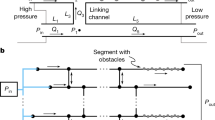
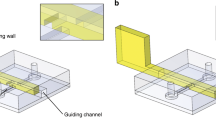
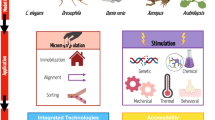
Abstract
Lab-on-a-chip devices leverage microfluidic technologies to enable chemical and biological processes at small scales. However, existing microfluidic channel networks are typically designed for the implementation of a single function or a well-defined protocol and do not allow the flexibility and real-time experimental decision-making essential to many scientific applications. In this Perspective, we highlight that reconfigurability and programmability of microfluidic platforms can support new functionalities that are beyond the reach of current lab-on-a-chip systems. We describe the ideal fully reconfigurable microfluidic device that can change its shape and function dynamically, which would allow researchers to tune a microscale experiment with the capacity to make real-time decisions. We review existing technologies that can dynamically control microscale flows, suggest additional physical mechanisms that could be leveraged towards the goal of reconfigurable microfluidics and highlight the importance of these efforts for the broad scientific community.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Terry, S. C., Jerman, J. H. & Angell, J. B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Devices 26, 1880–1886 (1979).
Manz, A., Graber, N. & Widmer, H. M. Miniaturized total chemical analysis systems: A novel concept for chemical sensing. Sens. Actuators B Chem. 1, 244–248 (1990).
Arango, Y., Temiz, Y., Gökçe, O. & Delamarche, E. Electro-actuated valves and self-vented channels enable programmable flow control and monitoring in capillary-driven microfluidics. Sci. Adv. 6, eaay8305 (2020).
Nelson, W. C. & Kim, C. J. Droplet actuation by electrowetting-on-dielectric (EWOD): a review. J. Adhes. Sci. Technol. 26, 1747–1771 (2012).
Castellanos, A., Ramos, A., González, A., Green, N. G. & Morgan, H. Electrohydrodynamics and dielectrophoresis in microsystems: scaling laws. J. Phys. D Appl. Phys. 36, 2584 (2003).
Karbalaei, A., Kumar, R. & Cho, H. J. Thermocapillarity in microfluidics — a review. Micromachines 7, 13 (2016).
Choi, K., Ng, A. H. C., Fobel, R. & Wheeler, A. R. Digital microfluidics. Annu. Rev. Anal. Chem. 5, 413–440 (2012).
Fair, R. B. Digital microfluidics: is a true lab-on-a-chip possible? Microfluid. Nanofluidics 3, 245–281 (2007).
Vittayarukskul, K. & Lee, A. P. A truly Lego®-like modular microfluidics platform. J. Micromech. Microeng. 27, 035004 (2017).
Yuen, P. K. SmartBuild–A truly plug-n-play modular microfluidic system. Lab Chip 8, 1374–1378 (2008).
Hahn, Y. K., Hong, D., Kang, J. H. & Choi, S. A reconfigurable microfluidics platform for microparticle separation and fluid mixing. Micromachines 7, 139 (2016).
Zhao, B., Moore, J. S. & Beebe, D. J. Surface-directed liquid flow inside microchannels. Science 291, 1023–1026 (2001).
Walsh, E. J. et al. Microfluidics with fluid walls. Nat. Commun. 8, 816 (2017).
Renaudot, R. et al. A programmable and reconfigurable microfluidic chip. Lab Chip 13, 4517–4524 (2013).
Dittrich, P. S. & Manz, A. Lab-on-a-chip: microfluidics in drug discovery. Nat. Rev. Drug Discov. 5, 210–218 (2006).
Vaucher, A. C. et al. Automated extraction of chemical synthesis actions from experimental procedures. Nat. Commun. 11, 3601 (2020).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365, eaax1566 (2019).
Bédard, A.-C. et al. Reconfigurable system for automated optimization of diverse chemical reactions. Science 361, 1220–1225 (2018).
Perez de Vargas Sansalvador, I. M. et al. Autonomous reagent-based microfluidic pH sensor platform. Sens. Actuators B Chem. 225, 369–376 (2016).
Guijt, R. M. et al. New approaches for fabrication of microfluidic capillary electrophoresis devices with on-chip conductivity detection. Electrophoresis 22, 235–241 (2001).
Ivanov, N. A., Kochmann, S. & Krylov, S. N. Visualization of streams of small organic molecules in continuous-flow electrophoresis. Anal. Chem. 92, 2907–2910 (2020).
Kitano, H. Systems biology: a brief overview. Science 295, 1662–1664 (2002).
Zare, R. N. & Kim, S. Microfluidic platforms for single-cell analysis. Annu. Rev. Biomed. Eng. 12, 187–201 (2010).
Anderson, J. & Quake, S. R. Microfluidic single-cell mRNA isolation and analysis. Anal. Chem. 78, 3084–3089 (2006).
Skelley, A. M., Kirak, O., Suh, H., Jaenisch, R. & Voldman, J. Microfluidic control of cell pairing and fusion. Nat. Methods 6, 147–152 (2009).
Brouzes, E. et al. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl Acad. Sci. USA 106, 14195–14200 (2009).
Barbulovic-Nad, I., Yang, H., Park, P. S. & Wheeler, A. R. Digital microfluidics for cell-based assays. Lab Chip 8, 519–526 (2008).
Cho, S. H., Chen, C. H., Tsai, F. S., Godin, J. M. & Lo, Y.-H. Human mammalian cell sorting using a highly integrated micro-fabricated fluorescence-activated cell sorter (microFACS). Lab Chip 10, 1567–1573 (2010).
Unger, M. A., Chou, H.-P., Thorsen, T., Scherer, A. & Quake, S. R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 288, 113–116 (2000).
Armingol, E., Officer, A., Harismendy, O. & Lewis, N. E. Deciphering cell–cell interactions and communication from gene expression. Nat. Rev. Genet. 22, 71–88 (2021).
Weigel, D. & Doerner, P. Cell–cell interactions: taking cues from the neighbors. Curr. Biol. 6, 10–12 (1996).
Fung, C. W., Chan, S. N. & Wu, A. R. Microfluidic single-cell analysis — toward integration and total on-chip analysis. Biomicrofluidics 14, 021502 (2020).
Haghighi, F., Talebpour, Z. & Nezhad, A. S. Towards fully integrated liquid chromatography on a chip: evolution and evaluation. Trends Anal. Chem. 105, 302–337 (2018).
Yuan, X. & Oleschuk, R. D. Advances in microchip liquid chromatography. Anal. Chem. 90, 283–301 (2018).
Wiederschain, G. Y. Handbook of capillary and microchip electrophoresis and associated microtechniques (3rd Edn). Biochem. Mosc. 73, 1350–1350 (2008).
Thorsen, T., Maerkl, S. J. & Quake, S. R. Microfluidic large-scale integration. Science 298, 580–584 (2002).
Grover, W. H., Skelley, A. M., Liu, C. N., Lagally, E. T. & Mathies, R. A. Monolithic membrane valves and diaphragm pumps for practical large-scale integration into glass microfluidic devices. Sens. Actuators B Chem. 89, 315–323 (2003).
Jönsson, U. et al. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. Biotechniques 11, 620–627 (1991).
Fu, L.-M., Yang, R.-J., Lee, G.-B. & Pan, Y.-J. Multiple injection techniques for microfluidic sample handling. Electrophoresis 24, 3026–3032 (2003).
Schasfoort, R. B. Field-effect flow control for microfabricated fluidic networks. Science 286, 942–945 (1999).
Lemoff, A. V. & Lee, A. P. An AC magnetohydrodynamic micropump. Sens. Actuators B Chem. 63, 178–185 (2000).
Bau, H. H., Zhu, J., Qian, S. & Xiang, Y. A magneto-hydrodynamically controlled fluidic network. Sens. Actuators B Chem. 88, 205–216 (2003).
Banerjee, A., Kreit, E., Liu, Y., Heikenfeld, J. & Papautsky, I. Reconfigurable virtual electrowetting channels. Lab Chip 12, 758–764 (2012).
Dhindsa, M. et al. Virtual electrowetting channels: electronic liquid transport with continuous channel functionality. Lab Chip 10, 832–836 (2010).
Beebe, D. J. et al. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 404, 588–590 (2000).
D’Eramo, L. et al. Microfluidic actuators based on temperature-responsive hydrogels. Microsyst. Nanoeng. 4, 17069 (2018).
Wada, H., Koike, Y., Yokoyama, Y. & Hayakawa, T. Evaluation of the response characteristics of on-chip gel actuators for various single cell manipulations. IEEE Robot. Autom. Lett. 5, 5205–5212 (2020).
Krishnan, M. & Erickson, D. Optically induced microfluidic reconfiguration. Lab Chip 12, 613–621 (2012).
Paratore, F., Bacheva, V., Kaigala, G. V. & Bercovici, M. Dynamic microscale flow patterning using electrical modulation of zeta potential. Proc. Natl Acad. Sci. USA 116, 10258–10263 (2019).
Bacheva, V., Paratore, F., Rubin, S., Kaigala, G. V. & Bercovici, M. Tunable bidirectional electroosmotic flow for diffusion-based separations. Angew. Chem. Int. Ed. 59, 12894–12899 (2020).
Cooksey, G. A., Sip, C. G. & Folch, A. A multi-purpose microfluidic perfusion system with combinatorial choice of inputs, mixtures, gradient patterns, and flow rates. Lab Chip 9, 417–426 (2009).
Taylor, P. D. & Kaigala, V. G. Reconfigurable microfluidics: real-time shaping of virtual channels through hydrodynamic forces. Lab Chip 20, 1720–1728 (2020).
Hadwen, B. et al. Programmable large area digital microfluidic array with integrated droplet sensing for bioassays. Lab Chip 12, 3305–3313 (2012).
Tanaka, T. Collapse of gels and the critical endpoint. Phys. Rev. Lett. 40, 820–823 (1978).
Beebe, D. J. et al. Microfluidic tectonics: a comprehensive construction platform for microfluidic systems. Proc. Natl Acad. Sci. USA 97, 13488–13493 (2000).
Hoffmann, J., Plötner, M., Kuckling, D. & Fischer, W.-J. Photopatterning of thermally sensitive hydrogels useful for microactuators. Sens. Actuators Phys. 77, 139–144 (1999).
Kuckling, D. et al. Photocrosslinking of thin films of temperature-sensitive polymers. Polym. Adv. Technol. 10, 345–352 (1999).
Baldi, A., Gu, Y., Loftness, P. E., Siegel, R. A. & Ziaie, B. in Technical Digest. MEMS 2002 IEEE International Conference. Fifteenth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No.02CH37266) 105–108 (IEEE, 2002).
Mutlu, S. et al. in TRANSDUCERS ’03. 12th International Conference on Solid-State Sensors, Actuators and Microsystems. Digest of Technical Papers (Cat. No.03TH8664) Vol. 1 802–805 (IEEE, 2003).
Richter, A., Kuckling, D., Howitz, S., Thomas, G. & Arndt, K. Electronically controllable microvalves based on smart hydrogels: magnitudes and potential applications. J. Microelectromech. Syst. 12, 748–753 (2003).
Richter, A. & Paschew, G. Optoelectrothermic control of highly integrated polymer-based MEMS applied in an artificial skin. Adv. Mater. 21, 979–983 (2009).
Li, L., Scheiger, J. M. & Levkin, P. A. Design and applications of photoresponsive hydrogels. Adv. Mater. 31, 1807333 (2019).
Zhang, Y.-Z. et al. MXene hydrogels: fundamentals and applications. Chem. Soc. Rev. 49, 7229–7251 (2020).
Shadish, J. A., Benuska, G. M. & DeForest, C. A. Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater. 18, 1005–1014 (2019).
Prince, E. & Kumacheva, E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 4, 99–115 (2019).
Goy, C. B., Chaile, R. E. & Madrid, R. E. Microfluidics and hydrogel: A powerful combination. React. Funct. Polym. 145, 104314 (2019).
Hornbeck, L. J. in Proc. SPIE 1150, Spatial Light Modulators and Applications III 86–103 (International Society for Optics and Photonics, 1990).
Qiu, Y., Lu, Z. & Pei, Q. Refreshable tactile display based on a bistable electroactive polymer and a stretchable serpentine Joule heating electrode. ACS Appl. Mater. Interfaces 10, 24807–24815 (2018).
Besse, N., Rosset, S., Zarate, J. J. & Shea, H. Flexible active skin: large reconfigurable arrays of individually addressed shape memory polymer actuators. Adv. Mater. Technol. 2, 1700102 (2017).
Lee, H. S. et al. Design analysis and fabrication of arrayed tactile display based on dielectric elastomer actuator. Sens. Actuators Phys. 205, 191–198 (2014).
Marette, A. et al. Flexible zinc–tin oxide thin film transistors operating at 1 kV for integrated switching of dielectric elastomer actuators arrays. Adv. Mater. 29, 1700880 (2017).
Zhao, H. et al. Compact dielectric elastomer linear actuators. Adv. Funct. Mater. 28, 1804328 (2018).
Acome, E. et al. Hydraulically amplified self-healing electrostatic actuators with muscle-like performance. Science 359, 61–65 (2018).
Boyko, E., Rubin, S., Gat, A. D. & Bercovici, M. Flow patterning in Hele-Shaw configurations using non-uniform electro-osmotic slip. Phys. Fluids 27, 102001 (2015).
Boyko, E., Eshel, R., Gommed, K., Gat, A. D. & Bercovici, M. Elastohydrodynamics of a pre-stretched finite elastic sheet lubricated by a thin viscous film with application to microfluidic soft actuators. J. Fluid Mech. 862, 732–752 (2019).
Hunter, R. J. Zeta Potential in Colloid Science: Principles and Applications (Academic Press, 1988).
Ramos, A. Electrohydrodynamic pumping in microsystems. J. Phys. Conf. Ser. 301, 012028 (2011).
Herr, A. E. et al. Electroosmotic capillary flow with nonuniform zeta potential. Anal. Chem. 72, 1053–1057 (2000).
Squires, T. M. & Bazant, M. Z. Induced-charge electro-osmosis. J. Fluid Mech. 509, 217–252 (2004).
Ajdari, A. Pumping liquids using asymmetric electrode arrays. Phys. Rev. E 61, R45–R48 (2000).
González, A., Ramos, A., Green, N. G., Castellanos, A. & Morgan, H. Fluid flow induced by nonuniform ac electric fields in electrolytes on microelectrodes. II. A linear double-layer analysis. Phys. Rev. E 61, 4019–4028 (2000).
Ramos, A., Morgan, H., Green, N. G. & Castellanos, A. Ac electrokinetics: a review of forces in microelectrode structures. J. Phys. D Appl. Phys. 31, 2338 (1998).
Ramos, A., Morgan, H., Green, N. G. & Castellanos, A. AC electric-field-induced fluid flow in microelectrodes. J. Colloid Interface Sci. 217, 420–422 (1999).
Ramos, A., Morgan, H., Green, N. G., González, A. & Castellanos, A. Pumping of liquids with traveling-wave electroosmosis. J. Appl. Phys. 97, 084906 (2005).
Salari, A., Navi, M., Lijnse, T. & Dalton, C. AC electrothermal effect in microfluidics: a review. Micromachines 10, 762 (2019).
Green, N. G., Ramos, A., González, A., Castellanos, A. & Morgan, H. Electric field induced fluid flow on microelectrodes: the effect of illumination. J. Phys. D Appl. Phys. 33, L13 (2000).
Wang, D., Sigurdson, M. & Meinhart, C. D. Experimental analysis of particle and fluid motion in ac electrokinetics. Exp. Fluids 38, 1–10 (2005).
Sin, M. L. Y., Gau, V., Liao, J. C. & Wong, P. K. Electrothermal fluid manipulation of high-conductivity samples for laboratory automation applications. J. Assoc. Lab. Autom. 15, 426–432 (2010).
Velarde, M. C. & Kh. Zeytounian, R. Interfacial Phenomena and the Marangoni Effect (Springer, 2002).
Frumkin, V., Gommed, K. & Bercovici, M. Dipolar thermocapillary motor and swimmer. Phys. Rev. Fluids 4, 074002 (2019).
Amador, G. J. et al. Temperature gradients drive bulk flow within microchannel lined by fluid–fluid interfaces. Small 15, 1900472 (2019).
Baier, T., Steffes, C. & Hardt, S. Thermocapillary flow on superhydrophobic surfaces. Phys. Rev. E 82, 037301 (2010).
Rofman, B., Dehe, S., Frumkin, V., Hardt, S. & Bercovici, M. Intermediate states of wetting on hierarchical superhydrophobic surfaces. Langmuir 36, 5517–5523 (2020).
Garnier, N., Grigoriev, R. O. & Schatz, M. F. Optical manipulation of microscale fluid flow. Phys. Rev. Lett. 91, 054501 (2003).
Ding, X. et al. Surface acoustic wave microfluidics. Lab Chip 13, 3626–3649 (2013).
Cecchini, M., Girardo, S., Pisignano, D., Cingolani, R. & Beltram, F. Acoustic-counterflow microfluidics by surface acoustic waves. Appl. Phys. Lett. 92, 104103 (2008).
Masini, L. et al. Surface-acoustic-wave counterflow micropumps for on-chip liquid motion control in two-dimensional microchannel arrays. Lab Chip 10, 1997–2000 (2010).
Fallah, M. A. et al. Acoustic driven flow and lattice Boltzmann simulations to study cell adhesion in biofunctionalized μ-fluidic channels with complex geometry. Biomicrofluidics 4, 024106 (2010).
Homsy, A. et al. A high current density DC magnetohydrodynamic (MHD) micropump. Lab Chip 5, 466–471 (2005).
Qian, S. & Bau, H. H. Magnetohydrodynamic flow of RedOx electrolyte. Phys. Fluids 17, 067105 (2005).
Qian, S. & Bau, H. H. Magneto-hydrodynamics based microfluidics. Mech. Res. Commun. 36, 10–21 (2009).
Baigl, D. Photo-actuation of liquids for light-driven microfluidics: state of the art and perspectives. Lab Chip 12, 3637–3653 (2012).
Lee, J. N., Park, C. & Whitesides, G. M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 75, 6544–6554 (2003).
Washizu, M. Electrostatic actuation of liquid droplets for micro-reactor applications. IEEE Trans. Ind. Appl. 34, 732–737 (1998).
Martinez, A. W., Phillips, S. T., Butte, M. J. & Whitesides, G. M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 46, 1318–1320 (2007).
Carrell, C. et al. Beyond the lateral flow assay: a review of paper-based microfluidics. Microelectron. Eng. 206, 45–54 (2019).
Shang, L., Cheng, Y. & Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 117, 7964–8040 (2017).
Gelorme, J. D., Cox, R. J. & Gutierrez, S. A. R. Photoresist composition and printed circuit boards and packages made therewith. US Patent US4882245A (1989).
Duffy, D. C., McDonald, J. C., Schueller, O. J. A. & Whitesides, G. M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 70, 4974–4984 (1998).
Becker, H. & Gärtner, C. Polymer microfabrication methods for microfluidic analytical applications. Electrophoresis 21, 12–26 (2000).
Au, A. K., Huynh, W., Horowitz, L. F. & Folch, A. 3D-printed microfluidics. Angew. Chem. Int. Ed. 55, 3862–3881 (2016).
Fidalgo, L. M. & Maerkl, S. J. A software-programmable microfluidic device for automated biology. Lab Chip 11, 1612–1619 (2011).
Acknowledgements
This Perspective was greatly influenced by many discussions with Prof. H. Shea (EPFL) in the context of a joint research proposal. F.P., V.B. and G.V.K. acknowledge E. Delamarche and H. Riel for their continuous support. The authors thank Prof. S. N. Krylov (York University) for useful discussion and suggestions. They thank I. Pereiro for his help on the preparation of the graphical abstract. Finally, they thank L. Rudin for proofreading the manuscript. This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme, grant agreement 678734 (MetamorphChip). G.V.K. gratefully acknowledges funding from a SNF grant (CRSK-2_190877). F.P. gratefully acknowledges the BRIDGE Proof-of-Concept programme, project number 40B1-0 191549, funded by Innosuisse and the Swiss National Science Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Chemistry thanks E. Verpoorte and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paratore, F., Bacheva, V., Bercovici, M. et al. Reconfigurable microfluidics. Nat Rev Chem 6, 70–80 (2022). https://doi.org/10.1038/s41570-021-00343-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-021-00343-9
This article is cited by
-
Reconfigurable liquid devices from liquid building blocks
Nature Chemical Engineering (2024)
-
Reconfiguring liquid devices
Nature Chemical Engineering (2024)
-
Modular microfluidics for life sciences
Journal of Nanobiotechnology (2023)
-
Dynamic control of high-voltage actuator arrays by light-pattern projection on photoconductive switches
Microsystems & Nanoengineering (2023)
-
3D free-assembly modular microfluidics inspired by movable type printing
Microsystems & Nanoengineering (2023)