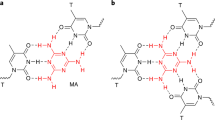
Abstract
Guanine and related nucleobases such as guanosine, deoxyguanosine and isoguanosine are notable molecular tools for designing functional supramolecular assemblies. This popularity originates in their ability to self-assemble via a unique topological pluralism — as isolated nucleobases, discrete macrocyclic quartets and virtually infinite linear ribbons — that endows them with a considerable functional versatility. Many programmes have been launched to fine-tune the chemical properties of guanine derivatives, to make them usable under different experimental conditions, such as in organic or aqueous environments, and responsive to external stimuli, such as ionic strength, pH, light or temperature. These strategies aim to translate the chemical information encoded in a basic guanine unit into programmable, higher-order supramolecular architectures. Spectacular results have been recently obtained in various chemical fields, from supramolecular chemistry to chemical biology, from soft matter to catalysis. In this Review, we detail these advances and demonstrate how these multidisciplinary investigations cast a bright light on the diversity that guanines, synthetic guanines and related nucleobases uniquely offer in terms of both structure and function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Change history
11 November 2019
This article has been corrected to add image credits to Figure 10. The permission line now reads Part b is adapted with permission from ref.147, CC-BY-4.0. Part c is adapted with permission from ref.148, OUP.
References
Davis, J. T. G-quartets 40 years later: from 5′-GMP to molecular biology and supramolecular chemistry. Angew. Chem. Int. Ed. 43, 668–698 (2004).
Watson, J. D. & Crick, F. H. Molecular structure of nucleic acids. Nature 171, 737–738 (1953).
Hoogsteen, K. The structure of crystals containing a hydrogen-bonded complex of 1-methylthymine and 9-methyladenine. Acta Cryst. 12, 822–823 (1959).
Tipson, R. S. The chemistry of the nucleic acids. Adv. Carbohydr. Chem. 1, 193–245 (1945).
Miescher, F. Hoppe-Seyler’s Med-chem. Untersuch 441, 502 (1871).
Hammarsten, O. Zur Kenntniss der Nucleoproteide. Z. Phys. Chem. 19, 19–37 (1895).
Levene, P. A. & Jacobs, W. A. Guanylic acid. Ber. Dtsch. Chem. Ges. 42, 2469–2473 (1909).
Bang, I. Untersuchungen über die Guanylsäure. Biochem. Z. 26, 293–311 (1910).
Buell, M. V. & Perkins, M. E. Crystalline guanine nucleotide. J. Biol. Chem. 72, 21–26 (1927).
Gellert, M., Lipsett, M. N. & Davies, R. D. Helix formation by guanylic acid. Proc. Natl Acad. Sci. USA 48, 2013–2018 (1962).
Maizels, N. G4-associated human diseases. EMBO Rep. 16, 910–922 (2015).
Cammas, A. & Millevoi, S. RNA G-quadruplexes: emerging mechanisms in disease. Nucleic Acids Res. 45, 1584–1595 (2017).
Balasubramanian, S., Hurley, L. H. & Neidle, S. Targeting G-quadruplexes in gene promoters: a novel anticancer strategy? Nat. Rev. Drug Discov. 10, 261–275 (2011).
Bochman, M. L., Paeschke, K. & Zakian, V. A. DNA secondary structures: stability and function of G-quadruplex structures. Nat. Rev. Genet. 13, 770–780 (2012).
Rhodes, D. & Lipps, H. J. G-quadruplexes and their regulatory roles in biology. Nucleic Acids Res. 43, 8627–8637 (2015).
Hänsel-Hertsch, R., Di Antonio, M. & Balasubramanian, S. DNA G-quadruplexes in the human genome: detection, functions and therapeutic potential. Nat. Rev. Mol. Cell Biol. 18, 279–284 (2017).
Kwok, C. K., Marsico, G. & Balasubramanian, S. Detecting RNA G-quadruplexes (rG4s) in the transcriptome. Cold Spring Harb. Persp. Biol. 10, a032284 (2018).
Davis, J. T., Tirumala, S., Jenssen, J. R., Radler, E. & Fabris, D. Self-assembled ionophores from isoguanosine. J. Org. Chem. 60, 4167–4176 (1995).
Gottarelli, G., Masiero, S. & Spada, G. P. Self-assembly in organic solvents of a deoxyguanosine derivative induced by alkali metal picrates. J. Chem. Soc. Chem. Commun. 24, 2555–2557 (1995).
Pinnavaia, T. et al. Alkali metal ion specificity in the solution ordering of a nucleotide, 5′-guanosine monophosphate. J. Am. Chem. Soc. 100, 3625–3627 (1978).
Cai, M. et al. Binding cesium ions with nucleosides: templated self-assembly of isoguanosine pentamers. Angew. Chem. Int. Ed. 39, 1283–1285 (2000).
Van Leeuwen, F. W., Verboom, W., Shi, X., Davis, J. T. & Reinhoudt, D. N. Selective 226Ra2+ ionophores provided by self-assembly of guanosine and isoguanosine derivatives. J. Am. Chem. Soc. 126, 16575–16581 (2004).
Gubala, V., Betancourt, J. E. & Rivera, J. M. Expanding the Hoogsteen edge of 2′-deoxyguanosine: consequences for G-quadruplex formation. Org. Lett. 6, 4735–4738 (2004).
Otero, R. et al. Guanine quartet networks stabilized by cooperative hydrogen bonds. Angew. Chem. Int. Ed. 44, 2270–2275 (2005).
Martín-Hidalgo, M. & Rivera, J. M. Metallo-responsive switching between hexadecameric and octameric supramolecular G-quadruplexes. Chem. Commun. 47, 12485–12487 (2011).
Lena, S., Neviani, P., Masiero, S., Pieraccini, S. & Spada, G. P. Triggering of guanosine self-assembly by light. Angew. Chem. Int. Ed. 49, 3657–3660 (2010).
González-Rodríguez, D. et al. G-quadruplex self-assembly regulated by Coulombic interactions. Nat. Chem. 1, 151–155 (2009).
Reddy, G. M. et al. Co-existence of distinct supramolecular assemblies in solution and in the solid state. Chem. Eur. J. 23, 2315–2322 (2017).
Kaucher, M. S. & Davis, J. T. N2, C8-disubstituted guanosine derivatives can form G-quartets. Tetrahedron Lett. 47, 6381–6384 (2006).
He, Y. et al. Construction of a cross-layer linked G-octamer via conformational control: a stable G-quadruplex in H-bond competitive solvent. Chem. Sci. 10, 4192–4199 (2019).
Chen, L., Sakai, N., Moshiri, S. T. & Matile, S. Toward supramolecular ion channels formed by oligonucleotide analogs: Hydrophobic guanine dimers. Tetrahedron Lett. 39, 3627–3630 (1998).
Sidorov, V., Kotch, F. W., El-Kouedi, M. & Davis, J. T. Toward artificial ion channels: self-assembled nanotubes from calix [4] arene–guanosine conjugates. Chem. Commun. 23, 2369–2370 (2000).
Cram, D. J., Jaeger, R. & Deshayes, K. Hemicarcerands that encapsulate hydrocarbons with molecular weights greater than two hundred. J. Am. Chem. Soc. 115, 10111–10116 (1993).
Nikan, M. & Sherman, J. C. Template-assembled synthetic G-quartets (TASQs). Angew. Chem. Int. Ed. 47, 4900–4902 (2008).
Nikan, M. & Sherman, J. C. Cation-complexation behavior of template-assembled synthetic G-quartets. J. Org. Chem. 74, 5211–5218 (2009).
Hightower, J. B., Olmos, D. R. & Walmsley, J. A. Supramolecular structure and polymorphism of alkali metal salts of guanosine 5′-monophosphate: SEM and NMR study. J. Phys. Chem. B 113, 12214–12219 (2009).
Wu, G., Kwan, I. C. M., Yan, Z., Huang, Y. & Ye, E. On the helical structure of guanosine 5′-monophosphate formed at pH 5: is it left- or right-handed? J. Nucleic Acids 2017, 6798759 (2017).
Wu, G. & Kwan, I. C. Helical structure of disodium 5′-guanosine monophosphate self-assembly in neutral solution. J. Am. Chem. Soc. 131, 3180–3182 (2009).
Nakayama, S., Kelsey, I., Wang, J. & Sintim, H. O. c-di-GMP can form remarkably stable G-quadruplexes at physiological conditions in the presence of some planar intercalators. Chem. Commun. 47, 4766–4768 (2011).
Nakayama, S. et al. Thiazole orange-induced c-di-GMP quadruplex formation facilitates a simple fluorescent detection of this ubiquitous biofilm regulating molecule. J. Am. Chem. Soc. 133, 4856–4864 (2011).
Bare, G. A. L., Liu, B. & Sherman, J. C. Synthesis of a single G-quartet platform in water. J. Am. Chem. Soc. 135, 11985–11989 (2013).
Gonnelli, A. et al. Small-angle X-ray scattering study of self-assembling lipophilic guanines in organic solvents: G-quadruplex formation and cation effects in cyclohexane. J. Phys. Chem. B 117, 1095–1103 (2013).
Kunstelj, K., Federiconi, F., Spindler, L. & Drevenšek-Olenik, I. Self-organization of guanosine 5′-monophosphate on mica. Colloids Surf. B Biointerfaces 59, 120–127 (2007).
Zhang, C. et al. Solventless formation of G-quartet complexes based on alkali and alkaline earth salts on Au(111). ChemPhysChem 16, 2099–2105 (2015).
González-Rodríguez, D. et al. Persistent, well-defined, monodisperse, π-conjugated organic nanoparticles via G-quadruplex self-assembly. J. Am. Chem. Soc. 132, 4710–4719 (2010).
Gao, M. et al. Temperature and pressure limits of guanosine monophosphate self-assemblies. Sci. Rep. 7, 9864 (2017).
Azargun, M. & Fridgen, T. D. Guanine tetrads: an IRMPD spectroscopy, energy resolved SORI-CID, and computational study of M(9-ethylguanine)4 + (M = Li, Na, K, Rb, Cs) in the gas phase. Phys. Chem. Chem. Phys. 17, 25778–25785 (2015).
Azargun, M., Jami-Alahmadi, Y. & Fridgen, T. D. The intrinsic stabilities and structures of alkali metal cationized guanine quadruplexes. Phys. Chem. Chem. Phys. 19, 1281–1287 (2017).
Fraschetti, C., Montagna, M., Guarcini, L., Guidoni, L. & Filippi, A. Spectroscopic evidence for a gas-phase librating G-quartet–Na+ complex. Chem. Commun. 50, 14767–14770 (2014).
Mudroňová, K., Římal, V. & Mojzeš, P. Effect of ribose versus 2′-deoxyribose residue in guanosine 5′-monophosphates on formation of G-quartets stabilized by potassium and sodium cations. Vib. Spectrosc. 82, 60–65 (2016).
Panda, M. & Walmsley, J. A. Circular dichroism study of supramolecular assemblies of guanosine 5′-monophosphate. J. Phys. Chem. B 115, 6377–6383 (2011).
Goncharova, I., Novotná, J. & Urbanová, M. Stacked and continuous helical self-assemblies of guanosine monophosphates detected by vibrational circular dichroism. Anal. Bioanal. Chem. 403, 2635–2644 (2012).
Kwan, I. C. M., Mo, X. & Wu, G. Probing hydrogen bonding and ion−carbonyl interactions by solid-state 17O NMR spectroscopy: G-ribbon and G-quartet. J. Am. Chem. Soc. 129, 2398–2407 (2007).
Wong, A., Kotch, F. W., Kwan, I. C. M., Davis, J. T. & Wu, G. Probing the Na+ binding site in a calix[4]arene–guanosine conjugate dimer by solid-state 23Na NMR and quantum chemical calculation. Chem. Commun. 16, 2154–2156 (2009).
Mukhopadhyay, T. K. & Datta, A. Design rules for the generation of stable quartet phases of nucleobases over two-dimensional materials. J. Phys. Chem. C 122, 28918–28933 (2018).
Šponer, J. et al. Folding of guanine quadruplex molecules–funnel-like mechanism or kinetic partitioning? An overview from MD simulation studies. Biochim. Biophys. Acta 1861, 1246–1263 (2017).
Gresh, N. et al. Channeling through two stacked guanine quartets of one and two alkali cations in the Li+, Na+, K+, and Rb+ series. Assessment of the accuracy of the SIBFA anisotropic polarizable molecular mechanics potential. J. Phys. Chem. B 121, 3997–4014 (2017).
Zaccaria, F., Paragi, G. & Fonseca Guerra, C. F. The role of alkali metal cations in the stabilization of guanine quadruplexes: why K+ is the best. Phys. Chem. Chem. Phys. 18, 20895–20904 (2016).
Paragi, G. & Fonseca Guerra, C. Cooperativity in the self-assembly of the guanine nucleobase into quartet and ribbon structures on surfaces. Chem. Eur. J. 23, 3042–3050 (2017).
Kotch, F. W. et al. Water-mediated association provides an ion pair receptor. J. Am. Chem. Soc. 125, 15140–15150 (2003).
Davis, J. T., Okunola, O. & Quesada, R. Recent advances in the transmembrane transport of anions. Chem. Soc. Rev. 39, 3843–3862 (2010).
Kaucher, M. S., Harrell, W. A. & Davis, J. T. A unimolecular G-quadruplex that functions as a synthetic transmembrane Na+ transporter. J. Am. Chem. Soc. 128, 38–39 (2006).
Sutyak, K. B., Lee, W., Zavalij, P. V., Gutierrez, O. & Davis, J. T. Templating and catalyzing [2+2] photocycloaddition in solution using a dynamic G-quadruplex. Angew. Chem. Int. Ed. 57, 17146–17150 (2018).
Sakai, N. et al. Dendritic folate rosettes as ion channels in lipid bilayers. J. Am. Chem. Soc. 128, 2218–2219 (2006).
Ma, L., Melegari, M., Colombini, M. & Davis, J. T. Large and stable transmembrane pores from guanosine−bile acid conjugates. J. Am. Chem. Soc. 130, 2938–2939 (2008).
Kumar, Y. P. et al. Triazole-tailored guanosine dinucleosides as biomimetic ion channels to modulate transmembrane potential. Chem. Eur. J. 20, 3023–3028 (2014).
Kumar, Y. P., Das, R. N., Schütte, O. M., Steinem, C. & Dash, J. Bis-triazolyl diguanosine derivatives as synthetic transmembrane ion channels. Nat. Protoc. 11, 1039–1056 (2016).
Ghosh, A., Parasar, B., Bhattacharyya, T. & Dash, J. Chiral carbon dots derived from guanosine 5′-monophosphate form supramolecular hydrogels. Chem. Commun. 52, 11159–11162 (2016).
Li, Z. & Mirkin, C. A. G-quartet-induced nanoparticle assembly. J. Am. Chem. Soc. 127, 11568–11569 (2005).
Wong, A., Ida, R., Spindler, L. & Wu, G. Disodium guanosine 5′-monophosphate self-associates into nanoscale cylinders at pH 8: a combined diffusion NMR spectroscopy and dynamic light scattering study. J. Am. Chem. Soc. 127, 6990–6998 (2005).
Hu, D., Ren, J. & Qu, X. Metal-mediated fabrication of new functional G-quartet-based supramolecular nanostructure and potential application as controlled drug release system. Chem. Sci. 2, 1356–1361 (2011).
Pu, F., Wu, L., Ran, X., Ren, J. & Qu, X. G-quartet-based nanostructure for mimicking light-harvesting antenna. Angew. Chem. Int. Ed. 54, 892–896 (2015).
Wu, Y.-L., Brown, K. E. & Wasielewski, M. R. Extending photoinduced charge separation lifetimes by using supramolecular design: guanine–perylenediimide G-quadruplex. J. Am. Chem. Soc. 135, 13322–13325 (2013).
Wu, Y.-L. et al. G-quadruplex organic frameworks. Nat. Chem. 9, 466–472 (2017).
Nakayama, S., Roelofs, K., Lee, V. T. & Sintim, H. O. A C-di-GMP–proflavine–hemin supramolecular complex has peroxidase activity—implication for a simple colorimetric detection. Mol. Biosyst. 8, 726–729 (2012).
Li, Y. & Sen, D. A catalytic DNA for porphyrin metallation. Nat. Struct. Biol. 3, 743–747 (1996).
Li, Y. & Sen, D. Toward an efficient DNAzyme. Biochemistry 36, 5589–5599 (1997).
Travascio, P., Li, Y. & Sen, D. DNA-enhanced peroxidase activity of a DNA aptamer-hemin complex. Chem. Biol. 5, 505–517 (1998).
Travascio, P., Witting, P. K., Mauk, A. G. & Sen, D. The peroxidase activity of a hemin–DNA oligonucleotide complex: free radical damage to specific guanine bases of the DNA. J. Am. Chem. Soc. 123, 1337–1348 (2001).
Stefan, L., Denat, F. & Monchaud, D. Insights into how nucleotide supplements enhance the peroxidase-mimicking DNAzyme activity of the G-quadruplex/hemin system. Nucleic Acids Res. 40, 8759–8772 (2012).
Yamamoto, Y. et al. Characterization of heme–DNA complexes composed of some chemically modified hemes and parallel G-quadruplex DNAs. Biochemistry 54, 7168–7177 (2015).
Chen, J. et al. How proximal nucleobases regulate the catalytic activity of G-quadruplex/hemin DNAzymes. ACS Catal. 8, 11352–11361 (2018).
Sen, D. & Poon, L. C. RNA and DNA complexes with hemin [Fe(III) heme] are efficient peroxidases and peroxygenases: how do they do it and what does it mean? Crit. Rev. Biochem. Mol. Biol. 46, 478–492 (2011).
Wang, F., Lu, C.-H. & Willner, I. From cascaded catalytic nucleic acids to enzyme–DNA nanostructures: controlling reactivity, sensing, logic operations, and assembly of complex structures. Chem. Rev. 114, 2881–2941 (2014).
Mergny, J.-L. & Sen, D. DNA quadruple helices in nanotechnology. Chem. Rev. 119, 6290–6325 (2019).
Flack, T. et al. Prefolded synthetic G-quartets display enhanced bioinspired properties. Chem. Eur. J. 22, 1760–1767 (2016).
Stefan, L., Xu, H.-J., Gros, C. P., Denat, F. & Monchaud, D. Harnessing nature’s insights: synthetic small molecules with peroxidase-mimicking DNAzyme properties. Chem. Eur. J. 17, 10857–10862 (2011).
Harraz, D. M. & Davis, J. T. A self-assembled peroxidase from 5′-GMP and heme. Chem. Commun. 54, 1587–1590 (2018).
Bhattacharyya, T., Kumar, Y. P. & Dash, J. Supramolecular hydrogel inspired from DNA structures mimics peroxidase activity. ACS Biomat. Sci. Eng. 3, 2358–2365 (2017).
Zhong, R. et al. Self-assembly of enzyme-like nanofibrous G-molecular hydrogel for printed flexible electrochemical sensors. Adv. Mater. 30, 1706887 (2018).
de Gennes, P. G. Soft matter (Nobel lecture). Angew. Chem. Int. Ed. 31, 842–845 (1992).
Nagel, S. R. Experimental soft-matter science. Rev. Mod. Phys. 89, 025002 (2017).
Peters, G. M. & Davis, J. T. Supramolecular gels made from nucleobase, nucleoside and nucleotide analogs. Chem. Soc. Rev. 45, 3188–3206 (2016).
Gottarelli, G. et al. The self-assembly of lipophilic guanosine derivatives in solution and on solid surfaces. Chem. Eur. J. 6, 3242–3248 (2000).
Mezzina, E. et al. The self-assembly of a lipophilic guanosine nucleoside into polymeric columnar aggregates: the nucleoside structure contains sufficient information to drive the process towards a strikingly regular polymer. Chem. Eur. J. 7, 388–395 (2001).
Pieraccini, S. et al. Columnar lyomesophases formed in hydrocarbon solvents by chiral lipophilic guanosine-alkali metal complexes. Chirality 13, 7–12 (2001).
Giorgi, T. et al. Gel-like lyomesophases formed in organic solvents by self-assembled guanine ribbons. Chem. Eur. J. 8, 2143–2152 (2002).
Gan, K. P., Yoshio, M., Sugihara, Y. & Kato, T. Guanine–oligothiophene conjugates: liquid-crystalline properties, photoconductivities and ion-responsive emission of their nanoscale assemblies. Chem. Sci. 9, 576–585 (2018).
Meng, L., Liu, K., Mo, S., Mao, Y. & Yi, T. From G-quartets to G-ribbon gel by concentration and sonication control. Org. Biomol. Chem. 11, 1525–1532 (2013).
Wang, X. et al. Reversible organogels triggered by dynamic K+ binding and release. J. Colloid Interface Sci. 353, 412–419 (2011).
Pieraccini, S., Masiero, S., Pandoli, O., Samorì, P. & Spada, G. P. Reversible interconversion between a supramolecular polymer and a discrete octameric species from a guanosine derivative by dynamic cation binding and release. Org. Lett. 8, 3125–3128 (2006).
Spada, G. P. et al. Guanosine-based hydrogen-bonded scaffolds: controlling the assembly of oligothiophenes. Adv. Mater. 20, 2433–2438 (2008).
Mihai, S., Le Duc, Y., Cot, D. & Barboiu, M. Sol–gel selection of hybrid G-quadruplex architectures from dynamic supramolecular guanosine libraries. J. Mat. Chem. 20, 9443–9448 (2010).
Meffre, A., Petit, E., Cot, D. & Barboiu, M. Kinetic selection of polymeric guanosine architectures from dynamic supramolecular libraries. C. R. Chim. 18, 960–965 (2015).
Zhang, J. et al. GMP-quadruplex-based hydrogels stabilized by lanthanide ions. Sci. China Chem. 61, 604–612 (2018).
Carducci, F., Yoneda, J. S., Itri, R. & Mariani, P. On the structural stability of guanosine-based supramolecular hydrogels. Soft Matter 14, 2938–2948 (2018).
Nava, G. et al. Quadruplex knots as network nodes: nano-partitioning of guanosine derivates in supramolecular hydrogels. Soft Matter 15, 2315–2318 (2019).
Yu, Y., Nakamura, D., DeBoyace, K., Neisius, A. W. & McGown, L. B. Tunable thermoassociation of binary guanosine gels. J. Phys. Chem. B 112, 1130–1134 (2008).
Buerkle, L. E., Li, Z., Jamieson, A. M. & Rowan, S. J. Tailoring the properties of guanosine-based supramolecular hydrogels. Langmuir 25, 8833–8840 (2009).
Adhikari, B., Shah, A. & Kraatz, H.-B. Self-assembly of guanosine and deoxy-guanosine into hydrogels: monovalent cation guided modulation of gelation, morphology and self-healing properties. J. Mat. Chem. B 2, 4802–4810 (2014).
Tang, F. et al. Developing a self-healing supramolecular nucleoside hydrogel based on guanosine and isoguanosine. Chem. Asian. J. 13, 1962–1971 (2018).
Peters, G. M. et al. A G4·K+ hydrogel stabilized by an anion. J. Am. Chem. Soc. 136, 12596–12599 (2014).
Peters, G. M., Skala, L. P. & Davis, J. T. A molecular chaperone for G4-quartet hydrogels. J. Am. Chem. Soc. 138, 134–139 (2016).
Pieraccini, S. et al. Playing supramolecular dominoes with light: building and breaking a photoreversible G-quadruplex made from guanosine, boric acid and an azobenzene. Org. Biomol. Chem. 17, 2759–2769 (2019).
Ghoussoub, A. & Lehn, J.-M. Dynamic sol–gel interconversion by reversible cation binding and release in G-quartet-based supramolecular polymers. Chem. Commun. 46, 5763–5765 (2005).
Buchs, B. et al. Release of bioactive volatiles from supramolecular hydrogels: influence of reversible acylhydrazone formation on gel stability and volatile compound evaporation. Org. Biomol. Chem. 9, 2906–2919 (2011).
Buhler, E., Sreenivasachary, N., Candau, S.-J. & Lehn, J.-M. Modulation of the supramolecular structure of G-quartet assemblies by dynamic covalent decoration. J. Am. Chem. Soc. 129, 10058–10059 (2007).
Sreenivasachary, N. & Lehn, J.-M. Gelation-driven component selection in the generation of constitutional dynamic hydrogels based on guanine-quartet formation. Proc. Natl Acad. Sci. USA 102, 5938-5943 (2005).
Arnal-Hérault, C. et al. Functional G-quartet macroscopic membrane films. Angew. Chem. Int. Ed. 46, 8409–8413 (2007).
Dash, J., Patil, A. J., Das, R. N., Dowdall, F. L. & Mann, S. Supramolecular hydrogels derived from silver ion-mediated self-assembly of 5′-guanosine monophosphate. Soft Matter 7, 8120–8126 (2011).
Feng, H. et al. Silver ions blocking crystallization of guanosine-based hydrogel for potential antimicrobial applications. RSC Adv. 8, 15842–15852 (2018).
Sreenivasachary, N. & Lehn, J.-M. Structural selection in G-quartet-based hydrogels and controlled release of bioactive molecules. Chem. Asian J. 3, 134–139 (2008).
Das, R. N., Kumar, Y. P., Pagoti, S., Patil, A. J. & Dash, J. Diffusion and birefringence of bioactive dyes in a supramolecular guanosine hydrogel. Chem. Eur. J. 18, 6008–6014 (2012).
Plank, T. N. & Davis, J. T. A G4·K+ hydrogel that self-destructs. Chem. Commun. 52, 5037–5040 (2016).
Venkatesh, V. et al. Supramolecular photoactivatable anticancer hydrogels. J. Am. Chem. Soc. 139, 5656–5659 (2017).
Buerkle, L. E., von Recum, H. A. & Rowan, S. J. Toward potential supramolecular tissue engineering scaffolds based on guanosine derivatives. Chem. Sci. 3, 564–572 (2012).
Rotaru, A. et al. G-quartet hydrogels for effective cell growth applications. Chem. Commun. 53, 12668–12671 (2017).
Shendure, J. et al. DNA sequencing at 40: past, present and future. Nature 550, 345–353 (2017).
Chambers, V. S. et al. High-throughput sequencing of DNA G-quadruplex structures in the human genome. Nat. Biotechnol. 33, 877–881 (2015).
Hänsel-Hertsch, R. et al. G-quadruplex structures mark human regulatory chromatin. Nat. Genet. 48, 1267–1272 (2016).
Kwok, C. K., Marsico, G., Sahakyan, A. B., Chambers, V. S. & Balasubramanian, S. rG4-seq reveals widespread formation of G-quadruplex structures in the human transcriptome. Nat. Methods 13, 841–844 (2016).
Yang, S. Y. et al. Transcriptome-wide identification of transient RNA G-quadruplexes in human cells. Nat. Commun. 9, 4730 (2018).
Monchaud, D. & Teulade-Fichou, M.-P. A hitchhiker’s guide to G-quadruplex ligands. Org. Biomol. Chem. 6, 627–636 (2008).
Neidle, S. Quadruplex nucleic acids as targets for anticancer therapeutics. Nat. Rev. Chem. 1, 0041 (2017).
Neidle, S. Quadruplex nucleic acids as novel therapeutic targets. J. Med. Chem. 59, 5987–6011 (2016).
Drygin, D. et al. Anticancer activity of CX-3543: a direct inhibitor of rRNA biogenesis. Cancer Res. 69, 7653–7661 (2009).
Moore, M. J. B. et al. Trisubstituted acridines as G-quadruplex telomere targeting agents. Effects of extensions of the 3,6- and 9-side chains on quadruplex binding, telomerase activity, and cell proliferation. J. Med. Chem. 49, 582–599 (2006).
De Cian, A., DeLemos, E., Mergny, J.-L., Teulade-Fichou, M.-P. & Monchaud, D. Highly efficient G-quadruplex recognition by bisquinolinium compounds. J. Am. Chem. Soc. 129, 1856–1857 (2007).
Rodriguez, R. et al. A novel small molecule that alters shelterin integrity and triggers a DNA-damage response at telomeres. J. Am. Chem. Soc. 130, 15758–15759 (2008).
Stefan, L. et al. DOTASQ as a prototype of nature-inspired G-quadruplex ligand. Chem. Commun. 47, 4992–4994 (2011).
Lejault, P. et al. The scope of application of macrocyclic polyamines beyond metal chelation. Eur. J. Org. Chem. https://doi.org/10.1002/ejoc.201900870 (2019).
Haudecoeur, R., Stefan, L., Denat, F. & Monchaud, D. A model of smart G-quadruplex ligand. J. Am. Chem. Soc. 135, 550–553 (2013).
Newman, M. et al. The G-quadruplex-specific RNA helicase DHX36 regulates p53 pre-mRNA 3′-end processing following UV-induced DNA damage. J. Mol. Biol. 429, 3121–3131 (2017).
Laguerre, A. et al. A twice-as-smart synthetic G-quartet: PyroTASQ is both a smart quadruplex ligand and a smart fluorescent probe. J. Am. Chem. Soc. 136, 12406–12414 (2014).
Zhou, J. et al. Computational understanding and experimental characterization of twice-as-smart quadruplex ligands as chemical sensors of bacterial nucleotide second messengers. Sci. Rep. 6, 33888 (2016).
Laguerre, A. et al. Visualization of RNA-quadruplexes in live cells. J. Am. Chem. Soc. 137, 8521–8525 (2015).
Laguerre, A., Wong, J. M. Y. & Monchaud, D. Direct visualization of both DNA and RNA quadruplexes in human cells via an uncommon spectroscopic method. Sci. Rep. 6, 32141 (2016).
Roux, A. et al. Small-molecule affinity capture of DNA/RNA quadruplexes and their identification in vitro and in vivo through the G4RP protocol. Nucleic Acids Res. 47, 5502–5510 (2019).
Rivera-Sánchez, Md. C., García-Arriaga, M., Hobley, G., Morales-de-Echegaray, A. V. & Rivera, J. M. Small-molecule-based self-assembled ligands for G-quadruplex DNA surface recognition. ACS Omega 2, 6619–6627 (2017).
Pavan Kumar, Y. et al. Fluorescent dansyl-guanosine conjugates that bind c-MYC promoter G-quadruplex and downregulate c-MYC expression. ChemBioChem 17, 388–393 (2016).
Ross, P. et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325, 279–281 (1987).
Monchaud, D. in DNA in Supramolecular Chemistry and Nanotechnology Ch. 3.5 (eds Stulz, E. & Clever, G. H.) 229-246 (Wiley, 2015).
Gilli, G., Bellucci, F., Ferretti, V. & Bertolasi, V. Evidence for resonance-assisted hydrogen bonding from crystal-structure correlations on the enol form of the. beta.-diketone fragment. J. Am. Chem. Soc. 111, 1023–1028 (1989).
Fonseca Guerra, C., Zijlstra, H., Paragi, G. & Bickelhaupt, F. M. Telomere structure and stability: covalency in hydrogen bonds, not resonance assistance, causes cooperativity in guanine quartets. Chem. Eur. J. 17, 12612–12622 (2011).
Davis, J. T. & Spada, G. P. Supramolecular architectures generated by self-assembly of guanosine derivatives. Chem. Soc. Rev. 36, 296–313 (2007).
Dahm, R. Discovering DNA: Friedrich Miescher and the early years of nucleic acid research. Hum. Genet. 122, 565–581 (2008).
Avery, O. T., MacLeod, C. M. & McCarty, M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J. Exp. Med. 79, 137–158 (1944).
Crick, F. H. On protein synthesis. Symp. Soc. Exp. Biol. 12, 138–163 (1958).
Rodriguez, R. et al. Small-molecule-induced DNA damage identifies alternative DNA structures in human genes. Nat. Chem. Biol. 8, 301–310 (2012).
Biffi, G., Tannahill, D., McCafferty, J. & Balasubramanian, S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 5, 182–186 (2013).
Acknowledgements
L.S. and D.M. thank the Centre National de la Recherche Scientifique (CNRS) for funding. D.M. thanks the Agence Nationale de la Recherche (ANR-17-CE17-0010-01), the European Research Council (H2020-MSCA-IF-2016-750368), the Université de Bourgogne, Conseil Régional de Bourgogne (PARI) and the European Union (Pharmaco-imagerie et agents théranostiques, PO FEDER-FSE Bourgogne 2014–2020 programme) for financial support. The authors also thank their collaborators for the daily passionate scientific discussions and all scientists worldwide involved in the fascinating field of research, to make it lively, thrilling and always moving.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- G4ome
-
the G4ome comprises all G-quadruplex (G4)-forming sequences identified in the genome (G4-DNA) and both the coding and non-coding transcriptome (G4-RNA).
- LipoG
-
lipophilic guanine derivatives aimed at being used in organic solvents, masking hydrophilic moieties (e.g. the hydroxyl groups of the guanosine’s ribose) through the use of aliphatic protecting groups.
- c-di-GMP
-
3,3′-cyclic diguanylic acid discovered by Benziman and colleagues in 1987 (ref.151), originally as a regulator of cellulose production in bacteria and now known as a regulator of virulence factor production and biofilm formation.
- G4-ligands
-
low-molecular-weight molecules designed to interact specifically with DNA/RNA quadruplexes (G4s), mostly through π-stacking interactions with the G4’s accessible external quartets, but groove and loop binding has also been documented.
- Fluorescence upconversion
-
this fluorescence spectroscopy (also known as sum frequency generation), is a nonlinear, high-resolution technique characterized by an emission wavelength that is shorter than the excitation wavelength.
- Organic frameworks
-
three-dimensional organic structures in which building blocks are associated either through covalent bonds (covalent organic framework, or COF) or metal-mediated coordination (metal organic framework, or MOF).
- Logic gates
-
in electronics, a logic gate is a device that performs a logical operation on one or two inputs and produces a single output. Logic gates are primarily implemented as switches of different possible natures (AND, OR, NOT, INHIBIT etc.) in digital circuits.
- Lyotropic liquid crystal
-
property of an amphiphilic material (mesogen) that exhibits phase transitions upon dissolution in a suited solvent, in a concentration-dependent manner.
- Storage moduli
-
indications of the ability of the gel to withstand and store deformation energy in an elastic manner, being dependent on its crosslinking state.
- Thixotropy
-
property of a material (gel, fluid), which is thick or viscous under static conditions, to become thin and liquid when shaken or sheared.
Rights and permissions
About this article
Cite this article
Stefan, L., Monchaud, D. Applications of guanine quartets in nanotechnology and chemical biology. Nat Rev Chem 3, 650–668 (2019). https://doi.org/10.1038/s41570-019-0132-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-019-0132-0
This article is cited by
-
Programmable supramolecular chirality in non-equilibrium systems affording a multistate chiroptical switch
Nature Communications (2023)
-
In Vitro Antiproliferative Activity and Phytochemicals Screening of Extracts of the Freshwater Microalgae, Chlorochromonas danica
Applied Biochemistry and Biotechnology (2023)
-
Global mapping of RNA G-quadruplexes (G4-RNAs) using G4RP-seq
Nature Protocols (2022)