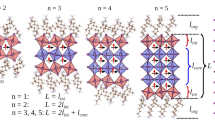
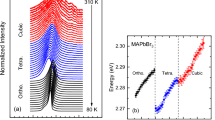
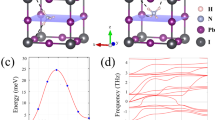
Abstract
Metal halide perovskites (MHPs) are characterized as strongly anharmonic and dynamic lattices. While there is a consensus on the solvation-like polarization effect in these materials, whether static polarization, that is, ferroelectricity, exists or not in 3D MHPs remains controversial. In this Review, we resolve this controversy by analysing the stereochemical expression (SE) of the ns2 electron pair (NSEP) on group IV metal cations. The SE-NSEP is key to lattice instability, which governs the breaking of inversion symmetry and induces ferroelectricity. The SE-NSEP is diminishingly small in commonly studied 3D lead iodide or bromide perovskites, indicating an absence of ferroelectricity. In contrast, 2D MHPs promote the SE-NSEP and produce unambiguous ferroelectricity or antiferroelectricity. Irrespective of ferroelectricity, the dynamic manifestation of the SE-NSEP provides the missing link to understanding polar fluctuations and efficient dielectric screening in MHPs, thus, contributing to the long carrier lifetimes and diffusion lengths.

Key points
-
The stereochemical expression of the ns2 electron pair (SE-NSEP) on group IV metal cations governs the breaking of inversion symmetry and induces ferroelectricity in metal halide perovskites.
-
The tendency of the SE-NSEP increases with lighter group IV cations, more electronegative halides and larger A-site cations.
-
The SE-NSEP is diminishingly small in commonly studied 3D lead halide perovskites, suggesting the absence of ferroelectricity.
-
Dimensionality reduction promotes the SE-NSEP and produces unambiguous ferroelectricity or antiferroelectricity in 2D lead halide perovskites.
-
The inherent driving force for the SE-NSEP in 3D perovskites results in dynamic symmetry breaking, strong phonon anharmonicity and polar fluctuations, giving rise to efficient dielectric screening of charge carriers.
-
Emerging halide perovskites with the SE-NSEP offer exciting systems to understand the origin of the remarkable photophysical properties in metal halide perovskites.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kojima, A., Teshima, K., Shirai, Y. & Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 131, 6050–6051 (2009).
Lee, M. M., Teuscher, J., Miyasaka, T., Murakami, T. N. & Snaith, H. J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338, 643–647 (2012).
Xing, G. et al. Low-temperature solution-processed wavelength-tunable perovskites for lasing. Nat. Mater. 13, 476–480 (2014).
Zhu, H. et al. Lead halide perovskite nanowire lasers with low lasing thresholds and high quality factors. Nat. Mater. 14, 636–642 (2015).
Fu, Y. et al. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 4, 169–188 (2019).
Yin, W.-J., Shi, T. & Yan, Y. Unusual defect physics in CH3NH3PbI3 perovskite solar cell absorber. Appl. Phys. Lett. 104, 63903 (2014).
Akkerman, Q. A., Rainò, G., Kovalenko, M. V. & Manna, L. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat. Mater. 17, 394–405 (2018).
Meggiolaro, D. et al. Iodine chemistry determines the defect tolerance of lead-halide perovskites. Energy Environ. Sci. 11, 702–713 (2018).
Kang, J. & Wang, L.-W. High defect tolerance in lead halide perovskite CsPbBr3. J. Phys. Chem. Lett. 8, 489–493 (2017).
Huang, H., Bodnarchuk, M. I., Kershaw, S. V., Kovalenko, M. V. & Rogach, A. L. Lead halide perovskite nanocrystals in the research spotlight: stability and defect tolerance. ACS Energy Lett. 2, 2071–2083 (2017).
Zhang, X., Turiansky, M. E. & Van de Walle, C. G. Correctly assessing defect tolerance in halide perovskites. J. Phys. Chem. C 124, 6022–6027 (2020).
Yaffe, O. et al. Local polar fluctuations in lead halide perovskite crystals. Phys. Rev. Lett. 118, 136001 (2017). This original paper provides important insights into the anharmonic, local polar fluctuations in lead halide perovskites.
Beecher, A. N. et al. Direct observation of dynamic symmetry breaking above room temperature in methylammonium lead iodide perovskite. ACS Energy Lett. 1, 880–887 (2016).
Wasylishen, R. E., Knop, O. & Macdonald, J. B. Cation rotation in methylammonium lead halides. Solid State Commun. 56, 581–582 (1985).
Whalley, L. D., Skelton, J. M., Frost, J. M. & Walsh, A. Phonon anharmonicity, lifetimes, and thermal transport in CH3NH3PbI3 from many-body perturbation theory. Phys. Rev. B 94, 220301 (2016).
Baikie, T. et al. A combined single crystal neutron/X-ray diffraction and solid-state nuclear magnetic resonance study of the hybrid perovskites CH3NH3PbX3 (X = I, Br and Cl). J. Mater. Chem. A 3, 9298–9307 (2015).
Franssen, W. M. J., van Es, S. G. D., Dervisoglu, R., de Wijs, G. A. & Kentgens, A. P. M. Symmetry, dynamics, and defects in methylammonium lead halide perovskites. J. Phys. Chem. Lett. 8, 61–66 (2017).
Marronnier, A. et al. Structural instabilities related to highly anharmonic phonons in halide perovskites. J. Phys. Chem. Lett. 8, 2659–2665 (2017).
Frost, J. M. et al. Atomistic origins of high-performance in hybrid halide perovskite solar cells. Nano Lett. 14, 2584–2590 (2014).
Ma, J. & Wang, L.-W. Nanoscale charge localization induced by random orientations of organic molecules in hybrid perovskite CH3NH3PbI3. Nano Lett. 15, 248–253 (2015).
Liu, S. et al. Ferroelectric domain wall induced band gap reduction and charge separation in organometal halide perovskites. J. Phys. Chem. Lett. 6, 693–699 (2015).
Miyata, K. et al. Large polarons in lead halide perovskites. Sci. Adv. 3, e1701217 (2017).
Zhu, X. Y. & Podzorov, V. Charge carriers in hybrid organic–inorganic lead halide perovskites might be protected as large polarons. J. Phys. Chem. Lett. 6, 4758–4761 (2015).
Anusca, I. et al. Dielectric response: answer to many questions in the methylammonium lead halide solar cell absorbers. Adv. Energy Mater. 7, 1700600 (2017).
Zhu, H. et al. Screening in crystalline liquids protects energetic carriers in hybrid perovskites. Science 353, 1409–1413 (2016).
Etienne, T., Mosconi, E. & De Angelis, F. Dynamical origin of the Rashba effect in organohalide lead perovskites: A key to suppressed carrier recombination in perovskite solar cells? J. Phys. Chem. Lett. 7, 1638–1645 (2016).
Zheng, F., Tan, L. Z., Liu, S. & Rappe, A. M. Rashba spin–orbit coupling enhanced carrier lifetime in CH3NH3PbI3. Nano Lett. 15, 7794–7800 (2015).
Hutter, E. M. et al. Direct–indirect character of the bandgap in methylammonium lead iodide perovskite. Nat. Mater. 16, 115–120 (2017).
Stranks, S. D. & Plochocka, P. The influence of the Rashba effect. Nat. Mater. 17, 381–382 (2018).
Miyata, K. & Zhu, X.-Y. Ferroelectric large polarons. Nat. Mater. 17, 379–381 (2018). This short note was the first to propose the ferroelectric polaron, in which the charge carrier induces local ordering of symmetry-breaking unit cells.
Wang, F. et al. Solvated electrons in solids — ferroelectric large polarons in lead halide perovskites. J. Am. Chem. Soc. 143, 5–16 (2021). This is an up-to-date review dealing with efficient dielectric screening in metal halide perovskites.
Grinberg, I. et al. Perovskite oxides for visible-light-absorbing ferroelectric and photovoltaic materials. Nature 503, 509–512 (2013).
Rappe, A. M., Grinberg, I. & Spanier, J. E. Getting a charge out of hybrid perovskites. Proc. Natl Acad. Sci. USA 114, 7191–7193 (2017).
Liu, Y. et al. Chemical nature of ferroelastic twin domains in CH3NH3PbI3 perovskite. Nat. Mater. 17, 1013–1019 (2018).
Gómez, A., Wang, Q., Goñi, A. R., Campoy-Quiles, M. & Abate, A. Ferroelectricity-free lead halide perovskites. Energy Environ. Sci. 12, 2537–2547 (2019).
Röhm, H., Leonhard, T., Hoffmann, M. J. & Colsmann, A. Ferroelectric domains in methylammonium lead iodide perovskite thin-films. Energy Environ. Sci. 10, 950–955 (2017).
Rakita, Y. et al. Tetragonal CH3NH3PbI3 is ferroelectric. Proc. Natl Acad. Sci. USA 114, 5504–5512 (2017).
Garten, L. M. et al. The existence and impact of persistent ferroelectric domains in MAPbI3. Sci. Adv. 5, eaas9311 (2019).
Gao, Z.-R. et al. Ferroelectricity of the orthorhombic and tetragonal MAPbBr3 single crystal. J. Phys. Chem. Lett. 10, 2522–2527 (2019).
G, S. et al. Is CH3NH3PbI3 polar? J. Phys. Chem. Lett. 7, 2412–2419 (2016).
Beilsten-Edmands, J., Eperon, G. E., Johnson, R. D., Snaith, H. J. & Radaelli, P. G. Non-ferroelectric nature of the conductance hysteresis in CH3NH3PbI3 perovskite-based photovoltaic devices. Appl. Phys. Lett. 106, 173502 (2015).
Benedek, N. A. & Fennie, C. J. Why are there so few perovskite ferroelectrics? J. Phys. Chem. C 117, 13339–13349 (2013).
Zhong, W. & Vanderbilt, D. Competing structural instabilities in cubic perovskites. Phys. Rev. Lett. 74, 2587–2590 (1995).
Woodward, P. Octahedral tilting in perovskites. I. Geometrical considerations. Acta Crystallogr. Sect. B 53, 32–43 (1997). This seminal paper provides a comprehensive understanding on the structural distortion in perovskite lattices.
Woodward, P. Octahedral tilting in perovskites. II. Structure stabilizing forces. Acta Crystallogr. Sect. B 53, 44–66 (1997).
Cohen, R. E. Origin of ferroelectricity in perovskite oxides. Nature 358, 136–138 (1992).
Angel, R. J., Zhao, J. & Ross, N. L. General rules for predicting phase transitions in perovskites due to octahedral tilting. Phys. Rev. Lett. 95, 25503 (2005).
Liao, W.-Q. et al. A lead-halide perovskite molecular ferroelectric semiconductor. Nat. Commun. 6, 7338 (2015). This original paper reports unambiguous ferroelectricity in a 2D lead halide perovskite.
Wang, S. et al. An unprecedented biaxial trilayered hybrid perovskite ferroelectric with directionally tunable photovoltaic effects. J. Am. Chem. Soc. 141, 7693–7697 (2019).
Li, L. et al. Bilayered hybrid perovskite ferroelectric with giant two-photon absorption. J. Am. Chem. Soc. 140, 6806–6809 (2018).
Wu, Z. et al. Discovery of an above-room-temperature antiferroelectric in two-dimensional hybrid perovskite. J. Am. Chem. Soc. 141, 3812–3816 (2019).
Li, L. et al. Two-dimensional hybrid perovskite-type ferroelectric for highly polarization-sensitive shortwave photodetection. J. Am. Chem. Soc. 141, 2623–2629 (2019).
Shi, P.-P. et al. Two-dimensional organic–inorganic perovskite ferroelectric semiconductors with fluorinated aromatic spacers. J. Am. Chem. Soc. 141, 18334–18340 (2019).
Han, S. et al. High-temperature antiferroelectric of lead iodide hybrid perovskites. J. Am. Chem. Soc. 141, 12470–12474 (2019).
Fabini, D. H. et al. Dynamic stereochemical activity of the Sn2+ lone pair in perovskite CsSnBr3. J. Am. Chem. Soc. 138, 11820–11832 (2016). This original paper reports dynamic B-cation off-centre displacement in CsSnBr3 upon heating.
Fabini, D. H., Seshadri, R. & Kanatzidis, M. G. The underappreciated lone pair in halide perovskites underpins their unusual properties. MRS Bull. 45, 467–477 (2020).
McCall, K. M., Morad, V., Benin, B. M. & Kovalenko, M. V. Efficient lone-pair-driven luminescence: structure–property relationships in emissive 5s2 metal halides. ACS Mater. Lett. 2, 1218–1232 (2020).
Stern, E. A. Character of order-disorder and displacive components in barium titanate. Phys. Rev. Lett. 93, 37601 (2004).
Sicron, N. et al. Nature of the ferroelectric phase transition in PbTiO3. Phys. Rev. B 50, 13168–13180 (1994).
Seshadri, R. & Hill, N. A. Visualizing the role of Bi 6s “lone pairs” in the off-center distortion in ferromagnetic BiMnO3. Chem. Mater. 13, 2892–2899 (2001).
Kuroiwa, Y. et al. Evidence for Pb-O covalency in tetragonal PbTiO3. Phys. Rev. Lett. 87, 217601 (2001).
Steigmeier, E. F. & Harbeke, G. Soft phonon mode and ferroelectricity in GeTe. Solid State Commun. 8, 1275–1279 (1970).
Waghmare, U. V., Spaldin, N. A., Kandpal, H. C. & Seshadri, R. First-principles indicators of metallicity and cation off-centricity in the IV-VI rocksalt chalcogenides of divalent Ge, Sn, and Pb. Phys. Rev. B 67, 125111 (2003).
Zhang, Y. et al. The first organic–inorganic hybrid luminescent multiferroic: (pyrrolidinium)MnBr3. Adv. Mater. 27, 3942–3946 (2015).
Zalar, B., Laguta, V. V. & Blinc, R. NMR evidence for the coexistence of order-disorder and displacive components in barium titanate. Phys. Rev. Lett. 90, 37601 (2003).
Fons, P. et al. Phase transition in crystalline GeTe: pitfalls of averaging effects. Phys. Rev. B 82, 155209 (2010).
Mao, L., Stoumpos, C. C. & Kanatzidis, M. G. Two-dimensional hybrid halide perovskites: principles and promises. J. Am. Chem. Soc. 141, 1171–1190 (2019).
Saparov, B. & Mitzi, D. B. Organic–inorganic perovskites: structural versatility for functional materials design. Chem. Rev. 116, 4558–4596 (2016).
Zhang, Y., Ke, X., Kent, P. R. C., Yang, J. & Chen, C. Anomalous lattice dynamics near the ferroelectric instability in PbTe. Phys. Rev. Lett. 107, 175503 (2011).
Liao, W.-Q., Tang, Y.-Y., Li, P.-F., You, Y.-M. & Xiong, R.-G. Competitive halogen bond in the molecular ferroelectric with large piezoelectric response. J. Am. Chem. Soc. 140, 3975–3980 (2018).
Ye, H.-Y. et al. High-temperature ferroelectricity and photoluminescence in a hybrid organic–inorganic compound: (3-pyrrolinium)MnCl3. J. Am. Chem. Soc. 137, 13148–13154 (2015).
Rakita, Y. et al. CH3NH3PbBr3 is not pyroelectric, excluding ferroelectric-enhanced photovoltaic performance. APL Mater. 4, 51101 (2016).
Sun, Z. et al. A photoferroelectric perovskite-type organometallic halide with exceptional anisotropy of bulk photovoltaic effects. Angew. Chem. Int. Ed. 55, 6545–6550 (2016).
Li, L. et al. A potential Sn-based hybrid perovskite ferroelectric semiconductor. J. Am. Chem. Soc. 142, 1159–1163 (2020).
Borriello, I., Cantele, G. & Ninno, D. Ab initio investigation of hybrid organic-inorganic perovskites based on tin halides. Phys. Rev. B 77, 235214 (2008).
Radha, S. K., Bhandari, C. & Lambrecht, W. R. L. Distortion modes in halide perovskites: to twist or to stretch, a matter of tolerance and lone pairs. Phys. Rev. Mater. 2, 63605 (2018).
Bechtel, J. S. & Van der Ven, A. Octahedral tilting instabilities in inorganic halide perovskites. Phys. Rev. Mater. 2, 25401 (2018).
Yang, R. X., Skelton, J. M., da Silva, E. L., Frost, J. M. & Walsh, A. Assessment of dynamic structural instabilities across 24 cubic inorganic halide perovskites. J. Chem. Phys. 152, 24703 (2020).
Boschker, J. E., Wang, R. & Calarco, R. GeTe: a simple compound blessed with a plethora of properties. CrystEngComm 19, 5324–5335 (2017).
Marronnier, A. et al. Anharmonicity and disorder in the black phases of cesium lead iodide used for stable inorganic perovskite solar cells. ACS Nano 12, 3477–3486 (2018).
Whitfield, P. S. et al. Structures, phase transitions and tricritical behavior of the hybrid perovskite methyl ammonium lead iodide. Sci. Rep. 6, 35685 (2016).
Poglitsch, A. & Weber, D. Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy. J. Chem. Phys. 87, 6373–6378 (1987).
Stoumpos, C. C., Malliakas, C. D. & Kanatzidis, M. G. Semiconducting tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 52, 9019–9038 (2013).
Frohna, K. et al. Inversion symmetry and bulk Rashba effect in methylammonium lead iodide perovskite single crystals. Nat. Commun. 9, 1829 (2018).
Morrow, D. J. et al. Disentangling second harmonic generation from multiphoton photoluminescence in halide perovskites using multidimensional harmonic generation. J. Phys. Chem. Lett. 11, 6551–6559 (2020).
Breternitz, J., Lehmann, F., Barnett, S. A., Nowell, H. & Schorr, S. Role of the iodide–methylammonium interaction in the ferroelectricity of CH3NH3PbI3. Angew. Chem. Int. Ed. 59, 424–428 (2020).
Dang, Y. et al. Bulk crystal growth of hybrid perovskite material CH3NH3PbI3. CrystEngComm 17, 665–670 (2015).
Leguy, A. M. A. et al. The dynamics of methylammonium ions in hybrid organic–inorganic perovskite solar cells. Nat. Commun. 6, 7124 (2015).
Bakulin, A. A. et al. Real-time observation of organic cation reorientation in methylammonium lead iodide perovskites. J. Phys. Chem. Lett. 6, 3663–3669 (2015).
Selig, O. et al. Organic cation rotation and immobilization in pure and mixed methylammonium lead-halide perovskites. J. Am. Chem. Soc. 139, 4068–4074 (2017).
Chi, L. et al. The ordered phase of methylammonium lead chloride CH3ND3PbCl3. J. Solid State Chem. 178, 1376–1385 (2005).
Swainson, I. P., Hammond, R. P., Soullière, C., Knop, O. & Massa, W. Phase transitions in the perovskite methylammonium lead bromide, CH3ND3PbBr3. J. Solid State Chem. 176, 97–104 (2003).
Schueller, E. C. et al. Crystal structure evolution and notable thermal expansion in hybrid perovskites formamidinium tin iodide and formamidinium lead bromide. Inorg. Chem. 57, 695–701 (2018).
Keshavarz, M. et al. Tracking structural phase transitions in lead-halide perovskites by means of thermal expansion. Adv. Mater. 31, 1900521 (2019).
Müller, K. A. & Burkard, H. SrTiO3: an intrinsic quantum paraelectric below 4 K. Phys. Rev. B 19, 3593–3602 (1979).
Travis, W., Glover, E. N. K., Bronstein, H., Scanlon, D. O. & Palgrave, R. G. On the application of the tolerance factor to inorganic and hybrid halide perovskites: a revised system. Chem. Sci. 7, 4548–4556 (2016).
Kieslich, G., Sun, S. & Cheetham, A. K. Solid-state principles applied to organic–inorganic perovskites: new tricks for an old dog. Chem. Sci. 5, 4712–4715 (2014).
Ma̧czka, M. et al. Methylhydrazinium lead bromide: noncentrosymmetric three-dimensional perovskite with exceptionally large framework distortion and green photoluminescence. Chem. Mater. 32, 1667–1673 (2020).
Ma̧czka, M. et al. Three-dimensional perovskite methylhydrazinium lead chloride with two polar phases and unusual second-harmonic generation bistability above room temperature. Chem. Mater. 32, 4072–4082 (2020).
Fu, Y. et al. Incorporating large A cations into lead iodide perovskite cages: relaxed Goldschmidt tolerance factor and impact on exciton–phonon interaction. ACS Cent. Sci. 5, 1377–1386 (2019). This original paper reports the incorporation of various oversized A-cations into the perovskite cages, which induces unusually large octahedra distortions.
Fu, Y. et al. Cation engineering in two-dimensional Ruddlesden–Popper lead iodide perovskites with mixed large A-site cations in the cages. J. Am. Chem. Soc. 142, 4008–4021 (2020).
Li, X. et al. Negative pressure engineering with large cage cations in 2D halide perovskites causes lattice softening. J. Am. Chem. Soc. 142, 11486–11496 (2020).
Hautzinger, M. P. et al. Band edge tuning of two-dimensional Ruddlesden–Popper perovskites by A cation size revealed through nanoplates. ACS Energy Lett. 5, 1430–1437 (2020).
Ye, H.-Y. et al. Bandgap engineering of lead-halide perovskite-type ferroelectrics. Adv. Mater. 28, 2579–2586 (2016).
Lemmerer, A. & Billing, D. G. Synthesis, characterization and phase transitions of the inorganic–organic layered perovskite-type hybrids [(CnH2n+1NH3)2PbI4], n = 7, 8, 9 and 10. Dalton Trans. 41, 1146–1157 (2012).
Li, L. et al. Tailored engineering of an unusual (C4H9NH3)2(CH3NH3)2Pb3Br10 two-dimensional multilayered perovskite ferroelectric for a high-performance photodetector. Angew. Chem. Int. Ed. 56, 12150–12154 (2017).
Sha, T.-T. et al. Fluorinated 2D lead iodide perovskite ferroelectrics. Adv. Mater. 31, 1901843 (2019).
Park, I.-H. et al. Ferroelectricity and Rashba effect in a two-dimensional Dion-Jacobson hybrid organic–inorganic perovskite. J. Am. Chem. Soc. 141, 15972–15976 (2019).
Kamminga, M. E. et al. Confinement effects in low-dimensional lead iodide perovskite hybrids. Chem. Mater. 28, 4554–4562 (2016).
Yang, Y., Lou, F. & Xiang, H. Cooperative nature of ferroelectricity in two-dimensional hybrid organic–inorganic perovskites. Nano Lett. 21, 3170–3176 (2021).
Wu, Z. et al. Alloying n-butylamine into CsPbBr3 to give a two-dimensional bilayered perovskite ferroelectric material. Angew. Chem. Int. Ed. 57, 8140–8143 (2018).
Hautzinger, M. P. et al. Two-dimensional lead halide perovskites templated by a conjugated asymmetric diammonium. Inorg. Chem. 56, 14991–14998 (2017).
Swainson, I. et al. Orientational ordering, tilting and lone-pair activity in the perovskite methylammonium tin bromide, CH3NH3SnBr3. Acta Crystallogr. Sect. B 66, 422–429 (2010).
Laurita, G., Fabini, D. H., Stoumpos, C. C., Kanatzidis, M. G. & Seshadri, R. Chemical tuning of dynamic cation off-centering in the cubic phases of hybrid tin and lead halide perovskites. Chem. Sci. 8, 5628–5635 (2017).
Božin, E. S. et al. Entropically stabilized local dipole formation in lead chalcogenides. Science 330, 1660–1663 (2010).
Xie, H. et al. All-inorganic halide perovskites as potential thermoelectric materials: dynamic cation off-centering induces ultralow thermal conductivity. J. Am. Chem. Soc. 142, 9553–9563 (2020).
Lee, W. et al. Ultralow thermal conductivity in all-inorganic halide perovskites. Proc. Natl Acad. Sci. USA 114, 8693–8697 (2017).
Zeier, W. G. et al. Thinking like a chemist: intuition in thermoelectric materials. Angew. Chem. Int. Ed. 55, 6826–6841 (2016).
Delaire, O. et al. Giant anharmonic phonon scattering in PbTe. Nat. Mater. 10, 614–619 (2011).
Sharma, R. et al. Elucidating the atomistic origin of anharmonicity in tetragonal CH3NH3PbI3 with Raman scattering. Phys. Rev. Mater. 4, 92401 (2020).
Wilson, J. N., Frost, J. M., Wallace, S. K. & Walsh, A. Dielectric and ferroic properties of metal halide perovskites. APL Mater. 7, 10901 (2019).
Herz, L. M. How lattice dynamics moderate the electronic properties of metal-halide perovskites. J. Phys. Chem. Lett. 9, 6853–6863 (2018).
Burkhard, H., Geick, R., Kästner, P. & Unkelbach, K.-H. Lattice vibrations and free carrier dispersion in PbSe. Phys. Status Solidi 63, 89–96 (1974).
Huang, L. & Lambrecht, W. R. L. Electronic band structure, phonons, and exciton binding energies of halide perovskites CsSnCl3, CsSnBr3, and CsSnI3. Phys. Rev. B 88, 165203 (2013).
Faizan, M., Bhamu, K.C., Murtaza, G. et al. Electronic and optical properties of vacancy ordered double perovskites A2BX6 (A = Rb, Cs; B = Sn, Pd, Pt; and X = Cl, Br, I): a first principles study. Sci. Rep. 11, 6965 (2021).
Young, K. F. & Frederikse, H. P. R. Compilation of the static dielectric constant of inorganic solids. J. Phys. Chem. Ref. Data 2, 313–410 (1973).
Guo, Y. et al. Dynamic emission Stokes shift and liquid-like dielectric solvation of band edge carriers in lead-halide perovskites. Nat. Commun. 10, 1175 (2019).
Kepenekian, M. et al. Rashba and Dresselhaus effects in hybrid organic–inorganic perovskites: from basics to devices. ACS Nano 9, 11557–11567 (2015).
Kepenekian, M. & Even, J. Rashba and Dresselhaus couplings in halide perovskites: accomplishments and opportunities for spintronics and spin–orbitronics. J. Phys. Chem. Lett. 8, 3362–3370 (2017).
Becker, M. A. et al. Bright triplet excitons in caesium lead halide perovskites. Nature 553, 189–193 (2018).
Wang, F. et al. Switchable Rashba anisotropy in layered hybrid organic–inorganic perovskite by hybrid improper ferroelectricity. NPJ Comput. Mater. 6, 183 (2020).
Jenkins, H. D. B. & Waddington, T. C. Lone electron pairs and stereochemistry. Nature 255, 623–625 (1975).
Pearson, R. G. The second-order Jahn-Teller effect. J. Mol. Struct.: THEOCHEM 103, 25–34 (1983).
Trinquier, G. Double bonds and bridged structures in the heavier analogs of ethylene. J. Am. Chem. Soc. 112, 2130–2137 (1990).
Orgel, L. E. 769. The stereochemistry of B subgroup metals. Part II. The inert pair. J. Chem. Soc. 3815–3819 (1959).
Wheeler, R. A. & Kumar, P. N. V. P. Stereochemically active or inactive lone pair electrons in some six-coordinate, group 15 halides. J. Am. Chem. Soc. 114, 4776–4784 (1992).
Walsh, A., Payne, D. J., Egdell, R. G. & Watson, G. W. Stereochemistry of post-transition metal oxides: revision of the classical lone pair model. Chem. Soc. Rev. 40, 4455–4463 (2011). This is an excellent review detailing the stereochemistry of ns2-lone-pair-bearing solids.
Stoltzfus, M. W., Woodward, P. M., Seshadri, R., Klepeis, J.-H. & Bursten, B. Structure and bonding in SnWO4, PbWO4, and BiVO4: lone pairs vs inert pairs. Inorg. Chem. 46, 3839–3850 (2007).
Payne, D. J. et al. Electronic origins of structural distortions in post-transition metal oxides: experimental and theoretical evidence for a revision of the lone pair model. Phys. Rev. Lett. 96, 157403 (2006).
Walsh, A. & Watson, G. W. The origin of the stereochemically active Pb(II) lone pair: DFT calculations on PbO and PbS. J. Solid State Chem. 178, 1422–1428 (2005).
Ganose, A. M., Butler, K. T., Walsh, A. & Scanlon, D. O. Relativistic electronic structure and band alignment of BiSI and BiSeI: candidate photovoltaic materials. J. Mater. Chem. A 4, 2060–2068 (2016).
Wang, X. & Liebau, F. Studies on bond and atomic valences. I. correlation between bond valence and bond angles in SbIII chalcogen compounds: the influence of lone-electron pairs. Acta Crystallogr. Sect. B 52, 7–15 (1996).
Skoug, E. J. & Morelli, D. T. Role of lone-pair electrons in producing minimum thermal conductivity in nitrogen-group chalcogenide compounds. Phys. Rev. Lett. 107, 235901 (2011).
Acknowledgements
X.-Y.Z. acknowledges the Vannevar Bush Faculty Fellowship through Office of Naval Research grant no. N00014-18-1-2080 and the US Department of Energy, Office of Energy Sciences, grant DE-SC0010692 for support at various stages of writing of this account. S.J. acknowledges support through the Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, under award DE-FG02-09ER46664.
Author information
Authors and Affiliations
Contributions
Y.F., S.J. and X.-Y.Z. discussed the content of the article and Y.F. wrote the first version of the manuscript. All authors edited the manuscript prior to submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Chemistry thanks Q. Tu, P. Saines and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Polaron
-
A quasiparticle consisting of an electron or a hole dressed by a cloud of local distortion of the lattice. When the distortion is mostly confined within the unit cell, it is a small polaron. If the distortion extends well beyond the unit cell, the result is a large polaron.
- Rashba effect
-
Momentum-dependent splitting of spin bands due to a combined effect of spin–orbit coupling and asymmetry of the crystal potential. In a non-centrosymmetric solid, the static electric field Lorentz transforms into a magnetic field in the reference frame of a moving electron, which then interacts with the electron spin and breaks the spin degeneracy.
- Displacive ferroelectrics
-
Refers to the scenario where ions are displaced from the equilibrium positions to create the spontaneous polarization at temperatures below the Curie temperature.
- Second-harmonic generation
-
A nonlinear optical process in which two photons with the same energy interacting with a nonlinear material are effectively ‘combined’ to form a new photon with twice the energy.
- Curie temperatures
-
The critical temperatures above which a ferroelectric material loses spontaneous polarization. The same concept applies to ferromagnetic materials.
- Orthorhombic phase
-
When the lattice parameters a ≠ b ≠ c and all the angles are 90°.
- Stokes shift
-
Describes the energy difference between the emission peak and the absorption peak.
Rights and permissions
About this article
Cite this article
Fu, Y., Jin, S. & Zhu, XY. Stereochemical expression of ns2 electron pairs in metal halide perovskites. Nat Rev Chem 5, 838–852 (2021). https://doi.org/10.1038/s41570-021-00335-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-021-00335-9
This article is cited by
-
Exciton engineering of 2D Ruddlesden–Popper perovskites by synergistically tuning the intra and interlayer structures
Nature Communications (2024)
-
Two-dimensional lead halide perovskite lateral homojunctions enabled by phase pinning
Nature Communications (2024)
-
Continuous electroproduction of formate via CO2 reduction on local symmetry-broken single-atom catalysts
Nature Communications (2023)
-
The chemistry and physics of organic—inorganic hybrid perovskite quantum wells
Science China Chemistry (2022)