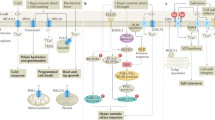
Abstract
Time is an often-neglected variable in biological research. Plants respond to biotic and abiotic stressors with a range of chemical signals, but as plants are non-equilibrium systems, single-point measurements often cannot provide sufficient temporal resolution to capture these time-dependent signals. In this article, we critically review the advances in continuous monitoring of chemical signals in living plants under stress. We discuss methods for sustained measurement of the most important chemical species, including ions, organic molecules, inorganic molecules and radicals. We examine analytical and modelling approaches currently used to identify and predict stress in plants. We also explore how the methods discussed can be used for applications beyond a research laboratory, in agricultural settings. Finally, we present the current challenges and future perspectives for the continuous monitoring of chemical signals in plants.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Geilfus, C. M. The pH of the apoplast: dynamic factor with functional impact under stress. Mol. Plant 10, 1371–1386 (2017).
Zhang, J. & Zhou, J.-M. Plant immunity triggered by microbial molecular signatures. Mol. Plant 3, 783–793 (2010).
Chisholm, S. T., Coaker, G., Day, B. & Staskawicz, B. J. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814 (2006).
Lam, E., Kato, N. & Lawton, M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature 411, 848–853 (2001).
Felle, H. H., Waller, F., Molitor, A. & Kogel, K. H. The mycorrhiza fungus Piriformospora indica induces fast root-surface pH signaling and primes systemic alkalinization of the leaf apoplast upon powdery mildew infection. Mol. Plant Microbe Interact. 22, 1179–1185 (2009).
Hussain, M., Malik, M. A., Farooq, M., Ashraf, M. Y. & Cheema, M. A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 194, 193–199 (2008).
Munné-Bosch, S. & Peñuelas, J. Photo- and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 217, 758–766 (2003).
Fichman, Y., Miller, G. & Mittler, R. Whole-plant live imaging of reactive oxygen species. Mol. Plant 12, 1203–1210 (2019).
Albert, M. & Fürst, U. in Plant Receptor Kinases. Methods in Molecular Biology Vol. 1621 (ed. Aalen, R.) 69–76 (Humana, 2017).
Giraldo, J. P., Wu, H., Newkirk, G. M. & Kruss, S. Nanobiotechnology approaches for engineering smart plant sensors. Nat. Nanotechnol. 14, 541–553 (2019).
Hill, O. The ultimate guide to crop disease and pest forecasting tools. Farmers Weekly https://www.fwi.co.uk/arable/find-latest-crop-disease-pest-forecasts-season (2016).
Liu, J. & Wang, X. Plant diseases and pests detection based on deep learning: a review. Plant Methods 17, 22 (2021).
Harakannanavar, S. S., Rudagi, J. M., Puranikmath, V. I., Siddiqua, A. & Pramodhini, R. Plant leaf disease detection using computer vision and machine learning algorithms. Glob. Transit. Proc. 3, 305–310 (2022).
Mohanty, S. P., Hughes, D. P. & Salathé, M. Using deep learning for image-based plant disease detection. Front. Plant Sci. 7, 1419 (2016).
Flynn, P. Biotic vs. abiotic - distinguishing disease problems. Iowa State University https://hortnews.extension.iastate.edu/biotic-vs-abiotic-distinguishing-disease-problems (2003).
Basu, S., Varsani, S. & Louis, J. Altering plant defenses: herbivore-associated molecular patterns and effector arsenal of chewing herbivores. Mol. Plant Microbe Interact. 31, 13–21 (2018).
Bi, G. et al. The ZAR1 resistosome is a calcium-permeable channel triggering plant immune signaling. Cell 184, 3528–3541.e12 (2021).
Ngou, B. P. M., Ahn, H. K., Ding, P. & Jones, J. D. G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115 (2021).
Barker, A. V & Pilbeam, D. J. Handbook of Plant Nutrition (CRC, 2015).
John Cram, B. W. et al. Handbook of reference methods for plant analysis. Crop Sci. 38, 1710–1711 (1998).
Crawford, N. M. Nitrate: nutrient and signal for plant growth. Plant Cell 7, 859–868 (1995).
Naveed, Z. A., Wei, X., Chen, J., Mubeen, H. & Ali, G. S. The PTI to ETI continuum in Phytophthora-plant interactions. Front. Plant Sci. 11, 593905 (2020).
Flowers, T. J. & Colmer, T. D. Salinity tolerance in halophytes. New Phytol. 179, 945–963 (2008).
He, M., He, C.-Q. & Ding, N.-Z. Abiotic stresses: general defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 9, 1771 (2018).
Colmenero-Flores, J. M., Franco-Navarro, J. D., Cubero-Font, P., Peinado-Torrubia, P. & Rosales, M. A. Chloride as a beneficial macronutrient in higher plants: new roles and regulation. Int. J. Mol. Sci. 20, 4686 (2019).
Broyer, T. C., Carlton, A. B., Johnson, C. M. & Stout, P. R. Chlorine — a micronutrient element for higher plants. Plant Physiol. 29, 526–532 (1954).
Yuan, P., Yang, T. & Poovaiah, B. W. Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci. 19, 3896 (2018).
Tuteja, N. & Mahajan, S. Calcium signaling network in plants: an overview. Plant Signal. Behav. 2, 79–85 (2007).
Muday, G. K. & Brown-Harding, H. Nervous system-like signaling in plant defense. Science 361, 1068–1069 (2018).
Toyota, M. et al. Glutamate triggers long-distance, calcium-based plant defense signaling. Science 361, 1112–1115 (2018). Revelation of a hormone-like role for glutamate in long-distance, time-dependent calcium signalling resulting from herbivore feeding and mechanical wounding.
Hanstein, S., de Beer, D. & Felle, H. H. Miniaturised carbon dioxide sensor designed for measurements within plant leaves. Sens. Actuators B Chem. 81, 107–114 (2001). Measurement of CO2 inside the stomatal pore enabled using a miniaturized glass-capillary electrochemical sensor.
Hu, S., Ding, Y. & Zhu, C. Sensitivity and responses of chloroplasts to heat stress in plants. Front. Plant Sci. 11, 375 (2020).
Im, H., Lee, S., Naqi, M., Lee, C. & Kim, S. Flexible PI-based plant drought stress sensor for real-time monitoring system in smart farm. Electronics 7, 114 (2018).
Ali, M., Cheng, Z., Ahmad, H. & Hayat, S. Reactive oxygen species (ROS) as defenses against a broad range of plant fungal infections and case study on ROS employed by crops against Verticillium dahliae wilts. J. Plant Interact. 13, 353–363 (2018).
Torres, M. A., Jones, J. D. G. & Dangl, J. L. Reactive oxygen species signaling in response to pathogens. Plant Physiol. 141, 373–378 (2006).
Lamb, C. & Dixon, R. A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 251–275 (1997).
Xu, Q. et al. In vivo monitoring of oxidative burst induced by ultraviolet A and C stress for oilseed rape by microbiosensor. Sens. Actuators B Chem. 141, 599–603 (2009).
Ding, Y., Shi, Y. & Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 222, 1690–1704 (2019).
Iovine, N. M. et al. Reactive nitrogen species contribute to innate host defense against Campylobacter jejuni. Infect. Immun. 76, 986–993 (2008).
Pauly, N. et al. Reactive oxygen and nitrogen species and glutathione: key players in the legume–Rhizobium symbiosis. J. Exp. Bot. 57, 1769–1776 (2006).
Parankusam, S., Adimulam, S. S., Bhatnagar-Mathur, P. & Sharma, K. K. Nitric oxide (NO) in plant heat stress tolerance: current knowledge and perspectives. Front. Plant Sci. 8, 1582 (2017).
Šimura, J. et al. Plant hormonomics: multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 177, 476–489 (2018).
Park, J., Lee, Y., Martinoia, E. & Geisler, M. Plant hormone transporters: what we know and what we would like to know. BMC Biol. 15, 93 (2017).
Shah, J. & Zeier, J. Long-distance communication and signal amplification in systemic acquired resistance. Front. Plant Sci. 4, 30 (2013).
McConn, M., Creelman, R. A., Bell, E., Mullet, J. E. & Browse, J. Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl Acad. Sci. USA 94, 5473–5477 (1997).
Snoeren, T. A. L. et al. The herbivore-induced plant volatile methyl salicylate negatively affects attraction of the parasitoid Diadegma semiclausum. J. Chem. Ecol. 36, 479–489 (2010).
Fong, D., Luo, S.-X., Andre, R. S. & Swager, T. M. Trace ethylene sensing via Wacker oxidation. ACS Cent. Sci. 6, 507–512 (2020).
Dudareva, N., Negre, F., Nagegowda, D. A. & Orlova, I. Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–440 (2006).
Raghava, T., Ravikumar, P., Hegde, R. & Kush, A. Spatial and temporal volatile organic compound response of select tomato cultivars to herbivory and mechanical injury. Plant Sci. 179, 520–526 (2010).
Taiz, L., Zeiger, E., Moller, I. M. & Mirphy, A. Plant Physiology and Development (Sinauer Associates, 2015).
Farmer, E. E. & Ryan, C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl Acad. Sci. USA 87, 7713–7716 (1990).
Altangerel, N. et al. In vivo diagnostics of early abiotic plant stress response via Raman spectroscopy. Proc. Natl Acad. Sci. USA 114, 3393–3396 (2017).
Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 70, 1–9 (1999).
Kovinich, N. et al. Not all anthocyanins are born equal: distinct patterns induced by stress in Arabidopsis. Planta 240, 931–940 (2014).
Wang, M. et al. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2, 16151 (2016).
Kim, G., LeBlanc, M. L., Wafula, E. K., DePamphilis, C. W. & Westwood, J. H. Genomic-scale exchange of mRNA between a parasitic plant and its hosts. Science 345, 808–811 (2014).
Chen, Y. et al. An aphid RNA transcript migrates systemically within plants and is a virulence factor. Proc. Natl Acad. Sci. USA 117, 12763–12771 (2020).
Qiao, Y. et al. Oomycete pathogens encode RNA silencing suppressors. Nat. Genet. 45, 330–333 (2013).
Hou, Y. et al. A Phytophthora effector suppresses trans-kingdom RNAi to promote disease susceptibility. Cell Host Microbe 25, 153–165.e5 (2019).
Yin, C. et al. A novel fungal effector from Puccinia graminis suppressing RNA silencing and plant defense responses. New Phytol. 222, 1561–1572 (2019).
Moroz, N. et al. Extracellular alkalinization as a defense response in potato cells. Front. Plant Sci. 8, 32 (2017).
González-Sánchez, M. I. et al. Electrochemical detection of extracellular hydrogen peroxide in Arabidopsis thaliana: a real-time marker of oxidative stress. Plant Cell Environ. 36, 869–878 (2013).
Roper, J. M., Garcia, J. F. & Tsutsui, H. Emerging technologies for monitoring plant health in vivo. ACS Omega 6, 5101–5107 (2021).
Schornack, S. et al. Ancient class of translocated oomycete effectors targets the host nucleus. Proc. Natl Acad. Sci. USA 107, 17421–17426 (2010).
Kikkert, J. R., Vidal, J. R. & Reisch, B. I. in Transgenic Plants: Methods and Protocols (ed. Peña, L.) 61–78 (Humana, 2004).
Wong, M. H. et al. Lipid exchange envelope penetration (LEEP) of nanoparticles for plant engineering: a universal localization mechanism. Nano Lett. 16, 1161–1172 (2016).
Gupta, S. et al. Portable Raman leaf-clip sensor for rapid detection of plant stress. Sci. Rep. 10, 20206 (2020). A portable, handheld Raman sensor designed to clip onto leaves was used for rapid detection of nutrient deficiency in a range of crops.
Montanha, G. S. et al. X-ray fluorescence spectroscopy (XRF) applied to plant science: challenges towards in vivo analysis of plants. Metallomics 12, 183–192 (2020).
Sadoine, M. et al. Designs, applications, and limitations of genetically encoded fluorescent sensors to explore plant biology. Plant Physiol. 187, 485–503 (2021).
Dakir, A., Zahra, B. F. & Omar, A. B. Optical satellite images services for precision agricultural use: a review. Adv. Sci. Technol. Eng. Syst. J. 6, 326–331 (2021).
Ruwanpathirana, G. P. et al. Continuous monitoring of plant sodium transport dynamics using clinical PET. Plant Methods 17, 8 (2021). PET enables continuous, whole-plant, 3D visualization of long-distance Na+ transport dynamics.
Sophocleous, M. & Atkinson, J. K. A review of screen-printed silver/silver chloride (Ag/AgCl) reference electrodes potentially suitable for environmental potentiometric sensors. Sens. Actuators A Phys. 267, 106–120 (2017).
Inzelt, G. in Handbook of Reference Electrodes (eds Inzelt, G., Lewenstam, A. & Scholz, F.) 331–332 (Springer, 2013).
Waleed Shinwari, M. et al. Microfabricated reference electrodes and their biosensing applications. Sensors 10, 1679–1715 (2010).
Morales, M. A. & Halpern, J. M. Guide to selecting a biorecognition element for biosensors. Bioconjug. Chem. 29, 3231–3239 (2018).
Westbroek, P. in Analytical Electrochemistry in Textiles (eds Westbroek, P., Priniotakis, G. & Kiekens, P.) 3–36 (2005).
Søpstad, S., Johannessen, E. A. & Imenes, K. Analytical errors in biosensors employing combined counter/pseudo-reference electrodes. Results Chem. 2, 100028 (2020).
Cao, S. et al. ISFET-based sensors for (bio)chemical applications: a review. Electrochem. Sci. Adv. https://doi.org/10.1002/ELSA.202100207 (2022).
Srinivasan, P., Ezhilan, M., Kulandaisamy, A. J., Babu, K. J. & Rayappan, J. B. B. Room temperature chemiresistive gas sensors: challenges and strategies — a mini review. J. Mater. Sci. Mater. Electron. 30, 15825–15847 (2019).
Esser, B., Schnorr, J. M. & Swager, T. M. Selective detection of ethylene gas using carbon nanotube-based devices: utility in determination of fruit ripeness. Angew. Chem. Int. Ed. 51, 5752–5756 (2012).
Li, Z. et al. Real-time monitoring of plant stresses via chemiresistive profiling of leaf volatiles by a wearable sensor. Matter 4, 2553–2570 (2021). Kirigami-inspired, lightweight, wearable, e-nose-style sensor for the detection and prediction of pathogenic infection by VOC release.
Cui, S., Ling, P., Zhu, H. & Keener, H. M. Plant pest detection using an artificial nose system: a review. Sensors 18, 378 (2018).
Yin, H. et al. Soil sensors and plant wearables for smart and precision agriculture. Adv. Mater. 33, 2007764 (2021).
Calisgan, S. D. et al. Micromechanical switch-based zero-power chemical detectors for plant health monitoring. J. Microelectromech. Syst. 29, 755–761 (2020).
Felle, H. Proton transport and pH control in Sinapis alba root hairs: a study carried out with double-barrelled pH micro-electrodes. J. Exp. Bot. 38, 340–354 (1987).
Felle, H. H. The apoplastic pH of the Zea mays root cortex as measured with pH-sensitive microelectrodes: aspects of regulation. J. Exp. Bot. 49, 987–995 (1998).
Izumi, R. et al. in Proc. 2017 IEEE Sensors (IEEE, 2017).
Martinière, A., Desbrosses, G., Sentenac, H. & Paris, N. Development and properties of genetically encoded pH sensors in plants. Front. Plant Sci. 4, 523 (2013).
Gjetting, S. K., Ytting, C. K., Schulz, A. & Fuglsang, A. T. Live imaging of intra- and extracellular pH in plants using pHusion, a novel genetically encoded biosensor. J. Exp. Bot. 63, 3207–3218 (2012).
Tantama, M., Hung, Y. P. & Yellen, G. Imaging intracellular pH in live cells with a genetically encoded red fluorescent protein sensor. J. Am. Chem. Soc. 133, 10034–10037 (2011).
Fujimaki, S. et al. Base to tip and long-distance transport of sodium in the root of common reed [Phragmites australis (Cav.) Trin. ex Steud.] at steady state under constant high-salt conditions. Plant Cell Physiol. 56, 943–950 (2015).
Nyein, H. Y. Y. et al. A wearable electrochemical platform for noninvasive simultaneous monitoring of Ca2+ and pH. ACS Nano 10, 7216–7224 (2016).
Keene, S. T. et al. Wearable organic electrochemical transistor patch for multiplexed sensing of calcium and ammonium ions from human perspiration. Adv. Healthc. Mater. 8, 1901321 (2019).
Pirovano, P. et al. A wearable sensor for the detection of sodium and potassium in human sweat during exercise. Talanta 219, 121145 (2020).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Bandodkar, A. J. et al. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 54, 603–609 (2014).
Schazmann, B. et al. A wearable electrochemical sensor for the real-time measurement of sweat sodium concentration. Anal. Methods 2, 342–348 (2010).
Garcia-Cordero, E. et al. Three-dimensional integrated ultra-low-volume passive microfluidics with ion-sensitive field-effect transistors for multiparameter wearable sweat analyzers. ACS Nano 12, 12646–12656 (2018).
Parrilla, M. et al. Wearable potentiometric ion patch for on-body electrolyte monitoring in sweat: toward a validation strategy to ensure physiological relevance. Anal. Chem. 91, 8644–8651 (2019).
Demuru, S., Kunnel, B. P. & Briand, D. Real-time multi-ion detection in the sweat concentration range enabled by flexible, printed, and microfluidics-integrated organic transistor arrays. Adv. Mater. Technol. 5, 2000328 (2020).
Zhai, Q. et al. Vertically aligned gold nanowires as stretchable and wearable epidermal ion-selective electrode for noninvasive multiplexed sweat analysis. Anal. Chem. 92, 4647–4655 (2020).
Parrilla, M. et al. A textile-based stretchable multi-ion potentiometric sensor. Adv. Healthc. Mater. 5, 996–1001 (2016).
Kim, M.-Y. et al. Highly stable potentiometric sensor with reduced graphene oxide aerogel as a solid contact for detection of nitrate and calcium ions. J. Electroanal. Chem. 897, 115553 (2021).
Jiao, Y. et al. in Int. Conf. Solid-State Sensors Actuators Microsystems Eurosensors XXXIII 37–40 (IEEE, 2019).
Lu, Y. et al. Multimodal plant healthcare flexible sensor system. ACS Nano 14, 10966–10975 (2020).
Oren, S., Ceylan, H., Schnable, P. S. & Dong, L. High-resolution patterning and transferring of graphene-based nanomaterials onto tape toward roll-to-roll production of tape-based wearable sensors. Adv. Mater. Technol. 2, 1700223 (2017).
Kim, J. J., Allison, L. K. & Andrew, T. L. Vapor-printed polymer electrodes for long-term, on-demand health monitoring. Sci. Adv. 5, eaaw0463 (2019).
Lee, K. et al. In-situ synthesis of carbon nanotube–graphite electronic devices and their integrations onto surfaces of live plants and insects. Nano Lett. 14, 2647–2654 (2014). Direct application of electrochemical sensors onto the leaf surface for the detection of gaseous analytes.
Koman, V. B. et al. Persistent drought monitoring using a microfluidic-printed electro-mechanical sensor of stomata: in planta. Lab Chip 17, 4015–4024 (2017).
Chauhan, S., Srivastava, H. S. & Patel, P. Wheat crop biophysical parameters retrieval using hybrid-polarized RISAT-1 SAR data. Remote Sens. Environ. 216, 28–43 (2018).
Grimnes, S. & Martinsen, Ø. G. Bioimpedance and Bioelectricity Basics 179–254 (Elsevier, 2015).
Xu, Q. et al. Microsensor in vivo monitoring of oxidative burst in oilseed rape (Brassica napus L.) leaves infected by Sclerotinia sclerotiorum. Anal. Chim. Acta 632, 21–25 (2009). Insertable Pt-based sensor for detecting successive bursts of ROS in plants with fungal infection.
Tangkuaram, T., Ponchio, C., Kangkasomboon, T., Katikawong, P. & Veerasai, W. Design and development of a highly stable hydrogen peroxide biosensor on screen printed carbon electrode based on horseradish peroxidase bound with gold nanoparticles in the matrix of chitosan. Biosens. Bioelectron. 22, 2071–2078 (2007).
Lu, S.-Y., Chen, Y., Fang, X. & Feng, X. Hydrogen peroxide sensor based on electrodeposited Prussian blue film. J. Appl. Electrochem. 47, 1261–1271 (2017).
Niemeyer, J., Scheuring, D., Oestreicher, J., Morgan, B. & Schroda, M. Real-time monitoring of subcellular H2O2 distribution in Chlamydomonas reinhardtii. Plant Cell 33, 2935–2949 (2021).
Hernández-Barrera, A. et al. Using hyper as a molecular probe to visualize hydrogen peroxide in living plant cells: a method with virtually unlimited potential in plant biology. Methods Enzymol. 527, 275–290 (2013).
Ugalde, J. M., Schlößer, M., Dongois, A., Martinière, A. & Meyer, A. J. The latest HyPe(r) in plant H2O2 biosensing. Plant Physiol. 187, 480–484 (2021).
Lew, T. T. S. et al. Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nat. Plants 6, 404–415 (2020).
Giraldo, J. P. et al. Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat. Mater. 13, 400–408 (2014).
Giraldo, J. P. et al. A ratiometric sensor using single chirality near-infrared fluorescent carbon nanotubes: application to in vivo monitoring. Small 11, 3973–3984 (2015).
Gan, T., Hu, C., Chen, Z. & Hu, S. A disposable electrochemical sensor for the determination of indole-3-acetic acid based on poly(safranine T)-reduced graphene oxide nanocomposite. Talanta 85, 310–316 (2011).
Li, H. et al. A highly sensitive electrochemical impedance immunosensor for indole-3-acetic acid and its determination in sunflowers under salt stress. RSC Adv. 7, 54416–54421 (2017).
Sun, L. J. et al. Paper-based electroanalytical devices for in situ determination of salicylic acid in living tomato leaves. Biosens. Bioelectron. 60, 154–160 (2014).
Yang, L. et al. Ratiometric electrochemical sensor for accurate detection of salicylic acid in leaves of living plants. RSC Adv. 10, 38841–38846 (2020).
Li, Y.-W., Xia, K., Wang, R.-Z., Jiang, J.-H. & Xiao, L.-T. An impedance immunosensor for the detection of the phytohormone abscisic acid. Anal. Bioanal. Chem. 391, 2869–2874 (2008).
Brunoud, G. et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 482, 103–106 (2012).
Waadt, R. et al. FRET-based reporters for the direct visualization of abscisic acid concentration changes and distribution in Arabidopsis. eLife 3, e01739 (2014).
Larrieu, A. et al. A fluorescent hormone biosensor reveals the dynamics of jasmonate signalling in plants. Nat. Commun. 6, 6043 (2015).
Zhang, H. & Wang, J. Detection of age and insect damage incurred by wheat, with an electronic nose. J. Stored Prod. Res. 43, 489–495 (2007).
Li, C., Krewer, G. & Kays, S. J. in 2009 Reno, Nevada, June 21–June 24 Vol. 8 5289–5301 (American Society of Agricultural and Biological Engineers, 2009).
Sankaran, S., Mishra, A., Ehsani, R. & Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 72, 1–13 (2010).
Baker, M. J., Hughes, C. S. & Hollywood, K. A. Biophotonics: Vibrational Spectroscopic Diagnostics Ch. 3 (Morgan & Claypool, 2016).
Nißler, R. et al. Detection and imaging of the plant pathogen response by near-infrared fluorescent polyphenol sensors. Angew. Chem. Int. Ed. 61, e202108373 (2022).
Galieni, A. et al. Past and future of plant stress detection: an overview from remote sensing to positron emission tomography. Front. Plant Sci. 11, 609155 (2021).
Weiss, M., Jacob, F. & Duveiller, G. Remote sensing for agricultural applications: a meta-review. Remote Sens. Environ. 236, 111402 (2020).
Wójtowicz, M., Wójtowicz, A. & Piekarczyk, J. Application of remote sensing methods in agriculture. Commun. Biometry Crop Sci. 11, 31–50 (2016).
Huang, S., Tang, L., Hupy, J. P., Wang, Y. & Shao, G. A commentary review on the use of normalized difference vegetation index (NDVI) in the era of popular remote sensing. J. For. Res. 32, 1–6 (2021).
Beisel, N. S. et al. Utilization of single-image normalized difference vegetation index (SI-NDVI) for early plant stress detection. Appl. Plant Sci. 6, e01186 (2018).
Zubler, A. V. & Yoon, J. Y. Proximal methods for plant stress detection using optical sensors and machine learning. Biosensors 10, 193 (2020).
Gao, Z., Luo, Z., Zhang, W., Lv, Z. & Xu, Y. Deep learning application in plant stress imaging: a review. AgriEngineering 2, 430–446 (2020).
Ma, J. et al. A recognition method for cucumber diseases using leaf symptom images based on deep convolutional neural network. Comput. Electron. Agric. 154, 18–24 (2018).
Esgario, J. G. M., Krohling, R. A. & Ventura, J. A. Deep learning for classification and severity estimation of coffee leaf biotic stress. Comput. Electron. Agric. 169, 105162 (2020).
Pérez-Clemente, R. M. et al. Biotechnological approaches to study plant responses to stress. BioMed. Res. Int. 2013, 654120 (2013).
Arbona, V., Manzi, M., Ollas, Cde & Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 14, 4885–4911 (2013).
Shulaev, V., Cortes, D., Miller, G. & Mittler, R. Metabolomics for plant stress response. Physiol. Plant 132, 199–208 (2008).
Krishnan, P., Kruger, N. J. & Ratcliffe, R. G. Metabolite fingerprinting and profiling in plants using NMR. J. Exp. Bot. 56, 255–265 (2005).
Widodo, W. et al. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 60, 4089–4103 (2009).
Michaletti, A., Naghavi, M. R., Toorchi, M., Zolla, L. & Rinalducci, S. Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci. Rep. 8, 5710 (2018).
Djoukeng, J. D., Arbona, V., Argamasilla, R. & Gomez-Cadenas, A. Flavonoid profiling in leaves of citrus genotypes under different environmental situations. J. Agric. Food Chem. 56, 11087–11097 (2008).
Sprenger, H. et al. Metabolite and transcript markers for the prediction of potato drought tolerance. Plant Biotechnol. J. 16, 939–950 (2018).
Li, Z. et al. Non-invasive plant disease diagnostics enabled by smartphone-based fingerprinting of leaf volatiles. Nat. Plants 5, 856–866 (2019). A colorimetric-based VOC fingerprinting smartphone sensing setup with diagnosis of infection through modelling.
Lee, G., Wei, Q. & Zhu, Y. Emerging wearable sensors for plant health monitoring. Adv. Funct. Mater. 31, 2106475 (2021).
Nassar, J. M. et al. Compliant plant wearables for localized microclimate and plant growth monitoring. npj Flex. Electron. 2, 24 (2018).
Tang, W., Yan, T., Ping, J., Wu, J. & Ying, Y. Rapid fabrication of flexible and stretchable strain sensor by chitosan-based water ink for plants growth monitoring. Adv. Mater. Technol. 2, 1700021 (2017).
Diacci, C. et al. Diurnal in vivo xylem sap glucose and sucrose monitoring using implantable organic electrochemical transistor sensors. iScience 24, 101966 (2021).
Rawson, T. M. et al. Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: a first-in-human evaluation in healthy volunteers. Lancet Digit. Health 1, e335–e343 (2019).
Stavrinidou, E. et al. In vivo polymerization and manufacturing of wires and supercapacitors in plants. Proc. Natl Acad. Sci. USA 114, 2807–2812 (2017).
Liu, J. et al. Syringe-injectable electronics. Nat. Nanotechnol. 10, 629–636 (2015).
Olenik, S., Lee, H. S. & Güder, F. The future of near-field communication-based wireless sensing. Nat. Rev. Mater. 6, 286–288 (2021).
Romanholo, P. V. V. et al. Biomimetic electrochemical sensors: new horizons and challenges in biosensing applications. Biosens. Bioelectron. 185, 113242 (2021).
Li, B. et al. Toward long-term accurate and continuous monitoring of nitrate in wastewater using poly(tetrafluoroethylene) (PTFE)–solid-state ion-selective electrodes (S-ISEs). ACS Sens. 5, 3182–3193 (2020).
Liu, X., You, S., Ma, F. & Zhou, H. Characterization of electrode fouling during electrochemical oxidation of phenolic pollutant. Front. Environ. Sci. Eng. 15, 53 (2021).
Hanssen, B. L., Siraj, S. & Wong, D. K. Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 35, 1–28 (2016).
Kusoglu, A. & Weber, A. Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 117, 987–1104 (2017).
Kwak, S. Y. et al. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 14, 447–455 (2019).
Demirer, G. S. et al. High aspect ratio nanomaterials enable delivery of functional genetic material without DNA integration in mature plants. Nat. Nanotechnol. 14, 456–464 (2019).
Voke, E., Pinals, R. L., Goh, N. S. & Landry, M. P. In planta nanosensors: understanding biocorona formation for functional design. ACS Sens. 6, 2802–2814 (2021).
Xu, Y., Zhang, P., Liu, X., Wang, Z. & Li, S. Preparation and irreversible inhibition mechanism insight into a recombinant Kunitz trypsin inhibitor from Glycine max L. seeds. Appl. Biochem. Biotechnol. 191, 1207–1222 (2020).
Acknowledgements
F.G. thanks the Bill and Melinda Gates Foundation (Grand Challenges Explorations scheme under grant number OPP1212574) and the US Army (U.S. Army Foreign Technology (and Science) Assessment Support programme under grant number W911QY-20-R-0022) for their generous support. L.G.-M. acknowledges the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 101025390. F.G., P.C. and A.S.P.C. thank EPSRC (EP/L016702/1) and BBSRC DTP (reference 2177734). T.B. acknowledges BBSRC (BB/T006102/1) for their support. F.G. and P.C. thank the Imperial College Centre for Processable Electronics (CPE). F.G. also acknowledges Agri Futures Lab.
Author information
Authors and Affiliations
Contributions
P.C., L.G.-M. and F.G. conceived the structure of the manuscript. P.C. led the writing of the manuscript. All authors contributed to the writing and reviewed and agreed on the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks M. Teresa Fernández Abedul, Ramses V. Martinez and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Avirulent
-
Not capable of causing disease.
- Bioimpedance spectroscopy
-
A non-invasive electrochemical spectroscopic technique for the measurement of electrical impedance of biological samples.
- Effector-triggered immunity
-
(ETI). A stronger immune response triggered upon detection of effector proteins released by the pathogen.
- Electrical impedance
-
The opposition to electrical flow.
- Electrodic technique
-
A technique measuring properties at the electrode–electrolyte interface.
- Genetic transformations
-
Insertion and incorporation of exogenous genetic material into a host organism.
- Oomycetes
-
Fungus-like filamentous microorganisms.
- PAMP-triggered immunity
-
(PTI). The primary plant immunity response, triggered when PAMPs are detected by recognition receptors in plants.
- Pathogen-associated molecular patterns
-
(PAMPs). Structural molecular components of pathogens that are recognized by receptors in plants, triggering an immune response.
- Phloem
-
Living tissue that transports soluble organic compounds (especially sugars) produced during photosynthesis around the plant.
- Protease inhibitors
-
Large variety of antiherbivore molecules (mostly proteins) that inhibit protease enzyme function to reduce herbivore digestion.
- Stomata
-
Pores on the epidermis of leaves that control exchange of CO2 and water vapour with the environment.
- Stomatal aperture
-
The width of the pore size of a stoma as controlled by the two guard cells.
- Synthetic-aperture radar
-
A remote imaging technique involving the transmission and reception of sequential electromagnetic waves by a device on a moving platform.
- Virulent
-
Capable of causing disease.
- Xylem
-
Vascular tissue that transports water and dissolved nutrients up from the roots to other organs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Coatsworth, P., Gonzalez-Macia, L., Collins, A.S.P. et al. Continuous monitoring of chemical signals in plants under stress. Nat Rev Chem 7, 7–25 (2023). https://doi.org/10.1038/s41570-022-00443-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-022-00443-0
This article is cited by
-
A hybrid multifunctional physicochemical sensor suite for continuous monitoring of crop health
Scientific Reports (2023)