Abstract
The membrane proteins found in nature control many important cellular functions, including signal transduction and transmembrane ion transport, and these, in turn, are regulated by external stimuli, such as small molecules, membrane potential and light. Membrane proteins also find technological applications in fields ranging from optogenetics to synthetic biology. Synthetic supramolecular analogues have emerged as a complementary method to engineer functional membranes. This Review describes stimuli-responsive supramolecular systems developed for the control of ion transport, signal transduction and catalysis in lipid-bilayer-membrane systems. Recent advances towards achieving spatio-temporal control over activity in artificial and living cells are highlighted. Current challenges, the scope, limitations and future potential to exploit supramolecular systems for engineering stimuli-responsive lipid-bilayer membranes are discussed.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yeagle, P. L. The Structure of Biological Membranes (CRC Press, 2011).
Krauss, G. Biochemistry of Signal Transduction and Regulation (Wiley, 2006).
Hauser, A. S. et al. Pharmacogenomics of GPCR drug targets. Cell 172, 41–54.e19 (2018).
Miesenböck, G. Optogenetic control of cells and circuits. Annu. Rev. Cell Dev. Biol. 27, 731–758 (2011).
Branton, D. et al. The potential and challenges of nanopore sequencing. Nat. Biotechnol. 26, 1146–1153 (2008).
Villar, G., Graham, A. D. & Bayley, H. A tissue-like printed material. Science 340, 48–52 (2013).
Elani, Y., Law, R. V. & Ces, O. Vesicle-based artificial cells as chemical microreactors with spatially segregated reaction pathways. Nat. Commun. 5, 5305 (2014).
Adamala, K. P., Martin-Alarcon, D. A., Guthrie-Honea, K. R. & Boyden, E. S. Engineering genetic circuit interactions within and between synthetic minimal cells. Nat. Chem. 9, 431–439 (2017).
Barba-Bon, A., Nilam, M. & Hennig, A. Supramolecular chemistry in the biomembrane. ChemBioChem 21, 886–910 (2020).
Matile, S., Jentzsch, A. V., Montenegro, J. & Fin, A. Recent synthetic transport systems. Chem. Soc. Rev. 40, 2453–2474 (2011).
Barboiu, M. Artificial water channels – incipient innovative developments. Chem. Commun. 52, 5657–5665 (2016).
Vanuytsel, S., Carniello, J. & Wallace, M. I. Artificial signal transduction across membranes. ChemBioChem 20, 2569–2580 (2019).
Takeuchi, T., Montenegro, J., Hennig, A. & Matile, S. Pattern generation with synthetic sensing systems in lipid bilayer membranes. Chem. Sci. 2, 303–307 (2011).
Gale, P. A., Davis, J. T. & Quesada, R. Anion transport and supramolecular medicinal chemistry. Chem. Soc. Rev. 46, 2497–2519 (2017).
Matile, S. & Sakai, N. in Analytical Methods in Supramolecular Chemistry (ed. Schalley, C. A.) 711–742 (Wiley, 2012).
Díazde Greñu, B. et al. Synthetic prodiginine obatoclax (GX15-070) and related analogues: anion binding, transmembrane transport, and cytotoxicity properties. Chem. Eur. J. 17, 14074–14083 (2011).
Ko, S.-K. et al. Synthetic ion transporters can induce apoptosis by facilitating chloride anion transport into cells. Nat. Chem. 6, 885–892 (2014).
Li, H. et al. Efficient, non-toxic anion transport by synthetic carriers in cells and epithelia. Nat. Chem. 8, 24–32 (2016).
Busschaert, N. et al. A synthetic ion transporter that disrupts autophagy and induces apoptosis by perturbing cellular chloride concentrations. Nat. Chem. 9, 667–675 (2017).
Marsh, D. Thermodynamics of phospholipid self-assembly. Biophys. J. 102, 1079–1087 (2012).
Kučerka, N., Nieh, M.-P. & Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta 1808, 2761–2771 (2011).
Alenghat, F. J. & Golan, D. E. Membrane protein dynamics and functional implications in mammalian cells. Curr. Top. Membr. 72, 89–120 (2013).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Saggiomo, V. et al. The role of lipophilicity in transmembrane anion transport. Chem. Commun. 48, 5274–5276 (2012).
Busschaert, N. et al. Towards predictable transmembrane transport: QSAR analysis of anion binding and transport. Chem. Sci. 4, 3036–3045 (2013).
Voskuhl, J. & Ravoo, B. J. Molecular recognition of bilayer vesicles. Chem. Soc. Rev. 38, 495–505 (2009).
Jiang, H. & Smith, B. D. Dynamic molecular recognition on the surface of vesicle membranes. Chem. Commun. 2006, 1407–1409 (2006).
Mammen, M., Choi, S.-K. & Whitesides, G. M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 37, 2754–2794 (1998).
Doyle, E. L., Hunter, C. A., Phillips, H. C., Webb, S. J. & Williams, N. H. Cooperative binding at lipid bilayer membrane surfaces. J. Am. Chem. Soc. 125, 4593–4599 (2003).
Han, J., Pluhackova, K. & Böckmann, R. A. The multifaceted role of SNARE proteins in membrane fusion. Front. Physiol. 8, 5 (2017).
Gong, Y., Luo, Y. & Bong, D. Membrane activation: selective vesicle fusion via small molecule recognition. J. Am. Chem. Soc. 128, 14430–14431 (2006).
Ma, M., Gong, Y. & Bong, D. Lipid membrane adhesion and fusion driven by designed, minimally multivalent hydrogen-bonding lipids. J. Am. Chem. Soc. 131, 16919–16926 (2009).
Ma, M. & Bong, D. Controlled fusion of synthetic lipid membrane vesicles. Acc. Chem. Res. 46, 2988–2997 (2013).
Paleos, C. M. & Pantos, A. Molecular recognition and organizational and polyvalent effects in vesicles induce the formation of artificial multicompartment cells as model systems of eukaryotes. Acc. Chem. Res. 47, 1475–1482 (2014).
Richard, A. et al. Fusogenic supramolecular vesicle systems induced by metal ion binding to amphiphilic ligands. Proc. Natl Acad. Sci. USA 101, 15279–15284 (2004).
Haluska, C. K. et al. Time scales of membrane fusion revealed by direct imaging of vesicle fusion with high temporal resolution. Proc. Natl Acad. Sci. USA 103, 15841–15846 (2006).
Mart, R. J., Liem, K. P., Wang, X. & Webb, S. J. The effect of receptor clustering on vesicle–vesicle adhesion. J. Am. Chem. Soc. 128, 14462–14463 (2006).
Gruber, B., Balk, S., Stadlbauer, S. & König, B. Dynamic interface imprinting: high-affinity peptide binding sites assembled by analyte-induced recruiting of membrane receptors. Angew. Chem. Int. Ed. 51, 10060–10063 (2012).
Gruber, B., Kataev, E., Aschenbrenner, J., Stadlbauer, S. & König, B. Vesicles and micelles from amphiphilic zinc(II)–cyclen complexes as highly potent promoters of hydrolytic DNA cleavage. J. Am. Chem. Soc. 133, 20704–20707 (2011).
Banerjee, S. & König, B. Molecular imprinting of luminescent vesicles. J. Am. Chem. Soc. 135, 2967–2970 (2013).
Gadsby, D. C. Ion channels versus ion pumps: the principal difference, in principle. Nat. Rev. Mol. Cell Biol. 10, 344–352 (2009).
Weber, J. Toward the ATP synthase mechanism. Nat. Chem. Biol. 6, 794–795 (2010).
Ernst, O. P. et al. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem. Rev. 114, 126–163 (2014).
Kanai, R., Ogawa, H., Vilsen, B., Cornelius, F. & Toyoshima, C. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature 502, 201–206 (2013).
Bean, B. P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007).
Ketchem, R. R., Hu, W. & Cross, T. A. High-resolution conformation of gramicidin A in a lipid bilayer by solid-state NMR. Science 261, 1457–1460 (1993).
Ohkuma, S. et al. Prodigiosins uncouple lysosomal vacuolar-type ATPase through promotion of H+/Cl− symport. Biochem. J. 334, 731–741 (1998).
Lehn, J.-M. Toward complex matter: supramolecular chemistry and self-organization. Proc. Natl Acad. Sci. USA 99, 4763–4768 (2002).
Matile, S., Som, A. & Sordé, N. Recent synthetic ion channels and pores. Tetrahedron 60, 6405–6435 (2004).
Fyles, T. M. Synthetic ion channels in bilayer membranes. Chem. Soc. Rev. 36, 335–347 (2007).
Busschaert, N. & Gale, P. A. Small-molecule lipid-bilayer anion transporters for biological applications. Angew. Chem. Int. Ed. 52, 1374–1382 (2013).
Zheng, S.-P., Huang, L.-B., Sun, Z. & Barboiu, M. Self-assembled artificial ion-channels toward natural selection of functions. Angew. Chem. Int. Ed. https://doi.org/10.1002/ange.201915287 (2020).
Si, W., Xin, P., Li, Z.-T. & Hou, J.-L. Tubular unimolecular transmembrane channels: construction strategy and transport activities. Acc. Chem. Res. 48, 1612–1619 (2015).
Alfonso, I. & Quesada, R. Biological activity of synthetic ionophores: ion transporters as prospective drugs? Chem. Sci. 4, 3009–3019 (2013).
De Riccardis, F., Izzo, I., Montesarchio, D. & Tecilla, P. Ion transport through lipid bilayers by synthetic ionophores: modulation of activity and selectivity. Acc. Chem. Res. 46, 2781–2790 (2013).
Wu, X., Gilchrist, A. M. & Gale, P. A. Prospects and challenges in anion recognition and transport. Chem 6, 1296–1309 (2020).
Howorka, S. Building membrane nanopores. Nat. Nanotechnol. 12, 619–630 (2017).
Göpfrich, K. & Keyser, U. F. in Biological and Bio-inspired Nanomaterials: Properties and Assembly Mechanisms (eds Perrett, S., Buell, A. K. & Knowles, T. P. J.) 331–370 (Springer, 2019).
Bae, W., Kocabey, S. & Liedl, T. DNA nanostructures in vitro, in vivo and on membranes. Nano Today 26, 98–107 (2019).
Velema, W. A., Szymanski, W. & Feringa, B. L. Photopharmacology: beyond proof of principle. J. Am. Chem. Soc. 136, 2178–2191 (2014).
Ellis-Davies, G. C. R. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods 4, 619–628 (2007).
Koçer, A., Walko, M., Meijberg, W. & Feringa, B. L. A light-actuated nanovalve derived from a channel protein. Science 309, 755–758 (2005).
Bao, C., Ma, M., Meng, F., Lin, Q. & Zhu, L. Efficient synthetic supramolecular channels and their light-deactivated ion transport in bilayer lipid membranes. New J. Chem. 39, 6297–6302 (2015).
Malla, J. A. et al. A glutathione activatable ion channel induces apoptosis in cancer cells by depleting intracellular glutathione levels. Angew. Chem. Int. Ed. 59, 7944–7952 (2020).
Salunke, S. B., Malla, J. A. & Talukdar, P. Phototriggered release of a transmembrane chloride carrier from an o-nitrobenzyl-linked procarrier. Angew. Chem. Int. Ed. 58, 5354–5358 (2019).
Fares, M. et al. Stimuli-responsive cycloaurated “OFF-ON” switchable anion transporters. Angew. Chem. Int. Ed. 59, 17614–17621 (2020).
Russew, M.-M. & Hecht, S. Photoswitches: from molecules to materials. Adv. Mater. 22, 3348–3360 (2010).
Dhammika Bandara, H. M. & C. Burdette, S. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 41, 1809–1825 (2012).
Beharry, A. A. & Woolley, G. A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 40, 4422–4437 (2011).
Szymański, W., Beierle, J. M., Kistemaker, H. A. V., Velema, W. A. & Feringa, B. L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem. Rev. 113, 6114–6178 (2013).
Mart, R. J. & Allemann, R. K. Azobenzene photocontrol of peptides and proteins. Chem. Commun. 52, 12262–12277 (2016).
Asanuma, H. et al. Synthesis of azobenzene-tethered DNA for reversible photo-regulation of DNA functions: hybridization and transcription. Nat. Protoc. 2, 203–212 (2007).
Szymański, W., Yilmaz, D., Koçer, A. & Feringa, B. L. Bright ion channels and lipid bilayers. Acc. Chem. Res. 46, 2910–2923 (2013).
Kuiper, J. M. & Engberts, J. B. F. N. H-aggregation of azobenzene-substituted amphiphiles in vesicular membranes. Langmuir 20, 1152–1160 (2004).
Kuiper, J. M., Stuart, M. C. A. & Engberts, J. B. F. N. Photochemically induced disturbance of the alkyl chain packing in vesicular membranes. Langmuir 24, 426–432 (2008).
Ishii, K. et al. Reversible control of exo- and endo-budding transitions in a photosensitive lipid membrane. ChemBioChem 10, 251–256 (2009).
Beharry, A. A., Sadovski, O. & Woolley, G. A. Azobenzene photoswitching without ultraviolet light. J. Am. Chem. Soc. 133, 19684–19687 (2011).
Bléger, D., Schwarz, J., Brouwer, A. M. & Hecht, S. o -Fluoroazobenzenes as readily synthesized photoswitches offering nearly quantitative two-way isomerization with visible light. J. Am. Chem. Soc. 134, 20597–20600 (2012).
Banghart, M., Borges, K., Isacoff, E., Trauner, D. & Kramer, R. H. Light-activated ion channels for remote control of neuronal firing. Nat. Neurosci. 7, 1381–1386 (2004).
Fehrentz, T., Schönberger, M. & Trauner, D. Optochemical genetics. Angew. Chem. Int. Ed. 50, 12156–12182 (2011).
Kramer, R. H., Chambers, J. J. & Trauner, D. Photochemical tools for remote control of ion channels in excitable cells. Nat. Chem. Biol. 1, 360–365 (2005).
Tochitsky, I., Kienzler, M. A., Isacoff, E. & Kramer, R. H. Restoring vision to the blind with chemical photoswitches. Chem. Rev. 118, 10748–10773 (2018).
Gorostiza, P. & Isacoff, E. Y. Optical switches for remote and noninvasive control of cell signaling. Science 322, 395–399 (2008).
Hüll, K., Morstein, J. & Trauner, D. In vivo photopharmacology. Chem. Rev. 118, 10710–10747 (2018).
Lien, L., Jaikaran, D. C. J., Zhang, Z. & Woolley, G. A. Photomodulated blocking of gramicidin ion channels. J. Am. Chem. Soc. 118, 12222–12223 (1996).
Jog, P. V. & Gin, M. S. A light-gated synthetic ion channel. Org. Lett. 10, 3693–3696 (2008).
Zhou, Y. et al. Reversible photo-gated transmembrane channel assembled from an acylhydrazone-containing crown ether triad. Chem. Commun. 53, 3681–3684 (2017).
Shinkai, S., Nakaji, T., Ogawa, T., Shigematsu, K. & Manabe, O. Photoresponsive crown ethers. 2. Photocontrol of ion extraction and ion transport by a bis(crown ether) with a butterfly-like motion. J. Am. Chem. Soc. 103, 111–115 (1981).
Rin Choi, Y. et al. Azobenzene-based chloride transporters with light-controllable activities. Chem. Commun. 50, 15305–15308 (2014).
Ahmed, M., Metya, S., Das, A. & Talukdar, P. A sandwich azobenzene–diamide dimer for photoregulated chloride transport. Chem. Eur. J. 26, 8703–8708 (2020).
Kerckhoffs, A. & Langton, M. Reversible photo-control over transmembrane anion transport using visible-light responsive supramolecular carriers. Chem. Sci. 11, 6325–6331 (2020).
García-López, V. et al. Molecular machines open cell membranes. Nature 548, 567–572 (2017).
Koumura, N., Zijlstra, R. W. J., van Delden, R. A., Harada, N. & Feringa, B. L. Light-driven monodirectional molecular rotor. Nature 401, 152–155 (1999).
Kassem, S. et al. Artificial molecular motors. Chem. Soc. Rev. 46, 2592–2621 (2017).
Liu, D. et al. Near-infrared light activates molecular nanomachines to drill into and kill cells. ACS Nano 13, 6813–6823 (2019).
Tedesco, M. M., Ghebremariam, B., Sakai, N. & Matile, S. Modeling the selectivity of potassium channels with synthetic, ligand-assembled π slides. Angew. Chem. Int. Ed. 38, 540–543 (1999).
Gorteau, V. et al. Synthetic multifunctional pores with external and internal active sites for ligand gating and noncompetitive blockage. J. Am. Chem. Soc. 126, 13592–13593 (2004).
Talukdar, P., Bollot, G., Mareda, J., Sakai, N. & Matile, S. Ligand-gated synthetic ion channels. Chem. Eur. J. 11, 6525–6532 (2005).
Muraoka, T. et al. Reversible ion transportation switch by a ligand-gated synthetic supramolecular ion channel. J. Am. Chem. Soc. 136, 15584–15595 (2014).
P. Wilson, C. & J. Webb, S. Palladium(II)-gated ion channels. Chem. Commun. 34, 4007–4009 (2008).
Devi, U., Brown, J. R. D., Almond, A. & Webb, S. J. Pd(II)-mediated assembly of porphyrin channels in bilayer membranes. Langmuir 27, 1448–1456 (2011).
Zhu, J. et al. Reversible ligand-gated ion channel via the interconversion between hollow single helix and intertwined double helix. Angew. Chem. Int. Ed. 59, 13602–13607 (2020).
Boccalon, M., Iengo, E. & Tecilla, P. Metal–organic transmembrane nanopores. J. Am. Chem. Soc. 134, 20310–20313 (2012).
Haynes, C. J. E. et al. Blockable Zn10L15 ion channels through subcomponent self-assembly. Angew. Chem. Int. Ed. 56, 15388–15392 (2017).
Haswell, E. S., Phillips, R. & Rees, D. C. Mechanosensitive channels: what can they do and how do they do it? Structure 19, 1356–1369 (2011).
Muraoka, T. et al. Mechano-sensitive synthetic ion channels. J. Am. Chem. Soc. 139, 18016–18023 (2017).
Chen, W.-H. & Regen, S. L. Thermally gated liposomes. J. Am. Chem. Soc. 127, 6538–6539 (2005).
Danial, M., Tran, C. M.-N., Jolliffe, K. A. & Perrier, S. Thermal gating in lipid membranes using thermoresponsive cyclic peptide–polymer conjugates. J. Am. Chem. Soc. 136, 8018–8026 (2014).
Gouaux, E. & MacKinnon, R. Principles of selective ion transport in channels and pumps. Science 310, 1461–1465 (2005).
Bezanilla, F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80, 555–592 (2000).
Si, W., Li, Z.-T. & Hou, J.-L. Voltage-driven reversible insertion into and leaving from a lipid bilayer: tuning transmembrane transport of artificial channels. Angew. Chem. Int. Ed. 53, 4578–4581 (2014).
Chen, L. et al. Chiral selective transmembrane transport of amino acids through artificial channels. J. Am. Chem. Soc. 135, 2152–2155 (2013).
Fyles, T. M., Loock, D. & Zhou, X. A voltage-gated ion channel based on a bis-macrocyclic bolaamphiphile. J. Am. Chem. Soc. 120, 2997–3003 (1998).
Goto, C., Yamamura, M., Satake, A. & Kobuke, Y. Artificial ion channels showing rectified current behavior. J. Am. Chem. Soc. 123, 12152–12159 (2001).
Sakai, N., Gerard, D. & Matile, S. Electrostatics of cell membrane recognition: structure and activity of neutral and cationic rigid push-pull rods in isoelectric, anionic, and polarized lipid bilayer membranes. J. Am. Chem. Soc. 123, 2517–2524 (2001).
Wu, X. et al. Voltage-switchable HCl transport enabled by lipid headgroup–transporter interactions. Angew. Chem. Int. Ed. 58, 15142–15147 (2019).
Busschaert, N. et al. Thiosquaramides: pH switchable anion transporters. Chem. Sci. 5, 3617–3626 (2014).
Elmes, R. B. P., Busschaert, N., Czech, D. D., Gale, P. A. & Jolliffe, K. A. pH switchable anion transport by an oxothiosquaramide. Chem. Commun. 51, 10107–10110 (2015).
Hernando, E. et al. Small molecule anionophores promote transmembrane anion permeation matching CFTR activity. Sci. Rep. 8, 2608 (2018).
Biswas, O. et al. Chloride ion transport by PITENINs across the phospholipid bilayers of vesicles and cells. ACS Appl. Bio Mater. 3, 935–944 (2020).
Choi, Y. R., Lee, B., Park, J., Namkung, W. & Jeong, K.-S. Enzyme-responsive procarriers capable of transporting chloride ions across lipid and cellular membranes. J. Am. Chem. Soc. 138, 15319–15322 (2016).
Mann, S. Life as a nanoscale phenomenon. Angew. Chem. Int. Ed. 47, 5306–5320 (2008).
van Rossum, S. A. P., Tena-Solsona, M., van Esch, J. H., Eelkema, R. & Boekhoven, J. Dissipative out-of-equilibrium assembly of man-made supramolecular materials. Chem. Soc. Rev. 46, 5519–5535 (2017).
Boekhoven, J., Hendriksen, W. E., Koper, G. J. M., Eelkema, R. & van Esch, J. H. Transient assembly of active materials fueled by a chemical reaction. Science 349, 1075–1079 (2015).
Maiti, S., Fortunati, I., Ferrante, C., Scrimin, P. & Prins, L. J. Dissipative self-assembly of vesicular nanoreactors. Nat. Chem. 8, 725–731 (2016).
della Sala, F., Maiti, S., Bonanni, A., Scrimin, P. & Prins, L. J. Fuel-selective transient activation of nanosystems for signal generation. Angew. Chem. Int. Ed. 57, 1611–1615 (2018).
Biagini, C. et al. Dissipative catalysis with a molecular machine. Angew. Chem. Int. Ed. 58, 9876–9880 (2019).
Bennett, I. M. et al. Active transport of Ca2+ by an artificial photosynthetic membrane. Nature 420, 398–401 (2002).
Steinberg-Yfrach, G. et al. Conversion of light energy to proton potential in liposomes by artificial photosynthetic reaction centres. Nature 385, 239–241 (1997).
Steinberg-Yfrach, G. et al. Light-driven production of ATP catalysed by F0F1-ATP synthase in an artificial photosynthetic membrane. Nature 392, 479–482 (1998).
Bhosale, S. et al. Photoproduction of proton gradients with π-stacked fluorophore scaffolds in lipid bilayers. Science 313, 84–86 (2006).
Howe, E. N. W. & Gale, P. A. Fatty acid fueled transmembrane chloride transport. J. Am. Chem. Soc. 141, 10654–10660 (2019).
Dambenieks, A. K., Vu, P. H. Q. & Fyles, T. M. Dissipative assembly of a membrane transport system. Chem. Sci. 5, 3396–3403 (2014).
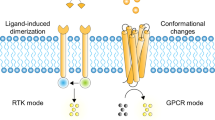
Simon, M. I., Strathmann, M. P. & Gautam, N. Diversity of G proteins in signal transduction. Science 252, 802–808 (1991).
Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 103, 211–225 (2000).
Kikuchi, J.-I. & Murakami, Y. Steroid cyclophanes as artificial cell-surface receptors. Molecular recognition and its consequence in signal transduction behavior. J. Incl. Phenom. Mol. Recognit. Chem. 32, 209–221 (1998).
Kikuchi, J., Ariga, K., Sasaki, Y. & Ikeda, K. Control of enzymic activity by artificial cell-surface receptors. J. Mol. Catal. B Enzym. 11, 977–984 (2001).
Tian, W.-J., Sasaki, Y., Fan, S.-D. & Kikuchi, J.-I. Switching of enzymatic activity through functional connection of molecular recognition on lipid bilayer membranes. Supramol. Chem. 17, 113–119 (2005).
Kikuchi, J., Ariga, K. & Ikeda, K. Signal transduction mediated by artificial cell-surface receptors: activation of lactate dehydrogenase triggered by molecular recognition and phase reorganization of bile acid derivatives embedded in a synthetic bilayer membrane. Chem. Commun. 1999, 547–548 (1999).
Mukai, M. et al. Propagation and amplification of molecular information using a photoresponsive molecular switch. Supramol. Chem. 21, 284–291 (2009).
Barton, P., Hunter, C. A., Potter, T. J., Webb, S. J. & Williams, N. H. Transmembrane signalling. Angew. Chem. Int. Ed. 41, 3878–3881 (2002).
Dijkstra, H. P. et al. Transmission of binding information across lipid bilayers. Chem. Eur. J. 13, 7215–7222 (2007).
Bernitzki, K. & Schrader, T. Entirely artificial signal transduction with a primary messenger. Angew. Chem. Int. Ed. 48, 8001–8005 (2009).
Bernitzki, K., Maue, M. & Schrader, T. Artificial signal transduction with primary and secondary messengers. Chem. Eur. J. 18, 13412–13417 (2012).
Brioche, J. et al. Conformational switching of a foldamer in a multicomponent system by pH-filtered selection between competing noncovalent interactions. J. Am. Chem. Soc. 137, 6680–6691 (2015).
Eccles, N. et al. Remote conformational responses to enantiomeric excess in carboxylate-binding dynamic foldamers. Chem. Commun. 55, 9331–9334 (2019).
De Poli, M. et al. Conformational photoswitching of a synthetic peptide foldamer bound within a phospholipid bilayer. Science 352, 575–580 (2016).
Lister, F. G. A., Le Bailly, B. A. F., Webb, S. J. & Clayden, J. Ligand-modulated conformational switching in a fully synthetic membrane-bound receptor. Nat. Chem. 9, 420–425 (2017).
Langton, M. J., Keymeulen, F., Ciaccia, M., Williams, N. H. & Hunter, C. A. Controlled membrane translocation provides a mechanism for signal transduction and amplification. Nat. Chem. 9, 426–430 (2017).
Langton, M. J., Williams, N. H. & Hunter, C. A. Recognition-controlled membrane translocation for signal transduction across lipid bilayers. J. Am. Chem. Soc. 139, 6461–6466 (2017).
Langton, M. J., Scriven, L. M., Williams, N. H. & Hunter, C. A. Triggered release from lipid bilayer vesicles by an artificial transmembrane signal transduction system. J. Am. Chem. Soc. 139, 15768–15773 (2017).
Ding, Y., Williams, N. H. & Hunter, C. A. A synthetic vesicle-to-vesicle communication system. J. Am. Chem. Soc. 141, 17847–17853 (2019).
Mora, N. L. et al. Targeted anion transporter delivery by coiled-coil driven membrane fusion. Chem. Sci. 7, 1768–1772 (2016).
Bayley, H. et al. Droplet interface bilayers. Mol. Biosyst. 4, 1191–1208 (2008).
Chen, S. et al. An artificial molecular shuttle operates in lipid bilayers for ion transport. J. Am. Chem. Soc. 140, 17992–17998 (2018).
Ren, C. et al. Molecular swings as highly active ion transporters. Angew. Chem. Int. Ed. 58, 8034–8038 (2019).
Ye, R. et al. Molecular ion fishers as highly active and exceptionally selective K+ transporters. J. Am. Chem. Soc. 141, 9788–9792 (2019).
Jentzsch, A. V. et al. Transmembrane anion transport mediated by halogen-bond donors. Nat. Commun. 3, 905 (2012).
Benz, S. et al. Anion transport with chalcogen bonds. J. Am. Chem. Soc. 138, 9093–9096 (2016).
Lee, L. M. et al. Anion transport with pnictogen bonds in direct comparison with chalcogen and halogen bonds. J. Am. Chem. Soc. 141, 810–814 (2019).
Bickerton, L. E., Sterling, A. J., Beer, P. D., Duarte, F. & Langton, M. J. Transmembrane anion transport mediated by halogen bonding and hydrogen bonding triazole anionophores. Chem. Sci. 11, 4722–4729 (2020).
Acknowledgements
M.J.L. is grateful for funding through a Royal Society University Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Transmembrane signal transduction
-
The transmission of molecular signals and information across a membrane, typically occurring without the signalling molecule crossing the membrane itself, maintaining cellular compartmentalization.
- Stimuli-responsive supramolecular systems
-
Synthetic molecules or molecular assemblies that exploit intermolecular interactions for their function, and their activity is regulated by changes in their chemical or physical environment.
- Mobile ion carriers
-
Lipid-soluble ion receptors (ionophores) that facilitate transmembrane ion transport by shuttling ions across the membrane.
- Photocaging
-
The use of photolabile protecting groups to temporarily mask the activity of a molecule. Subsequent photoirradiation removes the protecting group to reveal the functional molecule.
- Molecular photoswitches
-
Molecules that can be reversibly switched between at least two distinct states, which acquire different chemical and/or physical properties by absorbing photons.
Rights and permissions
About this article
Cite this article
Langton, M.J. Engineering of stimuli-responsive lipid-bilayer membranes using supramolecular systems. Nat Rev Chem 5, 46–61 (2021). https://doi.org/10.1038/s41570-020-00233-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-020-00233-6
This article is cited by
-
Bioinspired light-driven chloride pump with helical porphyrin channels
Nature Communications (2024)
-
Artificial molecular pumps
Nature Reviews Methods Primers (2024)
-
Rectifying artificial nanochannels with multiple interconvertible permeability states
Nature Communications (2024)
-
Photoinduced bidirectional switching in lipid membranes containing azobenzene glycolipids
Scientific Reports (2023)
-
Switching imidazole reactivity by dynamic control of tautomer state in an allosteric foldamer
Nature Communications (2023)