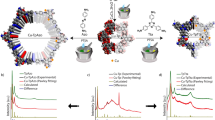
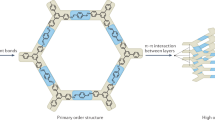
Abstract
Coordination polymers (CPs) and their subset, metal–organic frameworks (MOFs), can have porous structures and hybrid physicochemical properties that are useful for diverse applications. Although crystalline CPs and MOFs have received the most attention to date, their amorphous states are of growing interest as they can be directly synthesized under mild conditions. Directly synthesized amorphous CPs (aCPs) can be constructed from a wider range of metals and ligands than their crystalline and crystal-derived counterparts and demonstrate numerous unique material properties, such as higher mechanical robustness, increased stability and greater processability. This Review examines methods for the direct synthesis of aCPs and amorphous MOFs, as well as their properties and characterization routes, and offers a perspective on the opportunities for the widespread adoption of directly synthesized aCPs.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhou, H.-C., Long, J. R. & Yaghi, O. M. Introduction to metal–organic frameworks. Chem. Rev. 112, 673–674 (2012).
Kitagawa, S., Kitaura, R. & Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Maurin, G., Serre, C., Cooper, A. & Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 46, 3104–3107 (2017).
Michal, B. T., McKenzie, B. M., Felder, S. E. & Rowan, S. J. Metallo-, thermo-, and photoresponsive shape memory and actuating liquid crystalline elastomers. Macromolecules 48, 3239–3246 (2015).
Han, X., Yang, S. & Schröder, M. Porous metal–organic frameworks as emerging sorbents for clean air. Nat. Rev. Chem. 3, 108–118 (2019).
Du, Q. et al. Preparation of modified zirconium-based metal–organic frameworks (Zr-MOFs) supported metals and recent application in environment: a review and perspectives. Surf. Interfaces 28, 101647 (2022).
Miyasaka, H. Charge manipulation in metal–organic frameworks: toward designer functional molecular materials. Bull. Chem. Soc. Jpn. 94, 2929–2955 (2021).
Winter, A. & Schubert, U. S. Synthesis and characterization of metallo-supramolecular polymers. Chem. Soc. Rev. 45, 5311–5357 (2016).
Kumar, P. et al. Regeneration, degradation, and toxicity effect of MOFs: opportunities and challenges. Environ. Res. 176, 108488 (2019).
Feng, L., Wang, K.-Y., Day, G. S., Ryder, M. R. & Zhou, H.-C. Destruction of metal–organic frameworks: positive and negative aspects of stability and lability. Chemi. Rev. 120, 13087–13133 (2020).
Ettlinger, R. et al. Toxicity of metal–organic framework nanoparticles: from essential analyses to potential applications. Chem. Soc. Rev. 51, 464–484 (2022).
Sánchez-González, E., Tsang, M. Y., Troyano, J., Craig, G. A. & Furukawa, S. Assembling metal–organic cages as porous materials. Chem. Soc. Rev. 51, 4876–4889 (2022).
Hosono, N. & Kitagawa, S. Modular design of porous soft materials via self-organization of metal–organic cages. Acc. Chem. Res. 51, 2437–2446 (2018).
Chen, Y. et al. Shaping of metal–organic frameworks: from fluid to shaped bodies and robust foams. J. Am. Chem. Soc. 138, 10810–10813 (2016).
Howarth, A. J. et al. Chemical, thermal and mechanical stabilities of metal–organic frameworks. Nat. Rev. Mater. 1, 15018 (2016).
Simonov, A. & Goodwin, A. L. Designing disorder into crystalline materials. Nat. Rev. Chem. 4, 657–673 (2020).
Lin, W., Rieter, W. J. & Taylor, K. M. Modular synthesis of functional nanoscale coordination polymers. Angew. Chem. Int. Ed. 48, 650–658 (2009).
Ahmed, H. et al. Acoustomicrofluidic assembly of oriented and simultaneously activated metal–organic frameworks. Nat. Commun. 10, 2282 (2019).
Hosono, N. Design of porous coordination materials with dynamic properties. Bull. Chem. Soc. Jpn. 94, 60–69 (2021).
Bennett, T. D., Coudert, F.-X., James, S. L. & Cooper, A. I. The changing state of porous materials. Nat. Mater. 20, 1179–1187 (2021). A perspective highlighting topological disorder as a key future research direction of porous materials.
Ashworth, C. Characterizing amorphous MOFs. Nat. Rev. Chem. 5, 298–298 (2021).
Bennett, T. D. & Horike, S. Liquid, glass and amorphous solid states of coordination polymers and metal–organic frameworks. Nat. Rev. Mater. 3, 431–440 (2018). A review emphasizing various ways to post-synthetically form aCPs and aMOFs.
Spokoyny, A. M., Kim, D., Sumrein, A. & Mirkin, C. A. Infinite coordination polymer nano- and microparticle structures. Chem. Soc. Rev. 38, 1218–1227 (2009). A review summarizing the synthesis, morphologies and applications of aCP particles.
Oh, M. & Mirkin, C. A. Chemically tailorable colloidal particles from infinite coordination polymers. Nature 438, 651–654 (2005). A pioneering study on synthesizing aCPs through the mixed solvent method.
Ma, N. & Horike, S. Metal–organic network-forming glasses. Chem. Rev. 122, 4163–4203 (2022).
Ma, Q., Jin, H. & Li, Y. Tuning the adsorption selectivity of ZIF-8 by amorphization. Chem. Eur. J. 26, 13137–13141 (2020).
Orellana-Tavra, C. et al. Amorphous metal–organic frameworks for drug delivery. Chem. Commun. 51, 13878–13881 (2015).
Katsenis, A. D. et al. In situ X-ray diffraction monitoring of a mechanochemical reaction reveals a unique topology metal‒organic framework. Nat. Commun. 6, 6662 (2015).
Ejima, H. et al. One-step assembly of coordination complexes for versatile film and particle engineering. Science 341, 154–157 (2013). A pivotal study demonstrating the direct synthesis of metal–phenolic coatings on various organic, inorganic and biological templates.
You, X. et al. Metal-coordinated sub-10 nm membranes for water purification. Nat. Commun. 10, 4160 (2019).
Horike, S., Nagarkar, S. S., Ogawa, T. & Kitagawa, S. A new dimension for coordination polymers and metal–organic frameworks: towards functional glasses and liquids. Angew. Chem. Int. Ed. 59, 6652–6664 (2020).
Zhong, Q.-Z. et al. Oxidation-mediated kinetic strategies for engineering metal–phenolic networks. Angew. Chem. Int. Ed. 58, 12563–12568 (2019).
Saha, S., Wiebcke, M. & Huber, K. Insight into fast nucleation and growth of zeolitic imidazolate framework-71 by in situ static light scattering at variable temperature and kinetic modeling. Cryst. Growth Des. 18, 4653–4661 (2018). An investigation into the formation and crystallization of MOFs and aMOF seeds.
Gao, Z. et al. Topological distortion driven amorphous spherical MOFs for high quality single‐mode microlasers. Angew. Chem. Int. Ed. 60, 6362–6366 (2021).
Thyagarajan, R. & Sholl, D. S. A database of porous rigid amorphous materials. Chem. Mater. 32, 8020–8033 (2020).
Khare, E., Holten-Andersen, N. & Buehler, M. J. Transition-metal coordinate bonds for bioinspired macromolecules with tunable mechanical properties. Nat. Rev. Mater. 6, 421–436 (2021).
Guo, J. et al. Engineering multifunctional capsules through the assembly of metal–phenolic networks. Angew. Chem. Int. Ed. 53, 5546–5551 (2014).
Guan, B. Y. et al. Coordination polymers derived general synthesis of multishelled mixed metal-oxide particles for hybrid supercapacitors. Adv. Mater. 29, 1605902 (2017).
Geng, H. et al. Metal ion-directed functional metal–phenolic materials. Chem. Rev. 122, 11432–11473 (2022).
Liu, B., Zhang, J. & Li, L. Metal–DNA coordination-driven self-assembly: a conceptual methodology to expand the repertoire of DNA nanobiotechnology. Eur. J. Chem. 25, 13452–13457 (2019). A review summarizing the synthesis of aCPs through metal–DNA coordination.
Liu, C. et al. Self-assembly of copper–DNAzyme nanohybrids for dual-catalytic tumor therapy. Angew. Chem. Int. Ed. 133, 14324–14328 (2021).
Lopez, A. & Liu, J. Self-assembly of nucleobase, nucleoside and nucleotide coordination polymers: from synthesis to applications. ChemNanoMat 3, 670–684 (2017).
Nishiyabu, R. et al. Nanoparticles of adaptive supramolecular networks self-assembled from nucleotides and lanthanide ions. J. Am. Chem. Soc. 131, 2151–2158 (2009).
Wei, H., Li, B., Du, Y., Dong, S. & Wang, E. Nucleobase–metal hybrid materials: preparation of submicrometer-scale, spherical colloidal particles of adenine–gold(III) via a supramolecular hierarchical self-assembly approach. Chem. Mater. 19, 2987–2993 (2007).
Nishiyabu, R., Aimé, C., Gondo, R., Noguchi, T. & Kimizuka, N. Confining molecules within aqueous coordination nanoparticles by adaptive molecular self-assembly. Angew. Chem. Int. Ed. 48, 9465–9468 (2009).
Wang, C., Di, Z., Xiang, Z., Zhao, J. & Li, L. Coordination-driven assembly of proteins and nucleic acids in a single architecture for carrier-free intracellular co-delivery. Nano Today 38, 101140 (2021).
Pires, M. M. & Chmielewski, J. Self-assembly of collagen peptides into microflorettes via metal coordination. J. Am. Chem. Soc. 131, 2706–2712 (2009).
Aronsson, C., Selegård, R. & Aili, D. Zinc-triggered hierarchical self-assembly of fibrous helix–loop–helix peptide superstructures for controlled encapsulation and release. Macromolecules 49, 6997–7003 (2016).
Przybyla, D. E., Rubert Pérez, C. M., Gleaton, J., Nandwana, V. & Chmielewski, J. Hierarchical assembly of collagen peptide triple helices into curved disks and metal ion-promoted hollow spheres. J. Am. Chem. Soc. 135, 3418–3422 (2013).
Richardson, J. J. et al. Continuous metal–organic framework biomineralization on cellulose nanocrystals: extrusion of functional composite filaments. ACS Sustain. Chem. Eng. 7, 6287–6294 (2019).
Park, S. H. & Lee, S. J. Versatile amorphous structures of phosphonate metal–organic framework/alginate composite for tunable sieving of ions. Adv. Funct. Mater. 29, 1904016 (2019).
Sun, Z. et al. Multistimuli-responsive, moldable supramolecular hydrogels cross-linked by ultrafast complexation of metal ions and biopolymers. Angew. Chem. Int. Ed. 54, 7944–7948 (2015).
Nadar, S. S., Vaidya, L., Maurya, S. & Rathod, V. K. Polysaccharide based metal organic frameworks (polysaccharide-MOF): a review. Coord. Chem. Rev. 396, 1–21 (2019).
Liang, K. et al. Biomimetic mineralization of metal–organic frameworks around polysaccharides. Chem. Commun. 53, 1249–1252 (2017).
Wang, X. et al. Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces. Environ. Sci. Nano 4, 2193–2204 (2017).
Ma, T.-Y., Li, H., Tang, A.-N. & Yuan, Z.-Y. Ordered, mesoporous metal phosphonate materials with microporous crystalline walls for selective separation techniques. Small 7, 1827–1837 (2011).
Ma, T.-Y., Lin, X.-Z. & Yuan, Z.-Y. Periodic mesoporous titanium phosphonate hybrid materials. J. Mater. Chem. 20, 7406–7415 (2010).
Haskouri, J. E. et al. S+I– ionic formation mechanism to new mesoporous aluminum phosphonates and diphosphonates. Chem. Mater. 16, 4359–4372 (2004).
Ai, Y. et al. General construction of 2D ordered mesoporous iron-based metal–organic nanomeshes. Small 16, 2002701 (2020).
Zhu, Y.-P., Ren, T.-Z. & Yuan, Z.-Y. Hollow cobalt phosphonate spherical hybrid as high-efficiency Fenton catalyst. Nanoscale 6, 11395–11402 (2014).
Lee, H. J. et al. Morphological and structural evolutions of metal–organic framework particles from amorphous spheres to crystalline hexagonal rods. Angew. Chem. Int. Ed. 54, 10564–10568 (2015). A report on the synthesis and comparison of compositionally similar crystalline CPs and aCPs.
Kaminker, R., Popovitz-Biro, R. & van der Boom, M. E. Coordination-polymer nanotubes and spheres: a ligand-structure effect. Angew. Chem. Int. Ed. 50, 3224–3226 (2011).
Wang, Y.-M., Liu, W. & Yin, X.-B. Self-limiting growth nanoscale coordination polymers for fluorescence and magnetic resonance dual-modality imaging. Adv. Funct. Mater. 26, 8463–8470 (2016).
Zhang, Z., Nguyen, H. T. H., Miller, S. A. & Cohen, S. M. polyMOFs: a class of interconvertible polymer–metal–organic-framework hybrid materials. Angew. Chem. Int. Ed. 54, 6152–6157 (2015).
Zhang, Z. et al. Polymer–metal–organic frameworks (polyMOFs) as water tolerant materials for selective carbon dioxide separations. J. Am. Chem. Soc. 138, 920–925 (2016).
Cazacu, M. et al. Hydrophobic, amorphous metal–organic network readily prepared by complexing the aluminum ion with a siloxane spaced dicarboxylic acid in aqueous medium. J. Appl. Polym. Sci. 136, 47144 (2019).
Rahim, M. A. et al. Surface-confined amorphous films from metal–coordinated simple phenolic ligands. Chem. Mater. 27, 5825–5832 (2015).
Lin, Z. et al. Ordered mesoporous metal–phenolic network particles. J. Am. Chem. Soc. 142, 335–341 (2020). A report on engineering aMOFs with ordered porous structures.
Yun, G. et al. Self-assembly of nano- to macroscopic metal–phenolic materials. Chem. Mater. 30, 5750–5758 (2018).
Mitsuka, Y. et al. Fabrication of integrated copper-based nanoparticles/amorphous metal–organic framework by a facile spray-drying method: highly enhanced CO2 hydrogenation activity for methanol synthesis. Angew. Chem. Int. Ed. 133, 22457–22462 (2021).
Li, L., Zhang, G. & Su, Z. One-step assembly of phytic acid metal complexes for superhydrophilic coatings. Angew. Chem. Int. Ed. 55, 9093–9096 (2016).
Ping, Y. et al. pH-responsive capsules engineered from metal–phenolic networks for anticancer drug delivery. Small 11, 2032–2036 (2015).
Zhang, W. et al. Cobalt-directed assembly of antibodies onto metal–phenolic networks for enhanced particle targeting. Nano Lett. 20, 2660–2666 (2020).
Zhou, J. et al. Polyphenol-mediated assembly for particle engineering. Acc. Chem. Res. 53, 1269–1278 (2020).
Gagnon, K. J., Perry, H. P. & Clearfield, A. Conventional and unconventional metal–organic frameworks based on phosphonate ligands: MOFs and UMOFs. Chem. Rev. 112, 1034–1054 (2012).
Li, B. & Zeng, H. C. Synthetic chemistry and multifunctionality of an amorphous Ni-MOF-74 shell on a Ni/SiO2 hollow catalyst for efficient tandem reactions. Chem. Mater. 31, 5320–5330 (2019).
Zhang, Q. et al. In situ growth of ultrathin Co-MOF nanosheets on α-Fe2O3 hematite nanorods for efficient photoelectrochemical water oxidation. Sol. Energy 171, 388–396 (2018).
Li, L. et al. Fabricating nano-IrO2@amorphous Ir-MOF composites for efficient overall water splitting: a one-pot solvothermal approach. J. Mater. Chem. A 8, 25687–25695 (2020).
Zhao, M. et al. Core–shell palladium nanoparticle@metal–organic frameworks as multifunctional catalysts for cascade reactions. J. Am. Chem. Soc. 136, 1738–1741 (2014).
Jo, C., Lee, H. J. & Oh, M. One-pot synthesis of silica@coordination polymer core–shell microspheres with controlled shell thickness. Adv. Mater. 23, 1716–1719 (2011).
Wu, X. et al. Packaging and delivering enzymes by amorphous metal–organic frameworks. Nat. Commun. 10, 5165 (2019). An article comparing the performance of enzyme-loaded aMOFs and crystalline MOFs.
Zhang, Y., Xu, L. & Ge, J. Multienzyme system in amorphous metal–organic frameworks for intracellular lactate detection. Nano Lett. 22, 5029–5036 (2022).
Hammi, N. et al. Polysaccharide templated biomimetic growth of hierarchically porous metal–organic frameworks. Microporous Mesoporous Mater. 306, 110429 (2020).
Liu, F. et al. Gram-scale synthesis of coordination polymer nanodots with renal clearance properties for cancer theranostic applications. Nat. Commun. 6, 8003 (2015).
Gong, X. et al. Rapid generation of metal–organic framework phase diagrams by high-throughput transmission electron microscopy. J. Am. Chem. Soc. 144, 6674–6680 (2022).
Madsen, R. S. K. et al. Ultrahigh-field 67Zn NMR reveals short-range disorder in zeolitic imidazolate framework glasses. Science 367, 1473–1476 (2020).
Lee, T. et al. Large-area synthesis of ultrathin, flexible, and transparent conductive metal–organic framework thin films via a microfluidic-based solution shearing process. Adv. Mater. 34, 2107696 (2022).
Ogata, A. F. et al. Direct observation of amorphous precursor phases in the nucleation of protein–metal–organic frameworks. J. Am. Chem. Soc. 142, 1433–1442 (2020).
Liu, X. et al. Three-step nucleation of metal–organic framework nanocrystals. Proc. Natl Acad. Sci. USA 118, e2008880118 (2021).
Venna, S. R., Jasinski, J. B. & Carreon, M. A. Structural evolution of zeolitic imidazolate framework-8. J. Am. Chem. Soc. 132, 18030–18033 (2010). A study highlighting that crystalline MOFs often first start as amorphous materials.
Goesten, M. G. et al. Evidence for a chemical clock in oscillatory formation of UiO-66. Nat. Commun. 7, 11832 (2016).
Surblé, S., Millange, F., Serre, C., Férey, G. & Walton, R. I. An EXAFS study of the formation of a nanoporous metal–organic framework: evidence for the retention of secondary building units during synthesis. Chem. Commun. 14, 1518–1520 (2016).
Cerasale, D. J., Ward, D. C. & Easun, T. L. MOFs in the time domain. Nat. Rev. Chem. 6, 9–30 (2022).
Cravillon, J. et al. Fast nucleation and growth of ZIF-8 nanocrystals monitored by time-resolved in situ small-angle and wide-angle X-ray scattering. Angew. Chem. Int. Ed. 50, 8067–8071 (2011).
Richardson, J. J. & Liang, K. Nano-biohybrids: in vivo synthesis of metal–organic frameworks inside living plants. Small 14, 1702958 (2018).
Terban, M. W. et al. Early stage structural development of prototypical zeolitic imidazolate framework (ZIF) in solution. Nanoscale 10, 4291–4300 (2018).
Pan, S. et al. Rational modulating electronegativity of substituents in amorphous metal–organic frameworks for water oxidation catalysis. Int. J. Hydrog. Energy 45, 9723–9732 (2020).
Xu, H., Sommer, S., Broge, N. L. N., Gao, J. & Iversen, B. B. The chemistry of nucleation: in situ pair distribution function analysis of secondary building units during UiO-66 MOF formation. Chem. Eur. J. 25, 2051–2058 (2019).
Zhang, T. et al. Amorphous Fe/Mn bimetal–organic frameworks: outer and inner structural designs for efficient arsenic(III) removal. J. Mater. Chem. A 7, 2845–2854 (2019).
Zhang, Y., Gao, T., Jin, Z., Chen, X. & Xiao, D. A robust water oxidation electrocatalyst from amorphous cobalt–iron bimetallic phytate nanostructures. J. Mater. Chem. A 4, 15888–15895 (2016).
Xing, W. et al. Rational synthesis of amorphous iron–nickel phosphonates for highly efficient photocatalytic water oxidation with almost 100% yield. Angew. Chem. Int. Ed. 59, 1171–1175 (2020).
Hidalgo, T. et al. Crystal structure dependent in vitro antioxidant activity of biocompatible calcium gallate MOFs. J. Mater. Chem. B 5, 2813–2822 (2017). A study demonstrating how the final structure can affect the properties of MOFs with the same composition.
Cooper, L. et al. A biocompatible porous Mg–gallate metal–organic framework as an antioxidant carrier. Chem. Commun. 51, 5848–5851 (2015).
Sonnauer, A. et al. Giant pores in a chromium 2,6-naphthalenedicarboxylate open-framework structure with MIL-101 topology. Angew. Chem. Int. Ed. 48, 3791–3794 (2009).
Zhang, Z. et al. Well-defined metal–organic framework hollow nanocages. Angew. Chem. Int. Ed. 53, 429–433 (2014).
Xin, Z., Chen, X., Wang, Q., Chen, Q. & Zhang, Q. Nanopolyhedrons and mesoporous supra-structures of zeolitic imidazolate framework with high adsorption performance. Microporous Mesoporous Mater. 169, 218–221 (2013).
Jeon, Y.-M., Heo, J. & Mirkin, C. A. Dynamic interconversion of amorphous microparticles and crystalline rods in salen-based homochiral infinite coordination polymers. J. Am. Chem. Soc. 129, 7480–7481 (2007). A study demonstrating that aCPs can be converted into crystalline CPs and vice versa through the choice of solvent.
Zou, L., Hou, C.-C., Liu, Z., Pang, H. & Xu, Q. Superlong single-crystal metal–organic framework nanotubes. J. Am. Chem. Soc. 140, 15393–15401 (2018).
Wu, S., Zhuang, G., Wei, J., Zhuang, Z. & Yu, Y. Shape control of core–shell MOF@MOF and derived MOF nanocages via ion modulation in a one-pot strategy. J. Mater. Chem. A 6, 18234–18241 (2018).
Wang, B. et al. In situ structural evolution of the multi-site alloy electrocatalyst to manipulate the intermediate for enhanced water oxidation reaction. Energy Environ. Sci. 13, 2200–2208 (2020).
Zhu, Y.-P., Ma, T.-Y., Liu, Y.-L., Ren, T.-Z. & Yuan, Z.-Y. Metal phosphonate hybrid materials: from densely layered to hierarchically nanoporous structures. Inorg. Chem. Front. 1, 360–383 (2014).
Wang, H. et al. Structural, electronic, and dielectric properties of a large random network model of amorphous zeolitic imidazolate frameworks and its analogues. J. Am. Ceram. Soc. 102, 4602–4611 (2019).
Li, J. et al. Low-crystalline bimetallic metal–organic framework electrocatalysts with rich active sites for oxygen evolution. ACS Energy Lett. 4, 285–292 (2018).
Cao, S., Bennett, T. D., Keen, D. A., Goodwin, A. L. & Cheetham, A. K. Amorphization of the prototypical zeolitic imidazolate framework ZIF-8 by ball-milling. Chem. Commun. 48, 7805–7807 (2012). A study on the amorphization of pre-synthesized crystalline MOFs.
Chen, J. et al. Programmable permeability of metal–phenolic network microcapsules. Chem. Mater. 32, 6975–6982 (2020).
Lin, C.-E. et al. Ultrathin nanofilm with tailored pore size fabricated by metal–phenolic network for precise and rapid molecular separation. Sep. Purif. Technol. 207, 435–442 (2018).
Feller, R. K. & Cheetham, A. K. Fe(III), Mn(II), Co(II), and Ni(II) 3,4,5-trihydroxybenzoate (gallate) dihydrates; a new family of hybrid framework materials. Solid State Sci. 8, 1121–1125 (2006).
Zhang, R.-Z. et al. Effect of surface charge status of amorphous porous coordination polymer particles on the adsorption of organic dyes from an aqueous solution. J. Colloid Interface Sci. 525, 54–61 (2018).
Fonseca, J. & Choi, S. Synthesis of a novel amorphous metal organic framework with hierarchical porosity for adsorptive gas separation. Microporous Mesoporous Mater. 310, 110600 (2021).
Veliscek-Carolan, J., Rawal, A., Luca, V. & Hanley, T. L. Zirconium phosphonate sorbents with tunable structure and function. Microporous Mesoporous Mater. 252, 90–104 (2017).
Rahim, M. A. et al. Self-assembly of a metal–phenolic sorbent for broad-spectrum metal sequestration. ACS Appl. Mater. Interfaces 12, 3746–3754 (2020).
Eddaoudi, M. et al. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 295, 469–472 (2002).
Deng, H. et al. Large-pore apertures in a series of metal–organic frameworks. Science 336, 1018–1023 (2012).
Hamilton, T. D. et al. Thixotropic hydrogel derived from a product of an organic solid-state synthesis: properties and densities of metal–organic nanoparticles. J. Am. Chem. Soc. 133, 3365–3371 (2011).
Patterson, J. P. et al. Observing the growth of metal–organic frameworks by in situ liquid cell transmission electron microscopy. J. Am. Chem. Soc. 137, 7322–7328 (2015).
Cravillon, J. et al. Controlling zeolitic imidazolate framework nano- and microcrystal formation: insight into crystal growth by time-resolved in situ static light scattering. Chem. Mater. 23, 2130–2141 (2011).
Hikov, T., Schröder, C. A., Cravillon, J., Wiebcke, M. & Huber, K. In situ static and dynamic light scattering and scanning electron microscopy study on the crystallization of the dense zinc imidazolate framework ZIF-zni. Phys. Chem. Chem. Phys. 14, 511–521 (2012).
Saha, S. et al. Insight into fast nucleation and growth of zeolitic imidazolate framework-71 by in situ time-resolved light and X-ray scattering experiments. Cryst. Growth Des. 16, 2002–2010 (2016).
Castillo-Blas, C., Moreno, J. M., Romero-Muñiz, I. & Platero-Prats, A. E. Applications of pair distribution function analyses to the emerging field of non-ideal metal–organic framework materials. Nanoscale 12, 15577–15587 (2020).
Nayuk, R. et al. Modulated formation of MOF-5 nanoparticles — a SANS analysis. J. Phys. Chem. C 116, 6127–6135 (2012).
Moh, P. Y., Brenda, M., Anderson, M. W. & Attfield, M. P. Crystallisation of solvothermally synthesised ZIF-8 investigated at the bulk, single crystal and surface level. CrystEngComm 15, 9672–9678 (2013).
Seeber, G. et al. Following the self assembly of supramolecular MOFs using X-ray crystallography and cryospray mass spectrometry. Chem. Sci. 1, 62–67 (2010).
Van Vleet, M. J., Weng, T., Li, X. & Schmidt, J. In situ, time-resolved, and mechanistic studies of metal–organic framework nucleation and growth. Chem. Rev. 118, 3681–3721 (2018).
Frost, J. M. et al. From discrete molecule, to polymer, to MOF: mapping the coordination chemistry of CdII using 113Cd solid-state NMR. Chem. Commun. 52, 10680–10683 (2016).
Haouas, M., Volkringer, C., Loiseau, T., Férey, G. & Taulelle, F. In situ NMR, ex situ XRD and SEM study of the hydrothermal crystallization of nanoporous aluminum trimesates MIL-96, MIL-100, and MIL-110. Chem. Mater. 24, 2462–2471 (2012). An investigation of the crystallization process of different MOFs using an array of techniques.
Song, X. et al. Europium-based infinite coordination polymer nanospheres as an effective fluorescence probe for phosphate sensing. RSC Adv. 7, 8661–8669 (2017).
Elsabawy, K. M. & Fallatah, A. M. Synthesis of newly wings like structure non-crystalline Ni++-1,3,5-tribenzyl-1,3,5-triazine-2,4,6-(1H,3H,5H)-trione coordinated MOFs for CO2-capture. J. Mol. Struct. 1177, 255–259 (2019).
He, Q. et al. Solvent-free synthesis of uniform MOF shell‐derived carbon confined SnO2/Co nanocubes for highly reversible lithium storage. Small 13, 1701504 (2017).
Poryvaev, A. S., Polyukhov, D. M. & Fedin, M. V. Mitigation of pressure-induced amorphization in metal–organic framework ZIF-8 upon EPR control. ACS Appl. Mater. Interfaces 12, 16655–16661 (2020).
He, Z., Honeycutt, C. W., Zhang, T. & Bertsch, P. M. Preparation and FT-IR characterization of metal phytate compounds. J. Environ. Qual. 35, 1319–1328 (2006).
Ding, M., Cai, X. & Jiang, H.-L. Improving MOF stability: approaches and applications. Chem. Sci. 10, 10209–10230 (2019).
Liu, Y., Lv, S., Liu, D. & Song, F. Recent development of amorphous metal coordination polymers for cancer therapy. Acta Biomater. 116, 16–31 (2020).
Kim, S., Peterson, A. M. & Holten-Andersen, N. Enhanced water retention maintains energy dissipation in dehydrated metal-coordinate polymer networks: another role for Fe-catechol cross-links? Chem. Mater. 30, 3648–3655 (2018).
Zhou, C. et al. Metal–organic framework glasses with permanent accessible porosity. Nat. Commun. 9, 5042 (2018).
Yun, G., Richardson, J. J., Biviano, M. & Caruso, F. Tuning the mechanical behavior of metal–phenolic networks through building block composition. ACS Appl. Mater. Interfaces 11, 6404–6410 (2019).
Chen, J. et al. Metal–phenolic coatings as a platform to trigger endosomal escape of nanoparticles. ACS Nano 13, 11653–11664 (2019).
Björnmalm, M. et al. In vivo biocompatibility and immunogenicity of metal–phenolic gelation. Chem. Sci. 10, 10179–10194 (2019).
Ariga, K. Nanoarchitectonics: what’s coming next after nanotechnology? Nanoscale Horiz. 6, 364–378 (2021).
Park, J. H. et al. A cytoprotective and degradable metal–polyphenol nanoshell for single-cell encapsulation. Angew. Chem. Int. Ed. 53, 12420–12425 (2014).
Acknowledgements
This research was funded by the Australian Research Council (ARC) through the Discovery Project (DP200100713) scheme. J.J.R. acknowledges JSPS KAKENHI Grant Number 20F20373 and JSPS Fellowship P20373 and is a recipient of an ARC Future Fellowship (project number FT210100669). F.C. acknowledges the award of a National Health and Medical Research Council Senior Principal Research Fellowship (GNT1135806). The authors thank B. Abrahams for helpful discussions.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to discussion of the content and writing of the article and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, Z., Richardson, J.J., Zhou, J. et al. Direct synthesis of amorphous coordination polymers and metal–organic frameworks. Nat Rev Chem 7, 273–286 (2023). https://doi.org/10.1038/s41570-023-00474-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-023-00474-1