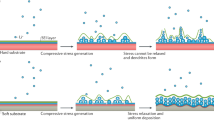
Abstract
Rechargeable metal–gas batteries have the promise of exceeding the energy densities of Li-ion batteries. An archetypal metal–gas system is the nonaqueous lithium–oxygen (Li–O2) battery, which was developed with a view to deploying it in electric vehicles. However, operating this battery comes with substantial challenges that include parasitic chemical reactivity and degrees of electrochemical irreversibility, which contribute to poor charging and cycling. To address these challenges, researchers began exploring new nonaqueous metal–gas battery paradigms by manipulating the underlying O2 redox behaviour through electrolyte and materials design, using non-Li-metal anodes to change the nature of the solid discharge phase and improve reversibility, and using other gaseous reactants as the cathode. This Review presents the new understanding of nonaqueous gas-to-solid electrochemistry that has emerged from these concerted efforts, along with new hurdles that have been revealed as cells have gradually been reformulated. The ultimate impact of new metal–gas batteries needs to be re-examined for applications beyond electric vehicles that are more amenable to the individual chemistries and performance characteristics.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
US DRIVE. Electrochemical energy storage technical team roadmap. Office of Energy Efficiency and Renewable Energy https://www.energy.gov/eere/vehicles/downloads/us-drive-electrochemical-energy-storage-technical-team-roadmap (2017).
Schmuch, R., Wagner, R., Hörpel, G., Placke, T. & Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 3, 267–278 (2018).
Whittingham, M. S. Ultimate limits to intercalation reactions for lithium batteries. Chem. Rev. 114, 11414–11443 (2014).
Nitta, N., Wu, F., Lee, J. T. & Yushin, G. Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015).
Wu, F. & Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 10, 435–459 (2017).
Cabana, J., Monconduit, L., Larcher, D. & Palacín, M. R. Beyond intercalation-based Li-ion batteries: the state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 22, E170–E192 (2010).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Pang, Q., Liang, X., Kwok, C. Y. & Nazar, L. Advances in lithium–sulfur batteries based on multifunctional cathodes and electrolytes. Nat. Energy 1, 16132 (2016).
Manthiram, A., Chung, S. H. & Zu, C. Lithium–sulfur batteries: progress and prospects. Adv. Mater. 27, 1980–2006 (2015).
Blurton, K. F. & Sammells, A. F. Metal/air batteries: their status and potential — a review. J. Power Sources 4, 263–279 (1979).
Meyers, W. F. & Simmons, J. W. Electric current-producing cell with anhydrous organic liquid electrolyte. US patent US3423242A (1969).
Abraham, K. M. & Jiang, Z. A polymer electrolyte-based rechargeable lithium/oxygen battery. J. Electrochem. Soc. 143, 1–5 (1996).
Ogasawara, T., Débart, A., Holzapfel, M., Novák, P. & Bruce, P. G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 128, 1390–1393 (2006).
Débart, A., Paterson, A. J., Bao, J. & Bruce, P. G. α-MnO2 nanowires: a catalyst for the O2 electrode in rechargeable lithium batteries. Angew. Chem. Int. Ed. 47, 4521–4524 (2008).
Lu, Y.-C. et al. Platinum–gold nanoparticles: a highly active bifunctional electrocatalyst for rechargeable lithium–air batteries. J. Am. Chem. Soc. 132, 12170–12171 (2010).
Kwak, W.-J. et al. Lithium–oxygen batteries and related systems: potential, status, and future. Chem. Rev. 120, 6626–6683 (2020).
Liu, T. et al. Current challenges and routes forward for nonaqueous lithium–air batteries. Chem. Rev. 120, 6558–6625 (2020). Kwak et al. (2020) and Liu et al. (2020) describe up-to-date advances in the reaction mechanisms and electrochemical performance of Li–O2 batteries.
Lu, Y.-C. et al. Lithium–oxygen batteries: bridging mechanistic understanding and battery performance. Energy Environ. Sci. 6, 750–768 (2013).
Lu, Y.-C. et al. The discharge rate capability of rechargeable Li–O2 batteries. Energy Environ. Sci. 4, 2999–3007 (2011).
Read, J. et al. Oxygen transport properties of organic electrolytes and performance of lithium/oxygen battery. J. Electrochem. Soc. 150, A1351 (2003).
Johnson, L. et al. The role of LiO2 solubility in O2 reduction in aprotic solvents and its consequences for Li–O2 batteries. Nat. Chem. 6, 1091–1099 (2014).
Mitchell, R. R., Gallant, B. M., Shao-Horn, Y. & Thompson, C. V. Mechanisms of morphological evolution of Li2O2 particles during electrochemical growth. J. Phys. Chem. Lett. 4, 1060–1064 (2013).
Lau, S. & Archer, L. A. Nucleation and growth of lithium peroxide in the Li–O2 battery. Nano Lett. 15, 5995–6002 (2015).
Gallant, B. M. et al. in The Lithium Air Battery: Fundamentals (eds Imanishi, N., Luntz, A. C. & Bruce, P. G.) 121–158 (Springer, 2014).
Rabenau, A. Lithium nitride and related materials case study of the use of modern solid state research techniques. Solid State Ion. 6, 277–293 (1982).
Girishkumar, G., McCloskey, B., Luntz, A. C., Swanson, S. & Wilcke, W. Lithium–air battery: promise and challenges. J. Phys. Chem. Lett. 1, 2193–2203 (2010).
Meini, S., Piana, M., Tsiouvaras, N., Garsuch, A. & Gasteiger, H. A. The effect of water on the discharge capacity of a non-catalyzed carbon cathode for Li–O2 batteries. Electrochem. Solid-State Lett. 15, A45 (2012).
Gallagher, K. G. et al. Quantifying the promise of lithium–air batteries for electric vehicles. Energy Environ. Sci. 7, 1555–1563 (2014).
Freunberger, S. A. et al. Reactions in the rechargeable lithium–O2 battery with alkyl carbonate electrolytes. J. Am. Chem. Soc. 133, 8040–8047 (2011).
McCloskey, B. D., Bethune, D. S., Shelby, R. M., Girishkumar, G. & Luntz, A. C. Solvents’ critical role in nonaqueous lithium–oxygen battery electrochemistry. J. Phys. Chem. Lett. 2, 1161–1166 (2011).
McCloskey, B. D. et al. On the efficacy of electrocatalysis in nonaqueous Li–O2 batteries. J. Am. Chem. Soc. 133, 18038–18041 (2011).
Aurbach, D., McCloskey, B. D., Nazar, L. F. & Bruce, P. G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 1, 16128 (2016).
Peng, Z. et al. Oxygen reactions in a non-aqueous Li+ electrolyte. Angew. Chem. Int. Ed. 50, 6351–6355 (2011).
Yu, Q. & Ye, S. In situ study of oxygen reduction in dimethyl sulfoxide (DMSO) solution: a fundamental study for development of the lithium–oxygen battery. J. Phys. Chem. C 119, 12236–12250 (2015).
Galloway, T. A. & Hardwick, L. J. Utilizing in situ electrochemical SHINERS for oxygen reduction reaction studies in aprotic electrolytes. J. Phys. Chem. Lett. 7, 2119–2124 (2016).
Viswanathan, V. et al. Electrical conductivity in Li2O2 and its role in determining capacity limitations in non-aqueous Li–O2 batteries. J. Chem. Phys. 135, 214704 (2011).
Gallant, B. M. et al. Influence of Li2O2 morphology on oxygen reduction and evolution kinetics in Li–O2 batteries. Energy Environ. Sci. 6, 2518–2528 (2013).
Laoire, C. O., Mukerjee, S., Abraham, K., Plichta, E. J. & Hendrickson, M. A. Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium–air battery. J. Phys. Chem. C 114, 9178–9186 (2010).
Burke, C. M., Pande, V., Khetan, A., Viswanathan, V. & McCloskey, B. D. Enhancing electrochemical intermediate solvation through electrolyte anion selection to increase nonaqueous Li–O2 battery capacity. Proc. Natl Acad. Sci. USA 112, 9293–9298 (2015).
Aetukuri, N. B. et al. Solvating additives drive solution-mediated electrochemistry and enhance toroid growth in non-aqueous Li–O2 batteries. Nat. Chem. 7, 50–56 (2015).
Schwenke, K. U., Metzger, M., Restle, T., Piana, M. & Gasteiger, H. A. The influence of water and protons on Li2O2 crystal growth in aprotic Li–O2 cells. J. Electrochem. Soc. 162, A573–A584 (2015).
Gao, X., Chen, Y., Johnson, L. & Bruce, P. G. Promoting solution phase discharge in Li–O2 batteries containing weakly solvating electrolyte solutions. Nat. Mater. 15, 882–888 (2016).
Lee, D. et al. Direct observation of redox mediator-assisted solution-phase discharging of Li–O2 battery by liquid-phase transmission electron microscopy. J. Am. Chem. Soc. 141, 8047–8052 (2019).
Liu, T. et al. The effect of water on quinone redox mediators in nonaqueous Li–O2 batteries. J. Am. Chem. Soc. 140, 1428–1437 (2018).
Peng, Z., Chen, Y., Bruce, P. G. & Xu, Y. Direct detection of the superoxide anion as a stable intermediate in the electroreduction of oxygen in a non-aqueous electrolyte containing phenol as a proton source. Angew. Chem. Int. Ed. 54, 8165–8168 (2015).
Gao, X., Jovanov, Z. P., Chen, Y., Johnson, L. R. & Bruce, P. G. Phenol-catalyzed discharge in the aprotic lithium–oxygen battery. Angew. Chem. Int. Ed. 129, 6639–6643 (2017).
Ko, Y. et al. Biological redox mediation in electron transport chain of bacteria for oxygen reduction reaction catalysts in lithium–oxygen batteries. Adv. Funct. Mater. 29, 1805623 (2019).
Zhang, Y. et al. High-capacity and high-rate discharging of a coenzyme Q10-catalyzed Li–O2 battery. Adv. Mater. 30, 1705571 (2018).
Park, J.-B., Lee, S. H., Jung, H.-G., Aurbach, D. & Sun, Y.-K. Redox mediators for Li–O2 batteries: status and perspectives. Adv. Mater. 30, 1704162 (2018). This paper reviews the recent developments of redox mediators for Li–O2 batteries.
Liang, Z., Zhou, Y. & Lu, Y.-C. Dynamic oxygen shield eliminates cathode degradation in lithium–oxygen batteries. Energy Environ. Sci. 11, 3500–3510 (2018).
Gallagher, K. G. et al. Optimizing areal capacities through understanding the limitations of lithium-ion electrodes. J. Electrochem. Soc. 163, A138 (2015).
Chen, S. et al. Critical parameters for evaluating coin cells and pouch cells of rechargeable Li-metal batteries. Joule 3, 1094–1105 (2019).
Lu, J. et al. Aprotic and aqueous Li–O2 batteries. Chem. Rev. 114, 5611–5640 (2014).
Chang, Z., Xu, J. & Zhang, X. Recent progress in electrocatalyst for Li–O2 batteries. Adv. Energy Mater. 7, 1700875 (2017).
Ko, Y., Park, H., Kim, B., Kim, J. S. & Kang, K. Redox mediators: a solution for advanced lithium–oxygen batteries. Trends Chem. 1, 349–360 (2019).
Khetan, A., Luntz, A. & Viswanathan, V. Trade-offs in capacity and rechargeability in nonaqueous Li–O2 batteries: solution-driven growth versus nucleophilic stability. J. Phys. Chem. Lett. 6, 1254–1259 (2015).
Wang, Y. et al. A solvent-controlled oxidation mechanism of Li2O2 in lithium–oxygen batteries. Joule 2, 2364–2380 (2018).
Ottakam Thotiyl, M. M., Freunberger, S. A., Peng, Z. & Bruce, P. G. The carbon electrode in nonaqueous Li–O2 cells. J. Am. Chem. Soc. 135, 494–500 (2012).
Black, R. et al. Screening for superoxide reactivity in Li–O2 batteries: effect on Li2O2/LiOH crystallization. J. Am. Chem. Soc. 134, 2902–2905 (2012).
Zhang, X. et al. LiO2: cryosynthesis and chemical/electrochemical reactivities. J. Phys. Chem. Lett. 8, 2334–2338 (2017).
Wandt, J., Jakes, P., Granwehr, J., Gasteiger, H. A. & Eichel, R.-A. Singlet oxygen formation during the charging process of an aprotic lithium–oxygen battery. Angew. Chem. Int. Ed. 55, 6892–6895 (2016).
Mahne, N. et al. Singlet oxygen generation as a major cause for parasitic reactions during cycling of aprotic lithium–oxygen batteries. Nat. Energy 2, 17036 (2017).
Mourad, E. et al. Singlet oxygen from cation driven superoxide disproportionation and consequences for aprotic metal–O2 batteries. Energy Environ. Sci. 12, 2559–2568 (2019). This work discusses the effect of peroxide disproportionation on the formation of 1O2 in alkali-metal–O2 batteries.
Mahne, N., Renfrew, S. E., McCloskey, B. D. & Freunberger, S. A. Electrochemical oxidation of lithium carbonate generates singlet oxygen. Angew. Chem. Int. Ed. 57, 5529–5533 (2018).
Freunberger, S. A. et al. The lithium–oxygen battery with ether-based electrolytes. Angew. Chem. Int. Ed. 50, 8609–8613 (2011).
Sharon, D. et al. Oxidation of dimethyl sulfoxide solutions by electrochemical reduction of oxygen. J. Phys. Chem. Lett. 4, 3115–3119 (2013).
McCloskey, B. D. et al. Twin problems of interfacial carbonate formation in nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 3, 997–1001 (2012).
Lu, Y.-C. & Shao-Horn, Y. Probing the reaction kinetics of the charge reactions of nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 4, 93–99 (2013).
Peng, Z., Freunberger, S. A., Chen, Y. & Bruce, P. G. A reversible and higher-rate Li–O2 battery. Science 337, 563–566 (2012).
Thotiyl, M. M. O. et al. A stable cathode for the aprotic Li–O2 battery. Nat. Mater. 12, 1050–1056 (2013).
Adams, B. D. et al. The importance of nanometric passivating films on cathodes for Li–air batteries. ACS Nano 8, 12483–12493 (2014).
Kundu, D., Black, R., Berg, E. J. & Nazar, L. F. A highly active nanostructured metallic oxide cathode for aprotic Li–O2 batteries. Energy Environ. Sci. 8, 1292–1298 (2015).
Chen, Y., Freunberger, S. A., Peng, Z., Fontaine, O. & Bruce, P. G. Charging a Li–O2 battery using a redox mediator. Nat. Chem. 5, 489–494 (2013).
Lim, H.-D. et al. Rational design of redox mediators for advanced Li–O2 batteries. Nat. Energy 1, 16066 (2016).
Chen, Y., Gao, X., Johnson, L. R. & Bruce, P. G. Kinetics of lithium peroxide oxidation by redox mediators and consequences for the lithium–oxygen cell. Nat. Commun. 9, 767 (2018).
McCloskey, B. D. & Addison, D. A viewpoint on heterogeneous electrocatalysis and redox mediation in nonaqueous Li–O2 batteries. ACS Catal. 7, 772–778 (2017).
Wang, Y., Liang, Z., Zou, Q., Cong, G. & Lu, Y.-C. Mechanistic insights into catalyst-assisted nonaqueous oxygen evolution reaction in lithium–oxygen batteries. J. Phys. Chem. C 120, 6459–6466 (2016).
Liang, Z. & Lu, Y.-C. Critical role of redox mediator in suppressing charging instabilities of lithium–oxygen batteries. J. Am. Chem. Soc. 138, 7574–7583 (2016).
Lim, H.-D. et al. Superior rechargeability and efficiency of lithium–oxygen batteries: hierarchical air electrode architecture combined with a soluble catalyst. Angew. Chem. Int. Ed. 53, 3926–3931 (2014).
Liu, T. et al. Cycling Li–O2 batteries via LiOH formation and decomposition. Science 350, 530–533 (2015).
Tułodziecki, M. et al. The role of iodide in the formation of lithium hydroxide in lithium–oxygen batteries. Energy Environ. Sci. 10, 1828–1842 (2017).
Kwak, W.-J. et al. Deactivation of redox mediators in lithium–oxygen batteries by singlet oxygen. Nat. Commun. 10, 1380 (2019).
Tikekar, M. D., Choudhury, S., Tu, Z. & Archer, L. A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114 (2016).
Assary, R. S. et al. The effect of oxygen crossover on the anode of a Li–O2 battery using an ether-based solvent: insights from experimental and computational studies. ChemSusChem 6, 51–55 (2013).
Lopez, N. et al. Reversible reduction of oxygen to peroxide facilitated by molecular recognition. Science 335, 450–453 (2012).
Lu, J. et al. A lithium–oxygen battery based on lithium superoxide. Nature 529, 377–382 (2016).
Kwak, W.-J., Park, J.-B., Jung, H.-G. & Sun, Y.-K. Controversial topics on lithium superoxide in Li–O2 batteries. ACS Energy Lett. 2, 2756–2760 (2017).
Papp, J. K. et al. Poly(vinylidene fluoride) (PVDF) binder degradation in Li–O2 batteries: a consideration for the characterization of lithium superoxide. J. Phys. Chem. Lett. 8, 1169–1174 (2017).
Giordani, V. et al. A molten salt lithium–oxygen battery. J. Am. Chem. Soc. 138, 2656–2663 (2016).
Xia, C., Kwok, C. & Nazar, L. F. A high-energy-density lithium–oxygen battery based on a reversible four-electron conversion to lithium oxide. Science 361, 777–781 (2018). This paper discloses a Li–O2 battery operating with molten nitrate salt electrolyte, in which 4-electron redox between O2 and Li2O was reversibly demonstrated.
Giordani, V. et al. Rechargeable-battery chemistry based on lithium oxide growth through nitrate anion redox. Nat. Chem. 11, 1133–1138 (2019). This article describes a novel molten nitrate salt system with NO3− as the electrochemically active O atom source, with gaseous O2 not required.
Grimaud, A., Hong, W. T., Shao-Horn, Y. & Tarascon, J.-M. Anionic redox processes for electrochemical devices. Nat. Mater. 15, 121–126 (2016).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 3, 373–386 (2018).
Zhu, Z. et al. Anion-redox nanolithia cathodes for Li-ion batteries. Nat. Energy 1, 16111 (2016). This work reported solid-state redox of oxygen beginning in the fully reduced Li2O state, reversibly yielding Li2O2 and LiO2 on charge.
Qiao, Y., Jiang, K., Deng, H. & Zhou, H. A high-energy-density and long-life lithium-ion battery via reversible oxide–peroxide conversion. Nat. Catal. 2, 1035–1044 (2019).
Hartmann, P. et al. A rechargeable room-temperature sodium superoxide (NaO2) battery. Nat. Mater. 12, 228–232 (2013). This study reports the first Na–O2 battery, where dramatic improvements in O2 redox reversibility are achieved by changing the alkali metal from Li to Na.
Hartmann, P. et al. Discharge and charge reaction paths in sodium–oxygen batteries: Does NaO2 form by direct electrochemical growth or by precipitation from solution? J. Phys. Chem. C 119, 22778–22786 (2015).
Xia, C., Black, R., Fernandes, R., Adams, B. & Nazar, L. F. The critical role of phase-transfer catalysis in aprotic sodium oxygen batteries. Nat. Chem. 7, 496–501 (2015).
Xia, C. et al. Direct evidence of solution-mediated superoxide transport and organic radical formation in sodium–oxygen batteries. J. Am. Chem. Soc. 138, 11219–11226 (2016).
McCloskey, B. D., Garcia, J. M. & Luntz, A. C. Chemical and electrochemical differences in nonaqueous Li–O2 and Na–O2 batteries. J. Phys. Chem. Lett. 5, 1230–1235 (2014).
Bender, C. L., Schröder, D., Pinedo, R., Adelhelm, P. & Janek, J. One- or two-electron transfer? The ambiguous nature of the discharge products in sodium–oxygen batteries. Angew. Chem. Int. Ed. 55, 4640–4649 (2016).
Ortiz-Vitoriano, N. et al. Rate-dependent nucleation and growth of NaO2 in Na–O2 batteries. J. Phys. Chem. Lett. 6, 2636–2643 (2015).
Bender, C. L., Hartmann, P., Vračar, M., Adelhelm, P. & Janek, J. On the thermodynamics, the role of the carbon cathode, and the cycle life of the sodium superoxide (NaO2) battery. Adv. Energy Mater. 4, 1301863 (2014).
Kim, J. et al. Dissolution and ionization of sodium superoxide in sodium–oxygen batteries. Nat. Commun. 7, 10670 (2016).
Sayed, S. Y. et al. Revealing instability and irreversibility in nonaqueous sodium–O2 battery chemistry. Chem. Commun. 52, 9691–9694 (2016).
Landa-Medrano, I. et al. Sodium–oxygen battery: steps toward reality. J. Phys. Chem. Lett. 7, 1161–1166 (2016).
Adelhelm, P. et al. From lithium to sodium: cell chemistry of room temperature sodium–air and sodium–sulfur batteries. Beilstein J. Nanotechnol. 6, 1016–1055 (2015).
Nichols, J. E. & McCloskey, B. D. The sudden death phenomena in nonaqueous Na–O2 batteries. J. Phys. Chem. C 121, 85–96 (2017).
Black, R. et al. The nature and impact of side reactions in glyme-based sodium–oxygen batteries. ChemSusChem 9, 1795–1803 (2016).
Schafzahl, L. et al. Singlet oxygen during cycling of the aprotic sodium–O2 battery. Angew. Chem. Int. Ed. 56, 15728–15732 (2017).
Lutz, L. et al. Role of electrolyte anions in the Na–O2 battery: implications for NaO2 solvation and the stability of the sodium solid electrolyte interphase in glyme ethers. Chem. Mater. 29, 6066–6075 (2017).
Ren, X. & Wu, Y. A low-overpotential potassium–oxygen battery based on potassium superoxide. J. Am. Chem. Soc. 135, 2923–2926 (2013).
Qin, L., Schkeryantz, L., Zheng, J., Xiao, N. & Wu, Y. Superoxide-based K–O2 batteries: highly reversible oxygen redox solves challenges in air electrodes. J. Am. Chem. Soc. 142, 11629–11640 (2020). This review includes recent advances in K–O2 battery chemistry.
Wang, W., Lai, N.-C., Liang, Z., Wang, Y. & Lu, Y.-C. Superoxide stabilization and a universal KO2 growth mechanism in potassium–oxygen batteries. Angew. Chem. Int. Ed. 57, 5042–5046 (2018).
Xiao, N., Rooney, R. T., Gewirth, A. A. & Wu, Y. The long-term stability of KO2 in K–O2 batteries. Angew. Chem. Int. Ed. 57, 1227–1231 (2018).
Xiao, N., Gourdin, G. & Wu, Y. Simultaneous stabilization of potassium metal and superoxide in K–O2 batteries on the basis of electrolyte reactivity. Angew. Chem. Int. Ed. 57, 10864–10867 (2018).
Ren, X., He, M., Xiao, N., McCulloch, W. D. & Wu, Y. Greatly enhanced anode stability in K-oxygen batteries with an in situ formed solvent- and oxygen-impermeable protection layer. Adv. Energy Mater. 7, 1601080 (2017).
Qin, L., Xiao, N., Zhang, S., Chen, X. & Wu, Y. From K–O2 to K–air batteries: realizing superoxide batteries on the basis of dry ambient air. Angew. Chem. Int. Ed. 59, 10498–10501 (2020).
Cong, G., Wang, W., Lai, N.-C., Liang, Z. & Lu, Y.-C. A high-rate and long-life organic–oxygen battery. Nat. Mater. 18, 390–396 (2019).
Li, C. S., Sun, Y., Gebert, F. & Chou, S.-L. Current progress on rechargeable magnesium–air battery. Adv. Energy Mater. 7, 1700869 (2017).
Reinsberg, P., Bondue, C. J. & Baltruschat, H. Calcium–oxygen batteries as a promising alternative to sodium–oxygen batteries. J. Phys. Chem. C 120, 22179–22185 (2016).
Reddy, T. B. (ed.) Linden’s Handbook of Batteries 4th edn (McGraw-Hill, 2011).
Xing, H. et al. Ambient lithium–SO2 batteries with ionic liquids as electrolytes. Angew. Chem. Int. Ed. 53, 2099–2103 (2014).
Maricle, D. L. & Mohns, J. P. Electrochemical cell containing sulfur dioxide as the cathode depolarizer. US Patent US3567515A (1971).
Dey, A., Kuo, H., Piliero, P. & Kallianidis, M. Inorganic electrolyte Li/SO2 rechargeable system: development of a prototype hermetic C cell and evaluation of its performance and safety characteristics. J. Electrochem. Soc. 135, 2115 (1988).
Fey, G. T.-K. Li/SO2 rechargeable batteries. J. Power Sources 35, 153–162 (1991).
Lim, H.-D. et al. A new perspective on Li–SO2 batteries for rechargeable systems. Angew. Chem. Int. Ed. 54, 9663–9667 (2015). This work describes a Li–SO2(g) battery exhibiting reversible formation and decomposition of Li2S2O4 solid.
Park, H. et al. High-efficiency and high-power rechargeable lithium–sulfur dioxide batteries exploiting conventional carbonate-based electrolytes. Nat. Commun. 8, 14989 (2017).
Qiao, Y. et al. Li–CO2 electrochemistry: a new strategy for CO2 fixation and energy storage. Joule 1, 359–370 (2017).
Xu, S., Das, S. K. & Archer, L. A. The Li–CO2 battery: a novel method for CO2 capture and utilization. RSC Adv. 3, 6656–6660 (2013).
Xie, J. & Wang, Y. Recent development of CO2 electrochemistry from Li–CO2 batteries to Zn–CO2 batteries. Acc. Chem. Res. 6, 1721–1729 (2019).
Hou, Y. et al. Mo2C/CNT: an efficient catalyst for rechargeable Li–CO2 batteries. Adv. Funct. Mater. 27, 1700564 (2017).
Khurram, A., Yin, Y., Yan, L., Zhao, L. & Gallant, B. M. Governing role of solvent on discharge activity in lithium–CO2 batteries. J. Phys. Chem. Lett. 10, 6679–6687 (2019).
Gowda, S. R., Brunet, A., Wallraff, G. & McCloskey, B. D. Implications of CO2 contamination in rechargeable nonaqueous Li–O2 batteries. J. Phys. Chem. Lett. 4, 276–279 (2013).
Takechi, K., Shiga, T. & Asaoka, T. A Li–O2/CO2 battery. Chem. Commun. 47, 3463–3465 (2011).
Yin, W., Grimaud, A., Lepoivre, F., Yang, C. & Tarascon, J. M. Chemical vs electrochemical formation of Li2CO3 as a discharge product in Li–O2/CO2 batteries by controlling the superoxide intermediate. J. Phys. Chem. Lett. 8, 214–222 (2017).
Lim, H.-K. et al. Toward a lithium–“air” battery: the effect of CO2 on the chemistry of a lithium–oxygen cell. J. Am. Chem. Soc. 135, 9733–9742 (2013).
Zhao, Z., Su, Y. & Peng, Z. Probing lithium carbonate formation in trace-O2-assisted aprotic Li–CO2 batteries using in situ surface-enhanced Raman spectroscopy. J. Phys. Chem. Lett. 10, 322–328 (2019).
Khurram, A., He, M. & Gallant, B. M. Tailoring the discharge reaction in Li–CO2 batteries through incorporation of CO2 capture chemistry. Joule 2, 2649–2666 (2018).
Yang, S. et al. A reversible lithium–CO2 battery with Ru nanoparticles as a cathode catalyst. Energy Environ. Sci. 10, 972–978 (2017).
Liu, B. et al. Recent advances in understanding Li–CO2 electrochemistry. Energy Environ. Sci. 12, 887–922 (2019).
Yang, S., He, P. & Zhou, H. Exploring the electrochemical reaction mechanism of carbonate oxidation in Li–air/CO2 battery through tracing missing oxygen. Energy Environ. Sci. 9, 1650–1654 (2016).
Garcia-Lastra, J. M., Myrdal, J. S., Christensen, R., Thygesen, K. S. & Vegge, T. DFT+U study of polaronic conduction in Li2O2 and Li2CO3: implications for Li–air batteries. J. Phys. Chem. C 117, 5568–5577 (2013).
Ling, C., Zhang, R., Takechi, K. & Mizuno, F. Intrinsic barrier to electrochemically decompose Li2CO3 and LiOH. J. Phys. Chem. C 118, 26591–26598 (2014).
Zhang, Z. et al. Verifying the rechargeability of Li–CO2 batteries on working cathodes of Ni nanoparticles highly dispersed on N-doped graphene. Adv. Sci. 5, 1700567 (2018).
Ma, W., Lu, S., Lei, X., Liu, X. & Ding, Y. Porous Mn2O3 cathode for highly durable Li–CO2 batteries. J. Mater. Chem. A 6, 20829–20835 (2018).
Németh, K. & Srajer, G. CO2/oxalate cathodes as safe and efficient alternatives in high energy density metal–air type rechargeable batteries. RSC Adv. 4, 1879–1885 (2014).
Hughes, T., Smith, R. & Kiely, D. Stored chemical energy propulsion system for underwater applications. J. Energy 7, 128–133 (1983).
Li, Y., Khurram, A. & Gallant, B. M. A high-capacity lithium–gas battery based on sulfur fluoride conversion. J. Phys. Chem. C 122, 7128–7138 (2018). This work demonstrates the first Li–SF6 battery, where 8-electron transfer per SF6 molecule was achieved.
Kwabi, D. G. et al. Experimental and computational analysis of the solvent-dependent O2/Li+-O2− redox couple: standard potentials, coupling strength, and implications for lithium–oxygen batteries. Angew. Chem. Int. Ed. 55, 3129–3134 (2016).
Zámostná, L. & Braun, T. Catalytic degradation of sulfur hexafluoride by rhodium complexes. Angew. Chem. Int. Ed. 54, 10652–10656 (2015).
He, H. et al. Highly-efficient conversion of SF6 via an eight-electron transfer process in lithium batteries. Nano Energy 72, 104679 (2020).
Braun, T., Noveski, D., Neumann, B. & Stammler, H.-G. Conversion of hexafluoropropene into 1,1,1-trifluoropropane by rhodium-mediated C–F activation. Angew. Chem. Int. Ed. 41, 2745–2748 (2002).
Gao, H., Li, Y., Guo, R. & Gallant, B. M. Controlling fluoride-forming reactions for improved rate capability in lithium-perfluorinated gas conversion batteries. Adv. Energy Mater. 9, 1900393 (2019).
He, M., Li, Y., Guo, R. & Gallant, B. M. Electrochemical conversion of nitrogen trifluoride as a gas-to-solid cathode in Li batteries. J. Phys. Chem. Lett. 9, 4700–4706 (2018).
Ma, J.-L., Bao, D., Shi, M.-M., Yan, J.-M. & Zhang, X.-B. Reversible nitrogen fixation based on a rechargeable lithium–nitrogen battery for energy storage. Chem 2, 525–532 (2017).
Parr, R. G. & Pearson, R. G. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983).
Pearson, R. G. Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl Acad. Sci. USA 83, 8440–8441 (1986).
Pearson, R. G. Hard and soft acids and bases. J. Am. Chem. Soc. 85, 3533–3539 (1963).
Travers, M. J., Cowles, D. C. & Ellison, G. B. Reinvestigation of the electron affinities of O2 and NO. Chem. Phys. Lett. 164, 449–455 (1989).
Johnson, R. D. III (ed.) NIST computational chemistry comparison and benchmark database. NIST standard reference database number 101. National Institute of Standards and Technology https://cccbdb.nist.gov/ (2019).
Zintl, E., Harder, A. & Dauth, B. Gitterstruktur der oxyde, sulfide, selenide und telluride des lithiums, natriums und kaliums. Z. Elektrochem. Angew. Physik. Chem. 40, 588–593 (1934).
Vannerberg, N.-G. in Progress in Inorganic Chemistry Vol. 4 (ed. Cotton, F. A.) (Wiley, 1962).
Sun, B. et al. Hierarchical porous carbon spheres for high-performance Na–O2 batteries. Adv. Mater. 29, 1606816 (2017).
Zhang, Z. et al. The first introduction of graphene to rechargeable Li–CO2 batteries. Angew. Chem. Int. Ed. 54, 6550–6553 (2015).
Xiao, N., Ren, X., He, M., McCulloch, W. D. & Wu, Y. Probing mechanisms for inverse correlation between rate performance and capacity in K–O2 batteries. ACS Appl. Mater. Interfaces 9, 4301–4308 (2017).
Tatara, R. et al. Oxygen reduction reaction in highly concentrated electrolyte solutions of lithium bis(trifluoromethanesulfonyl)amide/dimethyl sulfoxide. J. Phys. Chem. C 121, 9162–9172 (2017).
Adams, B. D. et al. Current density dependence of peroxide formation in the Li–O2 battery and its effect on charge. Energy Environ. Sci. 6, 1772–1778 (2013).
Liu, Y., Wang, R., Lyu, Y., Li, H. & Chen, L. Rechargeable Li/CO2–O2 (2:1) battery and Li/CO2 battery. Energy Environ. Sci. 7, 677–681 (2014).
Black, R., Lee, J.-H., Adams, B., Mims, C. A. & Nazar, L. F. The role of catalysts and peroxide oxidation in lithium–oxygen batteries. Angew. Chem. Int. Ed. 52, 392–396 (2013).
Li, J. & Rogachev, A. Y. SO2 — yet another two-faced ligand. Phys. Chem. Chem. Phys. 17, 1987–2000 (2015).
Tang, R. & Callaway, J. Electronic structure of SF6. J. Chem. Phys. 84, 6854–6860 (1986).
McLean, A. Extended basis-set LCSTO—MO—SCF calculations on the ground state of carbon dioxide. J. Chem. Phys. 38, 1347–1355 (1963).
Jürgensen, A. & Cavell, R. G. Valence shell photoionization energies and cross-sections of NF3 and PF3. J. Electron. Spectrosc. Relat. Phenom. 128, 245–260 (2003).
Cade, P. E. & Wahl, A. C. Hartree–Fock–Roothaan wavefunctions for diatomic molecules: II. First-row homonuclear systems A2, A2±, and A2*. At. Data Nucl. Data Tables 13, 339–389 (1974).
Jain, A. et al. The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Acknowledgements
The authors gratefully acknowledge funding from the MIT Lincoln Laboratory and from the Army Research Office under award number W911NF-19-1-0311.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gao, H., Gallant, B.M. Advances in the chemistry and applications of alkali-metal–gas batteries. Nat Rev Chem 4, 566–583 (2020). https://doi.org/10.1038/s41570-020-00224-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-020-00224-7
This article is cited by
-
Pd cluster decorated free standing flexible cathode for high performance Li-oxygen batteries
Nano Research (2024)
-
A corrosion inhibiting layer to tackle the irreversible lithium loss in lithium metal batteries
Nature Communications (2023)
-
Metal sulfide heterojunction with tunable interfacial electronic structure as an efficient catalyst for lithium-oxygen batteries
Science China Materials (2023)
-
Electric Vehicles and the Burning Question: Reasons, Risks, Ramifications and Remedies—An Indian Perspective
Fire Technology (2023)
-
The highly dispersed Co-based nanoparticles encapsulated into porous N-doping carbon polyhedral with the low content of Ru modification as a promising cathode catalyst for long-life Li-O2 batteries
Nano Research (2022)