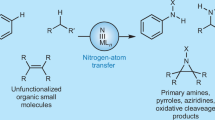
Abstract
Transition-metal-catalysed, non-enzymatic transformations of C–H and C=C bonds to C–N bonds through nitrene transfer (NT) are powerful synthetic tools to prepare valuable amine building blocks. Although the first examples of racemic NT were reported more than 50 years ago, catalysts that mediate enantioselective NT with a broad substrate scope have been slow to emerge. However, the past ten years have seen the discovery of several first-row, second-row and third-row transition metal catalysts for asymmetric NT. This Review covers recent developments in asymmetric aziridination and C–H bond amination reactions. We describe catalyst design principles, re-evaluate traditional catalyst architectures, show how the scope of nitrene precursors has expanded and present new mechanistic insights. Following this, we highlight remaining opportunities and challenges to developing more practical and general synthetic methodologies. Realizing chemoselective, site-selective and enantioselective intermolecular NT will streamline amine synthesis and allow us to explore new chemical space.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences (Wiley, 2008).
Nugent, T. C. (ed.) Chiral Amine Synthesis: Methods, Developments and Applications (Wiley, 2010).
Kwart, H. & Kahn, A. A. Copper-catalyzed decomposition of benzenesulfonyl azide in hydroxylic media. J. Am. Chem. Soc. 89, 1950–1951 (1967).
Kwart, H. & Khan, A. A. Copper-catalyzed decomposition of benzenesulfonyl azide in cyclohexene solution. J. Am. Chem. Soc. 89, 1951–1953 (1967).
Dodd, R. H. & Dauban, P. Iminoiodanes and C–N bond formation in organic synthesis. Synlett 11, 1571–1586 (2003).
Díaz-Requejo, M. M. & Pérez, P. J. Coinage metal catalyzed C–H bond functionalization of hydrocarbons. Chem. Rev. 108, 3379–3394 (2008).
Collet, F., Dodd, R. H. & Dauban, P. Catalytic C–H amination: recent progress and future directions. Chem. Commun. 2009, 5061–5074 (2009).
Fantauzzi, S., Caselli, A. & Gallo, E. Nitrene transfer reactions mediated by metallo-porphyrin complexes. Dalton Trans. 2009, 5434–5443 (2009).
Zalatan, D. N. & Du Bois, J. Metal-catalyzed oxidations of C–H to C–N bonds. Top. Curr. Chem. 292, 347–378 (2009).
Davies, H. M., Du Bois, J. & Yu, J.-Q. C–H functionalization in organic synthesis. Chem. Soc. Rev. 40, 1855–1856 (2011).
Dequirez, G., Pons, V. & Dauban, P. Nitrene chemistry in organic synthesis: still in its infancy? Angew. Chem. Int. Ed. 51, 7384–7395 (2012).
Che, C.-M.., Lo., K.-Y.; Zhou, C.-Y. in Comprehensive Organic Synthesis 2nd edn Vol. 7 (eds Knochel, P. & Molander, G. A.) 26–85 (Elsevier, 2014).
Darses, B., Rodrigues, R., Neuville, L., Mazurais, M. & Dauban, P. Transition metal-catalyzed iodine(iii)-mediated nitrene transfer reactions: efficient tools for challenging syntheses. Chem. Commun. 53, 493–508 (2017).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017). This is an excellent introduction to recent developments in transition-metal-catalyzed aminations of diverse C–H bonds that covers common catalysts and nitrene precursors.
Hazelard, D., Nocquet, P.-A. & Compain, P. Catalytic C–H amination at its limits: challenges and solutions. Org. Chem. Front. 4, 2500–2521 (2017).
Alderson, J. M., Corbin, J. R. & Schomaker, J. M. Tunable, chemo- and site-selective nitrene transfer reactions through the rational design of silver(i) catalysts. Acc. Chem. Res. 50, 2147–2158 (2017).
Kuijpers, P. F., van der Vlugt, J. I., Schneider, S. & de Bruin, B. Nitrene radical intermediates in catalytic synthesis. Chem. Eur. J. 23, 13819–13829 (2017).
Collet, F., Lescot, C. & Dauban, P. Catalytic C–H amination: the stereoselectivity issue. Chem. Soc. Rev. 40, 1926–1936 (2011).
Degennaro, L., Trinchera, P. & Luisi, R. Recent advances in the stereoselective synthesis of aziridines. Chem. Rev. 114, 7881–7929 (2014).
Hayashi, H. & Uchida, T. Nitrene transfer reactions for asymmetric C–H amination: recent development. Eur. J. Org. Chem. 2020, 909–916 (2020). This minireview focuses on recent strategies for asymmetric C–H amination with several nitrogen sources catalysed by molecular catalysts and artificial metalloenzymes.
Uchida, T. & Katsuki, T. Asymmetric nitrene transfer reactions: sulfimidation, aziridination and C–H amination using azide compounds as nitrene precursors. Chem. Rec. 14, 117–129 (2014).
Müller, P. & Fruit, C. Enantioselective catalytic aziridinations and asymmetric nitrene insertions into CH bonds. Chem. Rev. 103, 2905–2920 (2003). This review covers early efforts in catalytic asymmetric aziridination using nitrene and carbene transfer strategies.
Müller, P., Baud, C. & Naegeli, I. Rhodium(ii)-catalyzed nitrene transfer with phenyliodonium ylides. J. Phys. Org. Chem. 11, 597–601 (1998).
Fiori, K. W., Espino, C. G., Brodsky, B. H. & Du Bois, J. A mechanistic analysis of the Rh-catalyzed intramolecular C–H amination reaction. Tetrahedron 65, 3042–3051 (2009).
Harvey, M. E., Musaev, D. G. & Du Bois, J. A diruthenium catalyst for selective, intramolecular allylic C–H amination: reaction development and mechanistic insight gained through experiment and theory. J. Am. Chem. Soc. 133, 17207–17216 (2011).
Maestre, L., Sameera, W. M., Díaz-Requejo, M. M., Maseras, F. & Pérez, P. J. A general mechanism for the copper- and silver-catalyzed olefin aziridination reactions: concomitant involvement of the singlet and triplet pathways. J. Am. Chem. Soc. 135, 1338–1348 (2013).
Varela-Álvarez, A. et al. Rh2(ii,iii) catalysts with chelating carboxylate and carboxamidate supports: electronic structure and nitrene transfer reactivity. J. Am. Chem. Soc. 138, 2327–2341 (2016).
Weatherly, C., Alderson, J. M., Berry, J. F., Hein, J. E. & Schomaker, J. M. Catalyst-controlled nitrene transfer by tuning metal:ligand ratios: insight into the mechanisms of chemoselectivity. Organometallics 36, 1649–1661 (2017).
Liang, J.-L., Yuan, S.-X., Huang, J.-S. & Che, C.-M. Intramolecular C–N bond formation reactions catalyzed by ruthenium porphyrins: amidation of sulfamate esters and aziridination of unsaturated sulfonamides. J. Org. Chem. 69, 3610–3619 (2004).
Wentrup, C. Carbenes and nitrenes: recent developments in fundamental chemistry. Angew. Chem. Int. Ed. 57, 11508–11521 (2018).
Leung, S. K.-Y. et al. Imido transfer from bis(imido)ruthenium(vi) porphyrins to hydrocarbons: effect of imido substituents, C–H bond dissociation energies, and Ru(vi/v) reduction potentials. J. Am. Chem. Soc. 127, 16629–16640 (2005).
Lyaskovskyy, V. et al. Mechanism of cobalt(ii) porphyrin-catalyzed C–H amination with organic azides: radical nature and H-atom abstraction ability of the key cobalt(iii)–nitrene intermediates. J. Am. Chem. Soc. 133, 12264–12273 (2011).
Xing, Q., Chan, C.-M., Yeung, Y.-W. & Yu, W.-Y. Ruthenium(ii)-catalyzed enantioselective γ-lactams formation by intramolecular C–H amidation of 1,4,2-dioxazol-5-ones. J. Am. Chem. Soc. 141, 3849–3853 (2019).
Park, Y. & Chang, S. Asymmetric formation of γ-lactams via C–H amidation enabled by chiral hydrogen-bond-donor catalysts. Nat. Catal. 2, 219–227 (2019). Iridium catalysts with chiral H-bond-donor ligands use convenient 1,4,2-dioxazol-5-ones to produce γ-lactams in excellent ee using non-covalent interactions to control the stereochemical outcome.
Zhou, Z. et al. Non-C2-symmetric chiral-at-ruthenium catalyst for highly efficient enantioselective intramolecular C(sp3)–H amidation. J. Am. Chem. Soc. 141, 19048–19057 (2019).
Aguila, M. J., Badiei, Y. M. & Warren, T. H. Mechanistic insights into C–H amination via dicopper nitrenes. J. Am. Chem. Soc. 135, 9399–9406 (2013).
Llaveria, J. et al. Chemo-, regio-, and stereoselective silver-catalyzed aziridination of dienes: scope, mechanistic studies, and ring-opening reactions. J. Am. Chem. Soc. 136, 5342–5350 (2014).
Zhang, J. et al. Computational advances aiding mechanistic understanding of silver-catalyzed carbene/nitrene/silylene transfer reactions. Coord. Chem. Rev. 382, 69–84 (2019).
Lai, T.-S., Che, C.-M., Kwong, H.-L. & Peng, S.-M. Catalytic and asymmetric aziridination of alkenes catalysed by a chiral manganese porphyrin complex. Chem. Commun. 1997, 2373–2374 (1997).
Zhou, X.-G., Yu, X.-Q., Huang, J.-S. & Che, C.-M. Asymmetric amidation of saturated C–H bonds catalysed by chiral ruthenium and manganese porphyrins. Chem. Commun. 1999, 2377–2378 (1999).
Liang, J.-L., Yuan, S.-X., Huang, J.-S., Yu, W.-Y. & Che, C.-M. Highly diastereo- and enantioselective intramolecular amidation of saturated C–H bonds catalyzed by ruthenium porphyrins. Angew. Chem. Int. Ed. 41, 3465–3468 (2002).
Liang, J.-L., Huang, J.-S., Yu, X.-Q., Zhu, N. & Che, C.-M. Metalloporphyrin-mediated asymmetric nitrogen-atom transfer to hydrocarbons: aziridination of alkenes and amidation of saturated C–H bonds catalyzed by chiral ruthenium and manganese porphyrins. Chem. Eur. J. 8, 1563–1572 (2002). Early study that showed how chiral metalloporphyrins catalyse asymmetric aziridination of aromatic alkenes and asymmetric amidation of benzylic hydrocarbons.
Subbarayan, V., Ruppel, J. V., Zhu, S., Perman, J. A. & Zhang, X. P. Highly asymmetric cobalt-catalyzed aziridination of alkenes with trichloroethoxysulfonyl azide (TcesN3). Chem. Commun. 2009, 4266–4268 (2009).
Jones, J. E., Ruppel, J. V., Gao, G. Y., Moore, T. M. & Zhang, X. P. Cobalt-catalyzed asymmetric olefin aziridination with diphenylphosphoryl azide. J. Org. Chem. 73, 7260–7265 (2008).
Tao, J., Jin, L.-M. & Zhang, X. P. Synthesis of chiral N-phosphoryl aziridines through enantioselective aziridination of alkenes with phosphoryl azide via Co(ii)-based metalloradical catalysis. Beilstein J. Org. Chem. 10, 1282–1289 (2014).
Jin, L.-M. et al. Effective synthesis of chiral N-fluoroaryl aziridines through enantioselective aziridination of alkenes with fluoroaryl azides. Angew. Chem. Int. Ed. 52, 5309–5313 (2013).
Hopmann, K. H. & Ghosh, A. Mechanism of cobalt-porphyrin-catalyzed aziridination. ACS Catal. 1, 597–600 (2011).
Olivos Suarez, A. I., Jiang, H., Zhang, X. P. & de Bruin, B. The radical mechanism of cobalt(ii) porphyrin-catalyzed olefin aziridination and the importance of cooperative H-bonding. Dalton Trans. 40, 5697–5705 (2011).
Jin, L.-M., Xu, P., Xie, J. & Zhang, X. P. Enantioselective intermolecular radical C–H amination. J. Am. Chem. Soc. 142, 20828–20836 (2020). Co(ii) supported by D2-symmetric amidoporphyrinato ligands catalyses challenging asymmetric intermolecular radical C–H amination of esters with organic azides using non-covalent attractive interactions to achieve high ee.
Jiang, H., Lang, K., Lu, H., Wojtas, L. & Zhang, X. P. Asymmetric radical bicyclization of allyl azidoformates via cobalt(ii)-based metalloradical catalysis. J. Am. Chem. Soc. 139, 9164–9167 (2017).
Li, C. et al. Catalytic radical process for enantioselective amination of C(sp3)–H bonds. Angew. Chem. Int. Ed. 57, 16837–16841 (2018).
Hu, Y. et al. Enantioselective radical construction of 5-membered cyclic sulfonamides by metalloradical C–H amination. J. Am. Chem. Soc. 141, 18160–18169 (2019).
Lang, K., Li, C., Kim, I. & Zhang, X. P. Enantioconvergent amination of racemic tertiary C–H bonds. J. Am. Chem. Soc. 142, 20902–20911 (2020). This work describes the first Co(ii) catalysts for enantioconvergent radical amination of racemic tertiary C(sp3)-H bonds to create useful quaternary stereocentres.
Lang, K., Torker, S., Wojtas, L. & Zhang, X. P. Asymmetric induction and enantiodivergence in catalytic radical C–H amination via enantiodifferentiative H-atom abstraction and stereoretentive radical substitution. J. Am. Chem. Soc. 141, 12388–12396 (2019).
Hu, Y. et al. Next-generation D2-symmetric chiral porphyrins for cobalt(ii)-based metalloradical catalysis: catalyst engineering by distal bridging. Angew. Chem. Int. Ed. 58, 2670–2674 (2019).
Noda, K., Hosoya, N., Irie, R., Ito, Y. & Katsuki, T. Asymmetric aziridination by using optically active (salen)manganese(iii) complexes. Synlett 1993, 469–471 (1993).
Nishikori, H. & Katsuki, T. Catalytic and highly enantioselective aziridination of styrene derivatives. Tetrahedron Lett. 37, 9245–9248 (1996).
Kohmura, Y. & Katsuki, T. Mn(salen)-catalyzed enantioselective C–H amination. Tetrahedron Lett. 42, 3339–3342 (2001).
Omura, K., Murakami, M., Uchida, T., Irie, R. & Katsuki, T. Enantioselective aziridination and amination using p-toluenesulfonyl azide in the presence of Ru(salen)(CO) complex. Chem. Lett. 32, 354–355 (2003).
Omura, K., Uchida, T., Irie, R. & Katsuki, T. Design of a robust Ru(salen) complex: aziridination with improved turnover number using N-arylsulfonyl azides as precursors. Chem. Commun. https://doi.org/10.1039/B407693A (2004).
Kawabata, H., Omura, K. & Katsuki, T. Asymmetric aziridination: a new entry to optically active non-N-protected aziridines. Tetrahedron Lett. 47, 1571–1574 (2006).
Kawabata, H., Omura, K., Uchida, T. & Katsuki, T. Construction of robust ruthenium(salen)(CO) complexes and asymmetric aziridination with nitrene precursors in the form of azide compounds that bear easily removable N-sulfonyl groups. Chem. Asian J. 2, 248–256 (2007).
Kim, C., Uchida, T. & Katsuki, T. Asymmetric olefin aziridination using a newly designed Ru(CO)(salen) complex as the catalyst. Chem. Commun. 48, 7188–7190 (2012).
Ichinose, M. et al. Enantioselective intramolecular benzylic C–H bond amination: efficient synthesis of optically active benzosultams. Angew. Chem. Int. Ed. 50, 9884–9887 (2011).
Fukunaga, Y., Uchida, T., Ito, Y., Matsumoto, K. & Katsuki, T. Ru(CO)-salen-catalyzed synthesis of enantiopure aziridinyl ketones and formal asymmetric synthesis of (+)-PD 128907. Org. Lett. 14, 4658–4661 (2012).
Nishioka, Y., Uchida, T. & Katsuki, T. Enantio- and regioselective intermolecular benzylic and allylic C–H bond amination. Angew. Chem. Int. Ed. 52, 1739–1742 (2013).
Müller, P., Baud, C., Jacquier, Y., Moran, M. & Nägeli, I. Rhodium(ii)-catalyzed aziridinations and CH insertions with [N-(p-nitrobenzenesulfonyl)imino]phenyliodinane. J. Phys. Org. Chem. 9, 341–347 (1996).
Nägeli, I. et al. Rhodium(ii)-catalyzed CH insertions with {[(4-nitrophenyl)sulfonyl]imino}phenyl-λ3-iodane. Helv. Chim. Acta 80, 1087–1105 (1997).
Tsutsui, H. et al. Dirhodium(ii) tetrakis[N-tetrafluorophthaloyl-(S)-tert-leucinate]: an exceptionally effective Rh(ii) catalyst for enantiotopically selective aromatic C–H insertions of diazo ketoesters. Tetrahedron Asymmetry 14, 817–821 (2003).
Yasue, R. & Yoshida, K. Enantioselective desymmetrization of 1,3-disubstituted adamantane derivatives via rhodium-catalyzed C–H bond amination: access to optically active amino acids containing adamantane core. Adv. Synth. Catal. 363, 1662–1671 (2021).
Reddy, R. P. & Davies, H. M. Dirhodium tetracarboxylates derived from adamantylglycine as chiral catalysts for enantioselective C–H aminations. Org. Lett. 8, 5013–5016 (2006).
Nasrallah, A. et al. Catalytic enantioselective intermolecular benzylic C(sp3)–H amination. Angew. Chem. Int. Ed. 58, 8192–8196 (2019).
Nasrallah, A., Lazib, Y., Boquet, V., Darses, B. & Dauban, P. Catalytic intermolecular C(sp3)–H amination with sulfamates for the asymmetric synthesis of amines. Org. Process. Res. Dev. 24, 724–728 (2019).
Anada, M. et al. Catalytic enantioselective amination of silyl enol ethers using chiral dirhodium(ii) carboxylates: asymmetric formal synthesis of (−)-metazocine. Org. Lett. 9, 4559–4562 (2007).
Hashimoto, S., Tanaka, M., Nakamura, S. & Anada, M. Catalytic asymmetric synthesis of (−)-ritodrine hydrochloride via silyl enol ether amination using dirhodium(ii) tetrakis[tetrafluorophthaloyl-(S)-tert-leucinate]. Heterocycles 76, 1633–1645 (2008).
Tanaka, M., Kurosaki, Y., Washio, T., Anada, M. & Hashimoto, S. Enantioselective amination of silylketene acetals with (N-arylsulfonylimino)phenyliodinanes catalyzed by chiral dirhodium(ii) carboxylates: asymmetric synthesis of phenylglycine derivatives. Tetrahedron Lett. 48, 8799–8802 (2007).
Anada, M. et al. Asymmetric formal synthesis of (−)-pancracine via catalytic enantioselective C–H amination process. Tetrahedron 65, 3069–3077 (2009).
Miyazawa, T. et al. Chiral paddle-wheel diruthenium complexes for asymmetric catalysis. Nat. Catal. 3, 851–858 (2020). The first example of chiral paddle-wheel Ru2 complexes exhibiting reactivity and high ee in C–H amination under oxidizing conditions.
Zalatan, D. N. & Du Bois, J. A chiral rhodium carboxamidate catalyst for enantioselective C–H amination. J. Am. Chem. Soc. 130, 9220–9221 (2008). Early examples of Rh2 complexes supported by chiral lactams enable high site-selective and enantioselective C–H amination using sulfamate esters.
Lebel, H., Huard, K. & Lectard, S. N-tosyloxycarbamates as a source of metal nitrenes: rhodium-catalyzed C–H insertion and aziridination reactions. J. Am. Chem. Soc. 127, 14198–14199 (2005).
Espino, C. G., Fiori, K. W., Kim, M. & Du Bois, J. Expanding the scope of C–H amination through catalyst design. J. Am. Chem. Soc. 126, 15378–15379 (2004).
Fiori, K. W. & Du Bois, J. Catalytic intermolecular amination of C–H bonds: method development and mechanistic insights. J. Am. Chem. Soc. 129, 562–568 (2007).
Hoke, T., Herdtweck, E. & Bach, T. Hydrogen-bond mediated regio- and enantioselectivity in a C–H amination reaction catalysed by a supramolecular Rh(ii) complex. Chem. Commun. 49, 8009–8011 (2013).
Lonergan, D. G., Riego, J. & Deslongchamps, G. A convergent hydroxyimide module for molecular recognition. Tetrahedron Lett. 37, 6109–6112 (1996).
Lonergan, D. G., Halse, J. & Deslongchamps, G. Comparative probe for stacking interactions in simple A:T base pair mimics. Tetrahedron Lett. 39, 6865–6868 (1998).
Lonergan, D. G. & Deslongchamps, G. Tricyclic scaffolds for the rapid assembly of abiotic receptors. Tetrahedron 54, 14041–14052 (1998).
Zhong, F. & Bach, T. Enantioselective construction of 2,3-dihydrofuro[2,3-b]quinolines through supramolecular hydrogen bonding interactions. Chem. Eur. J. 20, 13522–13526 (2014).
Annapureddy, R. R., Jandl, C. & Bach, T. A chiral phenanthroline ligand with a hydrogen-bonding site: application to the enantioselective amination of methylene groups. J. Am. Chem. Soc. 142, 7374–7378 (2020).
Desimoni, G., Faita, G. & Jørgensen, K. A. Update 1 of: C2-symmetric chiral bis(oxazoline) ligands in asymmetric catalysis. Chem. Rev. 111, PR284–PR437 (2011).
Evans, D. A., Woerpel, K. A., Hinman, M. M. & Faul, M. M. Bis(oxazolines) as chiral ligands in metal-catalyzed asymmetric reactions. Catalytic, asymmetric cyclopropanation of olefins. J. Am. Chem. Soc. 113, 726–728 (1991).
Evans, D. A., Faul, M. M., Bilodeau, M. T., Anderson, B. A. & Barnes, D. M. Bis(oxazoline)–copper complexes as chiral catalysts for the enantioselective aziridination of olefins. J. Am. Chem. Soc. 115, 5328–5329 (1993).
Lowenthal, R. E. & Masamune, S. Asymmetric copper-catalyzed cyclopropanation of trisubstituted and unsymmetrical cis-1,2-disubstituted olefins: modified bis-oxazoline ligands. Tetrahedron Lett. 32, 7373–7376 (1991).
Adam, W., Roschmann, K. J. & Saha-Möller, C. R. Catalytic asymmetric aziridination of enol derivatives in the presence of chiral copper complexes to give optically active α-amino ketones. Eur. J. Org. Chem. 2000, 557–561 (2000).
Dauban, P., Dodd, R., Estéoule, A., Durán, F. & Retailleau, P. Enantioselective intramolecular copper-catalyzed aziridination of sulfamates. Synthesis 2007, 1251–1260 (2007).
Milczek, E., Boudet, N. & Blakey, S. Enantioselective C–H amination using cationic ruthenium(ii)–pybox catalysts. Angew. Chem. Int. Ed. 47, 6825–6828 (2008).
Ju, M., Weatherly, C. D., Guzei, I. A. & Schomaker, J. M. Chemo- and enantioselective intramolecular silver-catalyzed aziridinations. Angew. Chem. Int. Ed. 56, 9944–9948 (2017).
Ju, M. et al. Silver-catalyzed enantioselective propargylic C–H bond amination through rational ligand design. J. Am. Chem. Soc. 142, 12930–12936 (2020). This report showcases the first Ag-catalysed asymmetric nitrene transfer, allowing regioselective and enantioselective amination of propargylic C–H bonds using modular bis(oxazoline) ligands.
Zhang, Y.-Q., Yuan, Y.-A., Liu, G.-S. & Xu, H. Iron(ii)-catalyzed asymmetric intramolecular aminohydroxylation of indoles. Org. Lett. 15, 3910–3913 (2013).
Zhu, C.-L., Tian, J.-S., Gu, Z.-Y., Xing, G.-W. & Xu, H. Iron(ii)-catalyzed asymmetric intramolecular olefin aminochlorination using chloride ion. Chem. Sci. 6, 3044–3050 (2015).
Lu, D.-F., Zhu, C.-L., Sears, J. D. & Xu, H. Iron(ii)-catalyzed intermolecular aminofluorination of unfunctionalized olefins using fluoride ion. J. Am. Chem. Soc. 138, 11360–11367 (2016).
Zhu, C.-L., Lu, D.-F., Sears, J. D., Jia, Z.-X. & Xu, H. Practical synthetic procedures for the iron-catalyzed intermolecular olefin aminohydroxylation using functionalized hydroxylamines. Synthesis 48, 3031–3041 (2016).
Nie, X., Yan, Z., Ivlev, S. & Meggers, E. Ruthenium pybox-catalyzed enantioselective intramolecular C–H amination of sulfamoyl azides en route to chiral vicinal diamines. J. Org. Chem. 86, 750–761 (2021).
Li, L. et al. Complementing pyridine-2,6-bis(oxazoline) with cyclometalated N-heterocyclic carbene for asymmetric ruthenium catalysis. Angew. Chem. Int. Ed. 59, 12392–12395 (2020).
Ju, M. et al. Tunable catalyst-controlled syntheses of β- and γ-amino alcohols enabled by silver-catalysed nitrene transfer. Nat. Catal. 2, 899–908 (2019).
Charton, M. Nature of the ortho effect. II. Composition of the Taft steric parameters. J. Am. Chem. Soc. 91, 615–618 (1969).
Charton, M. Steric effects. I. Esterification and acid-catalyzed hydrolysis of esters. J. Am. Chem. Soc. 97, 1552–1556 (1975).
Charton, M. Steric effects. II. Base-catalyzed ester hydrolysis. J. Am. Chem. Soc. 97, 3691–3693 (1975).
Charton, M. Steric effects. 7. Additional V constants. J. Org. Chem. 41, 2217–2220 (1976).
Sigman, M. S. & Miller, J. J. Examination of the role of Taft-type steric parameters in asymmetric catalysis. J. Org. Chem. 74, 7633–7643 (2009).
Alderson, J. M., Phelps, A. M., Scamp, R. J., Dolan, N. S. & Schomaker, J. M. Ligand-controlled, tunable silver-catalyzed C–H amination. J. Am. Chem. Soc. 136, 16720–16723 (2014).
Dolan, N. S., Scamp, R. J., Yang, T., Berry, J. F. & Schomaker, J. M. Catalyst-controlled and tunable, chemoselective silver-catalyzed intermolecular nitrene transfer: experimental and computational studies. J. Am. Chem. Soc. 138, 14658–14667 (2016).
Huang, M., Yang, T., Paretsky, J. D., Berry, J. F. & Schomaker, J. M. Inverting steric effects: using “attractive” noncovalent interactions to direct silver-catalyzed nitrene transfer. J. Am. Chem. Soc. 139, 17376–17386 (2017).
Huang, M., Paretsky, J. & Schomaker, J. M. Rigidifying Ag(i) complexes for selective nitrene transfer. ChemCatChem 12, 3076–3081 (2020).
Nishiyama, H., Itoh, Y., Matsumoto, H., Park, S.-B. & Itoh, K. New chiral ruthenium bis(oxazolinyl)pyridine catalyst. Efficient asymmetric cyclopropanation of olefins with diazoacetates. J. Am. Chem. Soc. 116, 2223–2224 (1994).
Okamoto, K., Nanya, A., Eguchi, A. & Ohe, K. Asymmetric synthesis of 2H-azirines with a tetrasubstituted stereocenter by enantioselective ring contraction of isoxazoles. Angew. Chem. Int. Ed. 57, 1039–1043 (2018).
Hong, S. Y. et al. Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts. Science 359, 1016–1021 (2018).
Wang, H. et al. Iridium-catalyzed enantioselective C(sp3)–H amidation controlled by attractive noncovalent interactions. J. Am. Chem. Soc. 141, 7194–7201 (2019).
Fukagawa, S. et al. Enantioselective C(sp3)–H amidation of thioamides catalyzed by a cobaltiii/chiral carboxylic acid hybrid system. Angew. Chem. Int. Ed. 58, 1153–1157 (2019).
Sekine, D. et al. Chiral 2-aryl ferrocene carboxylic acids for the catalytic asymmetric C(sp3)–H activation of thioamides. Organometallics 38, 3921–3926 (2019).
Liu, Y.-H. et al. Cp*Co(iii)/MPAA-catalyzed enantioselective amidation of ferrocenes directed by thioamides under mild conditions. Org. Lett. 21, 1895–1899 (2019).
Fukagawa, S., Kojima, M., Yoshino, T. & Matsunaga, S. Catalytic enantioselective methylene C(sp3)–H amidation of 8-alkylquinolines using a Cp*Rhiii/chiral carboxylic acid system. Angew. Chem. Int. Ed. 58, 18154–18158 (2019).
Farr, C. M. B. et al. Designing a planar chiral rhodium indenyl catalyst for regio- and enantioselective allylic C–H amidation. J. Am. Chem. Soc. 142, 13996–14004 (2020). An exciting advance in the chemoselective, regioselective and enantioselective allylic C–H amidation of unactivated olefins using a planar-chiral indenyl Rh complex.
Zhang, L. & Meggers, E. Stereogenic-only-at-metal asymmetric catalysts. Chem. Asian J. 12, 2335–2342 (2017).
Zheng, Y. et al. Octahedral ruthenium complex with exclusive metal-centered chirality for highly effective asymmetric catalysis. J. Am. Chem. Soc. 139, 4322–4325 (2017).
Zhang, L. & Meggers, E. Steering asymmetric Lewis acid catalysis exclusively with octahedral metal-centered chirality. Acc. Chem. Res. 50, 320–330 (2017).
Qin, J., Zhou, Z., Cui, T., Hemming, M. & Meggers, E. Enantioselective intramolecular C–H amination of aliphatic azides by dual ruthenium and phosphine catalysis. Chem. Sci. 10, 3202–3207 (2019).
Zhou, Z. et al. Enantioselective ring-closing C–H amination of urea derivatives. Chem 6, 1851–1853 (2020).
Zhou, Z. et al. Catalytic enantioselective intramolecular C(sp3)–H amination of 2-azidoacetamides. Angew. Chem. Int. Ed. 58, 1088–1093 (2019).
Wang, G., Zhou, Z., Shen, X., Ivlev, S. & Meggers, E. Asymmetric catalysis with a chiral-at-osmium complex. Chem. Commun. 56, 7714–7717 (2020).
Acknowledgements
E. Zerull, J. Kim and T. A. Trinh are thanked for their helpful comments during the editing of this manuscript. J.M.S. is grateful to the NSF (Award number 1664374) for financial support for this research.
Author information
Authors and Affiliations
Contributions
M.J. researched data for the article and contributed to the content and writing of the manuscript. J.M.S. contributed to the discussion, writing and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ju, M., Schomaker, J.M. Nitrene transfer catalysts for enantioselective C–N bond formation. Nat Rev Chem 5, 580–594 (2021). https://doi.org/10.1038/s41570-021-00291-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-021-00291-4
This article is cited by
-
Expedited synthesis of α-amino acids by single-step enantioselective α-amination of carboxylic acids
Nature Synthesis (2023)
-
Unnatural α-amino acid synthesis
Nature Synthesis (2023)
-
Combining visible-light induction and copper catalysis for chemo-selective nitrene transfer for late-stage amination of natural products
Communications Chemistry (2022)
-
Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids
Nature Chemistry (2022)