metabolites 9, 156 (2019).
Cajka, T. & Fiehn, O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 88, 524–545 (2016).
Berthod, A. Chiral Recognition in Separation Methods (Springer, 2010).
Subramanian, G. Chiral Separation Techniques: A Practical Approach (Wiley, 2008).
Aswad, D. W. Determination of d- and l-aspartate in amino acid mixtures by high-performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. Anal. Biochem. 137, 405–409 (1984).
Fisher, G. et al. Free d- and l-amino acids in ventricular cerebrospinal fluid from Alzheimer and normal subjects. Amino Acids 15, 263–269 (1998).
Gibson, K. et al. Stable-isotope dilution analysis of d- and l-2-hydroxyglutaric acid: application to the detection and prenatal diagnosis of d- and l-2-hydroxyglutaric acidemias. Pediatr. Res. 34, 277–280 (1993).
El Zahar, N. M., Magdy, N., El-Kosasy, A. M. & Bartlett, M. G. Chromatographic approaches for the characterization and quality control of therapeutic oligonucleotide impurities. Biomed. Chromatogr. 32, e4088 (2018).
Kagan, H. & Fiaud, J. Kinetic resolution. Top. Stereochem 18, 249–330 (1988).
Rashed, M. S., AlAmoudi, M. & Aboul‐Enein, H. Y. Chiral liquid chromatography tandem mass spectrometry in the determination of the configuration of 2-hydroxyglutaric acid in urine. Biomed. Chromatogr. 14, 317–320 (2000).
Kimura, T. et al. Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci. Rep. 6, 26137 (2016).
Furusho, A. et al. Three-dimensional high-performance liquid chromatographic determination of Asn, Ser, Ala, and Pro enantiomers in the plasma of patients with chronic kidney disease. Anal. Chem. 91, 11569–11575 (2019).
Du, S., Wang, Y., Weatherly, C. A., Holden, K. & Armstrong, D. W. Variations of l- and d-amino acid levels in the brain of wild-type and mutant mice lacking d-amino acid oxidase activity. Anal. Bioanal. Chem. 410, 2971–2979 (2018).
Armstrong, D., Duncan, J. & Lee, S. Evaluation of d-amino acid levels in human urine and in commercial l-amino acid samples. Amino Acids 1, 97–106 (1991).
McNair, H. M., Miller, J. M. & Snow, N. H. Basic Gas Chromatography (Wiley, 2019).
Farajzadeh, M. A., Nouri, N. & Khorram, P. Derivatization and microextraction methods for determination of organic compounds by gas chromatography. Trends Analyt. Chem. 55, 14–23 (2014).
Charissou, A., Ait-Ameur, L. & Birlouez-Aragon, I. Evaluation of a gas chromatography/mass spectrometry method for the quantification of carboxymethyllysine in food samples. J. Chromatogr. A 1140, 189–194 (2007).
Creamer, J. S., Mora, M. F. & Willis, P. A. Stability of reagents used for chiral amino acid analysis during spaceflight missions in high‐radiation environments. Electrophoresis 39, 2864–2871 (2018).
Bernardo-Bermejo, S., Sánchez-López, E., Castro-Puyana, M. & Marina, M. L. Chiral capillary electrophoresis. Trends Analyt. Chem. 124, 115807 (2020).
Li, S., Yu, Q., Lu, X. & Zhao, S. Determination of d,l-serine in midbrain of Parkinson’s disease mouse by capillary electrophoresis with in-column light-emitting diode induced fluorescence detection. J. Sep. Sci. 32, 282–287 (2009).
Martineau, M. et al. Storage and uptake of d-serine into astrocytic synaptic-like vesicles specify gliotransmission. J. Neurosci. 33, 3413–3423 (2013).
Patel, A. V., Kawai, T., Wang, L., Rubakhin, S. S. & Sweedler, J. V. Chiral measurement of aspartate and glutamate in single neurons by large-volume sample stacking capillary electrophoresis. Anal. Chem. 89, 12375–12382 (2017).
Fanali, C. & Fanali, S. Chiral separations using miniaturized techniques: state of the art and perspectives. Isr. J. Chem. 56, 958–967 (2016).
Merola, G., Fu, H., Tagliaro, F., Macchia, T. & McCord, B. R. Chiral separation of 12 cathinone analogs by cyclodextrin‐assisted capillary electrophoresis with UV and mass spectrometry detection. Electrophoresis 35, 3231–3241 (2014).
Krait, S., Konjaria, M. L. & Scriba, G. K. Advances of capillary electrophoresis enantioseparations in pharmaceutical analysis (2017–2020). Electrophoresis 42, 1709–1725 (2021).
Beckonert, O. et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Protoc. 2, 2692 (2007).
Suh, E. H. et al. Detection of glucose-derived d- and l-lactate in cancer cells by the use of a chiral NMR shift reagent. Cancer Metab. 9, 38 (2021).
Bal, D., Gradowska, W. & Gryff-Keller, A. Determination of the absolute configuration of 2-hydroxyglutaric acid and 5-oxoproline in urine samples by high-resolution NMR spectroscopy in the presence of chiral lanthanide complexes. J. Pharm. Biomed. Anal. 28, 1061–1071 (2002).
Wenzel, T. J. & Wilcox, J. D. Chiral reagents for the determination of enantiomeric excess and absolute configuration using NMR spectroscopy. Chirality 15, 256–270 (2003).
Emwas, A.-H. et al. NMR spectroscopy for metabolomics research. Metabolites 9, 123 (2019).
Rosini, E., D’Antona, P. & Pollegioni, L. Biosensors for d-amino acids: detection methods and applications. Int. J. Mol. Sci. 21, 4574 (2020).
Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 6, 24–27 (1969).
Zhang, Z. et al. Non-invasive detection of gastric cancer relevant d-amino acids with luminescent DNA/silver nanoclusters. Nanoscale 9, 19367–19373 (2017).
Mothet, J. P. et al. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter d-serine. Proc. Natl Acad. Sci. USA 102, 5606–5611 (2005).
Fisher, G. H. et al. Free d-amino acids in human cerebrospinal fluid of Alzheimer disease, multiple sclerosis, and healthy control subjects. Mol. Chem. Neuropathol. 23, 115–124 (1994).
Shi, R et al. The interaction of K and O2 on Au(111): multiple growth modes of potassium oxide and their catalytic activity for CO oxidation. Angew. Chem. Int. Ed. 61, e202208666 (2022).
Ma, J., Zhang, X., Huang, X., Luo, S. & Meggers, E. Preparation of chiral-at-metal catalysts and their use in asymmetric photoredox chemistry. Nat. Protoc. 13, 605–632 (2018).
Shoja, Y., Rafati, A. A. & Ghodsi, J. Enzymatic biosensor based on entrapment of d-amino acid oxidase on gold nanofilm/MWCNTs nanocomposite modified glassy carbon electrode by sol–gel network: analytical applications for d-alanine in human serum. Enzyme Microb. Technol. 100, 20–27 (2017).
Nieh, C.-H., Kitazumi, Y., Shirai, O. & Kano, K. Sensitive d-amino acid biosensor based on oxidase/peroxidase system mediated by pentacyanoferrate-bound polymer. Biosens. Bioelectron. 47, 350–355 (2013).
Rabinovitch, B., March, W. & Adams, R. L. Noninvasive glucose monitoring of the aqueous humor of the eye: part I. Measurement of very small optical rotations. Diabetes Care 5, 254–258 (1982).
Berova, N., Nakanishi, K. & Woody, R. W. Circular Dichroism: Principles and Applications (Wiley, 2000).
Lecoeur-Lorin, M., Delepee, R., Ribet, J. P. & Morin, P. Chiral analysis of milnacipran by a nonchiral HPLC–circular dichroism: improvement of the linearity of dichroic response by temperature control. J. Sep. Sci. 31, 3009–3014 (2008).
Luo, Y. et al. A novel potential primary method for quantification of enantiomers by high performance liquid chromatography–circular dichroism. Sci. Rep. 8, 1–11 (2018).
Pelton, J. T. & McLean, L. R. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 277, 167–176 (2000).
Kypr, J., Kejnovska, I., Renciuk, D. & Vorlickova, M. Circular dichroism and conformational polymorphism of DNA. Nucleic Acids Res 37, 1713–1725 (2009).
Morvan, M. & Mikšík, I. Recent advances in chiral analysis of proteins and peptides. Separations 8, 112 (2021).
Micsonai, A. et al. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proc. Natl Acad. Sci.USA 112, E3095–E3103 (2015).
Kong, K., Kendall, C., Stone, N. & Notingher, I. Raman spectroscopy for medical diagnostics — from in-vitro biofluid assays to in-vivo cancer detection. Adv. Drug Deliv. Rev. 89, 121–134 (2015).
Nafie, L. A. Infrared and Raman vibrational optical activity: theoretical and experimental aspects. Annu. Rev. Phys. Chem. 48, 357–386 (1997).
Barron, L. D. The development of biomolecular Raman optical activity spectroscopy. Biomed. Spectrosc. Imaging 4, 223–253 (2015).
Zhu, F., Isaacs, N. W., Hecht, L. & Barron, L. D. Raman optical activity: a tool for protein structure analysis. Structure 13, 1409–1419 (2005).
Yamamoto, S. & Bouř, P. Detection of molecular chirality by induced resonance Raman optical activity in europium complexes. Angew. Chem. Int. Ed. 51, 11058–11061 (2012).
Stephens, P. J., Devlin, F. J. & Pan, J. J. The determination of the absolute configurations of chiral molecules using vibrational circular dichroism (VCD) spectroscopy. Chirality 20, 643–663 (2008).
Guo, C. et al. Determination of enantiomeric excess in samples of chiral molecules using Fourier transform vibrational circular dichroism spectroscopy: simulation of real-time reaction monitoring. Anal. Chem. 76, 6956–6966 (2004).
Armstrong, D. W., Yu, J., Cole, H. D., McFarland, S. A. & Nafie, J. Chiral resolution and absolute configuration determination of new metal-based photodynamic therapy antitumor agents. J. Pharm. Biomed. Anal. 204, 114233 (2021).
Zhang, P. & Polavarapu, P. L. Vibrational circular dichroism of matrix-assisted amino acid films in the mid-infrared region. Appl. Spectrosc. 60, 378–385 (2006).
Hentschel, M., Schaferling, M., Duan, X., Giessen, H. & Liu, N. Chiral plasmonics. Sci. Adv. 3, e1602735 (2017).
Solomon, M. L. et al. Nanophotonic platforms for chiral sensing and separation. Acc. Chem. Res. 53, 588–598 (2020).
Zhao, Y. et al. Chirality detection of enantiomers using twisted optical metamaterials. Nat. Commun. 8, 14180 (2017).
Wu, Z. & Zheng, Y. Moiré chiral metamaterials. Adv. Opt. Mater. 5, 1700034 (2017).
Wu, Z. & Zheng, Y. Moiré metamaterials and metasurfaces. Adv. Opt. Mater. 6, 1701057 (2018).
Hendry, E. et al. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 5, 783 (2010).
Liu, Y. et al. Label-free ultrasensitive detection of abnormal chiral metabolites in diabetes. ACS Nano 15, 6448–6456 (2021).
Wang, X. & Tang, Z. Circular dichroism studies on plasmonic nanostructures. Small 13, 1601115 (2017).
Zhang, Q. et al. Unraveling the origin of chirality from plasmonic nanoparticle–protein complexes. Science 365, 1475–1478 (2019).
Xu, L. et al. Highly selective recognition and ultrasensitive quantification of enantiomers. J. Mater. Chem. B 1, 4478–4483 (2013).
Hu, J., Lawrence, M. & Dionne, J. A. High quality factor dielectric metasurfaces for ultraviolet circular dichroism spectroscopy. ACS Photonics 7, 36–42 (2019).
Lee, Y. Y., Kim, R. M., Im, S. W., Balamurugan, M. & Nam, K. T. Plasmonic metamaterials for chiral sensing applications. Nanoscale 12, 58–66 (2020).
Yoo, S. & Park, Q.-H. Metamaterials and chiral sensing: a review of fundamentals and applications. Nanophotonics 8, 249–261 (2019).
Paiva-Marques, W. A., Reyes Gómez, F., Oliveira, O. N. & Mejía-Salazar, J. R. Chiral plasmonics and their potential for point-of-care biosensing applications. Sensors 20, 944 (2020).
Abdali, S. & Blanch, E. W. Surface enhanced Raman optical activity (SEROA). Chem. Soc. Rev. 37, 980–992 (2008).
Rocks, L. et al. Through-space transfer of chiral information mediated by a plasmonic nanomaterial. Nat. Chem. 7, 591 (2015).
Mitra, S. Sample Preparation Techniques in Analytical Chemistry Vol. 237 (Wiley, 2004).
Kumar, R. & Ismail, A. Fouling control on microfiltration/ultrafiltration membranes: effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 132, 42042 (2015).
Gilar, M., Bouvier, E. S. & Compton, B. J. Advances in sample preparation in electromigration, chromatographic and mass spectrometric separation methods. J. Chromatogr. A 909, 111–135 (2001).
Goodwin, R. J. Sample preparation for mass spectrometry imaging: small mistakes can lead to big consequences. J. Proteom. 75, 4893–4911 (2012).
Pusztai, L., Hatzis, C. & Andre, F. Reproducibility of research and preclinical validation: problems and solutions. Nat. Rev. Clin. Oncol. 10, 720–724 (2013).
Addona, T. A. et al. Multi-site assessment of the precision and reproducibility of multiple reaction monitoring–based measurements of proteins in plasma. Nat. Biotechnol. 27, 633–641 (2009).
Yager, P., Domingo, G. J. & Gerdes, J. Point-of-care diagnostics for global health. Annu. Rev. Biomed. Eng. 10, 107–144 (2008).
Luppa, P. B., Müller, C., Schlichtiger, A. & Schlebusch, H. Point-of-care testing (POCT): current techniques and future perspectives. Trends Analyt. Chem. 30, 887–898 (2011).
National Institute of Biomedical Imaging and Bioengineering/National Heart, Lung, and Blood Institute/National Science Foundation Workshop Faculty, Price, C. P. & Kricka, L. J. Improving healthcare accessibility through point-of-care technologies. Clin. Chem. 53, 1665–1675 (2007).
Gałuszka, A., Migaszewski, Z. M. & Namieśnik, J. Moving your laboratories to the field — advantages and limitations of the use of field portable instruments in environmental sample analysis. Environ. Res. 140, 593–603 (2015).
Kuehnbaum, N. L. & Britz-McKibbin, P. New advances in separation science for metabolomics: resolving chemical diversity in a post-genomic era. Chem. Rev. 113, 2437–2468 (2013).
Gowda, G. N. et al. Metabolomics-based methods for early disease diagnostics. Expert Rev. Mol. Diagn. 8, 617–633 (2008).
Polavarapu, P. L. Chiral Analysis: Advances in Spectroscopy, Chromatography and Emerging Methods (Elsevier, 2018).
Newgard, C. B. Metabolomics and metabolic diseases: where do we stand? Cell Metab. 25, 43–56 (2017).
Li, S. et al. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol. 9, e1003123 (2013).
van den Berg, R. A., Hoefsloot, H. C., Westerhuis, J. A., Smilde, A. K. & van der Werf, M. J. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics. 7, 142 (2006).
Lalkhen, A. G. & McCluskey, A. Clinical tests: sensitivity and specificity. Contin. Educ. Anaesth. Crit. Care Pain 8, 221–223 (2008).
Nie, S. & Emory, S. R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 275, 1102–1106 (1997).
Friedman, M. & Levin, C. E. Nutritional and medicinal aspects of d-amino acids. Amino Acids 42, 1553–1582 (2012).
Wang, Z. et al. Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis. Commun. Biol. 3, 39 (2020).
Nota, B. et al. Deficiency in SLC25A1, encoding the mitochondrial citrate carrier, causes combined d-2-and l-2-hydroxyglutaric aciduria. Am. J. Hum. Genet. 92, 627–631 (2013).
Strain, S. K., Groves, M. D., Olino, K. L. & Emmett, M. R. Measurement of 2-hydroxyglutarate enantiomers in serum by chiral gas chromatography–tandem mass spectrometry and its application as a biomarker for IDH mutant gliomas. Clin. Mass Spectrom. 15, 16–24 (2020).
Willekens, C. et al. Serum 2-hydroxyglutarate level can predict IDH2 mutation in myeloid sarcoma. Blood 126, 3835 (2015).
Struys, E. A. et al. Mutations in the d-2-hydroxyglutarate dehydrogenase gene cause d-2-hydroxyglutaric aciduria. Am. J. Hum. Genet. 76, 358–360 (2005).
Gibson, K. M., Craigen, W., Herman, G. E. & Jakobs, C. d-2-Hydroxyglutaric aciduria in a newborn with neurological abnormalities: a new neurometabolic disorder? J. Inherit. Metab. Dis. 16, 497–500 (1993).
Rodrigues, D. G. B. et al. Experimental evidence of oxidative stress in patients with l-2-hydroxyglutaric aciduria and that l-carnitine attenuates in vitro DNA damage caused by d-2-hydroxyglutaric and l-2-hydroxyglutaric acids. Toxicol. Vitr. 42, 47–53 (2017).
Huang, Y., Shi, M. & Zhao, S. Quantification of d-Asp and d-Glu in rat brain and human cerebrospinal fluid by microchip electrophoresis. J. Sep. Sci. 32, 3001–3006 (2009).
Hashimoto, K. et al. Possible role of d-serine in the pathophysiology of Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 385–388 (2004).
Ohnuma, T. et al. Changes in plasma glycine, l-serine, and d-serine levels in patients with schizophrenia as their clinical symptoms improve: results from the Juntendo University Schizophrenia Projects (JUSP). Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1905–1912 (2008).
Grant, S. L., Shulman, Y., Tibbo, P., Hampson, D. R. & Baker, G. B. Determination of d-serine and related neuroactive amino acids in human plasma by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B 844, 278–282 (2006).
Visser, W. F. et al. A sensitive and simple ultra-high-performance-liquid chromatography–tandem mass spectrometry based method for the quantification of d-amino acids in body fluids. J. Chromatogr. A 1218, 7130–7136 (2011).
Fuchs, S. A. et al. Two mass-spectrometric techniques for quantifying serine enantiomers and glycine in cerebrospinal fluid: potential confounders and age-dependent ranges. Clin. Chem. 54, 1443–1450 (2008).
Barbot, C. et al. l-2-Hydroxyglutaric aciduria: clinical, biochemical and magnetic resonance imaging in six Portuguese pediatric patients. Brain Dev. 19, 268–273 (1997).
Topcu, M. et al. l-2-hydroxyglutaric aciduria: a report of 29 patients. Turk. J. Pediatr. 47, 1–7 (2005).
Kranendijk, M. et al. Development and implementation of a novel assay for l-2-hydroxyglutarate dehydrogenase (l-2-HGDH) in cell lysates: l-2-HGDH deficiency in 15 patients with l-2-hydroxyglutaric aciduria. J. Inherit. Metab. Dis. 32, 713 (2009).
Zhao, S., Wang, B., He, M., Bai, W. & Chen, L. Determination of free d-alanine in the human plasma by capillary electrophoresis with optical fiber light-emitting diode-induced fluorescence detection. Anal. Chim. Acta 569, 182–187 (2006).
Scheijen, J. L. et al. l(+) and d(−) lactate are increased in plasma and urine samples of type 2 diabetes as measured by a simultaneous quantification of l(+) and d(−) lactate by reversed-phase liquid chromatography tandem mass spectrometry. Exp. Diabetes Res. 2012, 234812 (2012).
Brandt, R. B., Siegel, S. A., Waters, M. G. & Bloch, M. H. Spectrophotometric assay for d-(–)-lactate in plasma. Anal. Biochem. 102, 39–46 (1980).
Tsutsui, H. et al. Simultaneous determination of dl-lactic acid and dl-3-hydroxybutyric acid enantiomers in saliva of diabetes mellitus patients by high-throughput LC–ESI-MS/MS. Anal. Bioanal. Chem. 404, 1925–1934 (2012).
Pandey, R. et al. Novel strategy for untargeted chiral metabolomics using liquid chromatography–high resolution tandem mass spectrometry. Anal. Chem. 93, 5805–5814 (2021).
Takahashi, M., Morita, M., Niwa, O. & Tabei, H. Highly sensitive high-performance liquid chromatography detection of catecholamine with interdigitated array microelectrodes. J. Electroanal. Chem. 335, 253–263 (1992).
Creamer, J. S., Mora, M. F. & Willis, P. A. Enhanced resolution of chiral amino acids with capillary electrophoresis for biosignature detection in extraterrestrial samples. Anal. Chem. 89, 1329–1337 (2017).
Rosini, E., D’Antona, P. & Pollegioni, L. Biosensors for d-amino acids: detection methods and applications. Int. J. Mol. Sci. 21, 4574 (2020).
Acknowledgements
Y.L., Z.W. and Y.Z. acknowledge the financial support of the National Institute of General Medical Sciences of the National Institutes of Health (NIH) (R01GM146962) and National Science Foundation (NSF) (ECCS-2001650). D.W.A acknowledges the support of the R. A. Welch Foundation (Y-0026). H.W. acknowledges the financial support of Israel Science Foundation 337/19, Ministry of Health, and Allen and Jewell Prince Center for Neurodegenerative Disorders of the Brain.
Author information
Authors and Affiliations
Contributions
All authors collected data, wrote and edited the manuscript. Y.L. drafted the manuscript. D.W.A and H.W. provided substantial content. Y. L., Z. W. and Y. Z. conceived the content, and edited and finalized the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Jean-Pierre Mothet, Yang Zhao and the other anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Chiral
-
The geometric property of a molecule that precludes it from being superimposable on its mirror image.
- Chiral selectors
-
Chiral components in the chiral separation system that interact enantioselectively with the target chiral molecule.
- Chiroptical
-
The optical properties related to the interaction of chirality.
- Enantiomeric excess
-
The excess of one enantiomer over the other in a mixture of enantiomers.
- Enantiomers
-
A pair of compounds that are not superposable onto their own mirror image.
- Metabolites
-
Substances produced after metabolism (food, drugs or chemical digestion).
- Metabolomics
-
A systematic study of chemical processes involving metabolites, the small-molecule substrates, intermediates and products of cell metabolism.
- Metamaterials
-
Materials engineered to have a property that is not found in the equivalent naturally occurring materials.
- Metasurfaces
-
2D thin film materials that can modulate the propagation of electromagnetic waves.
- Multiplexed
-
A way of measuring the concentration of multiple biomarkers.
- Recovery rates
-
The percentages of molecular concentration that can be recovered from the total concentration after physical or chemical processes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Wu, Z., Armstrong, D.W. et al. Detection and analysis of chiral molecules as disease biomarkers. Nat Rev Chem 7, 355–373 (2023). https://doi.org/10.1038/s41570-023-00476-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00476-z
This article is cited by
-
Fast, sensitive LC–MS resolution of \({{\upalpha}}\)-hydroxy acid biomarkers via SPP-teicoplanin and an alternative UV detection approach
Analytical and Bioanalytical Chemistry (2024)
-
Stereoselective analysis and enantioseparation of isoproterenol in spiked human plasma
Chemical Papers (2024)
-
Variable fragmentation and ionization of amyloid-beta epimers and isomers
Analytical and Bioanalytical Chemistry (2023)
-
Occurrence of D-amino acids in natural products
Natural Products and Bioprospecting (2023)