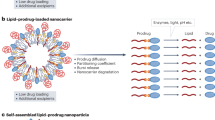
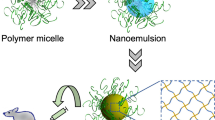
Abstract
Some Ru complexes have extremely promising anticancer or antibacterial properties, but the poor H2O solubility and/or low stability of many Ru complexes in aqueous solution under physiological conditions and/or metabolic or biodistribution profiles prevent their therapeutic use. To overcome these drawbacks, various strategies have been developed to improve the delivery of these compounds to their target tissues. The first strategy is based on physical encapsulation of Ru complexes in carriers, such as polymeric micelles, microparticles, nanoparticles and polymer–lipid hybrids, which enables the delivery and controlled release of the active Ru drug candidate. The second strategy involves covalent conjugation of the Ru complex to a polymer to give a prodrug that can be converted into the active drug at a more controllable rate. In this Review, we provide an overview of recent developments in polymer encapsulation of Ru complexes for biological and medicinal applications. We place particular emphasis on how polymer structure affects Ru delivery.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Grimley, B. & Lansing, E. The inhibition of growth or cell division in Escherichia by different ionic species of platinum(iv) complexes. J. Biol. Chem. 242, 1347–1352 (1967).
Galluzzi, L. et al. Molecular mechanisms of cisplatin resistance. Oncogene 31, 1869–1883 (2012).
Poynton, F. E. et al. The development of ruthenium polypyridyl complexes and conjugates for in vitro cellular and in vivo applications. Chem. Soc. Rev. 46, 5771–5804 (2017).
Notaro, A. & Gasser, G. Monomeric and dimeric coordinatively saturated and substitutionally inert Ru polypyridyl complexes as anticancer drug candidates. Chem. Soc. Rev. 46, 7317–7337 (2017).
Antonarakis, E. S. & Emadi, A. Ruthenium-based chemotherapeutics: are they ready for prime time? Cancer Chemother. Pharmacol. 66, 1–9 (2010).
Levina, A., Mitra, A. & Lay, P. A. Recent developments in ruthenium anticancer drugs. Metallomics 1, 458–470 (2009).
Kostova, I. Ruthenium complexes as anticancer agents. Curr. Med. Chem. 13, 1085–1107 (2006).
Li, F., Collins, J. G. & Keene, F. R. Ruthenium complexes as antimicrobial agents. Chem. Soc. Rev. 44, 2529–2542 (2015).
Meggers, E. From conventional to unusual enyzme inhibitor scaffolds: the quest for target specificity. Angew. Chem. Int. Ed. 50, 2442–2448 (2011).
Meggers, E. Targeting proteins with metal complexes. Chem. Commun. 7, 1001–1010 (2009).
Kilpin, K. J. & Dyson, P. J. Enzyme inhibition by metal complexes: concepts, strategies and applications. Chem. Sci. 4, 1410–1419 (2013).
Mari, C., Pierroz, V., Ferrari, S. & Gasser, G. Combination of Ru complexes and light: new frontiers in cancer therapy. Chem. Sci. 6, 2660–2686 (2015).
Heinemann, F., Karges, J. & Gasser, G. Critical overview of the use of Ru(ii) polypyridyl complexes as photosensitizers in one-photon and two-photon photodynamic therapy. Acc. Chem. Res. 50, 2727–2736 (2017).
Knoll, J. D. & Turro, C. Control and utilization of ruthenium and rhodium metal complex excited states for photoactivated cancer therapy. Coord. Chem. Rev. 282–283, 110–126 (2015).
Shi, G. et al. Ru(ii) dyads derived from α-oligothiophenes: a new class of potent and versatile photosensitizers for PDT. Coord. Chem. Rev. 282–283, 127–138 (2015).
Monro, S. et al. Transition metal complexes and photodynamic therapy from a tumor-centered approach: challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 119, 797–828 (2019).
Bastos, C. M., Gordon, K. A. & Ocain, T. D. Synthesis and immunosuppressive activity of ruthenium complexes. Bioorg. Med. Chem. Lett. 8, 147–150 (1998).
Gill, M. R. & Thomas, J. A. Ruthenium(ii) polypyridyl complexes and DNA—from structural probes to cellular imaging and therapeutics. Chem. Soc. Rev. 41, 3179–3192 (2012).
Puckett, C. A., Ernst, R. J. & Barton, J. K. Exploring the cellular accumulation of metal complexes. Dalton Trans. 39, 1159–1170 (2010).
Boynton, A. N., Marce, L. & Barton, J. K. [Ru(Me4phen)2dppz]2+, a light switch for dna mismatches. J. Am. Chem. Soc. 138, 5020–5023 (2016).
Martí, A. A. et al. Inorganic–organic hybrid luminescent binary probe for DNA detection based on spin-forbidden resonance energy transfer. J. Am. Chem. Soc. 129, 8680–8681 (2007).
Dwyer, F. P., Gyarfas, E. C., Rogers, W. P. & Koch, J. H. Biological activity of complex ions. Nature 170, 190–191 (1952).
Clarke, M. J. Oncological implications of the chemistry of ruthenium. Met. Ions. Biol. Syst. 11, 231–283 (1980).
Allardyce, C. S. & Dyson, P. J. Ruthenium in medicine: current clinical uses and future prospects. Platin. Met. Rev. 45, 62–69 (2001).
Allardyce, C. S., Dorcier, A., Scolaro, C. & Dyson, P. J. Development of organometallic (organo-transition metal) pharmaceuticals. Appl. Organomet. Chem. 19, 1–10 (2005).
Clarke, M. J. Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 232, 69–93 (2002).
Schluga, P. et al. Redox behavior of tumor-inhibiting ruthenium(iii) complexes and effects of physiological reductants on their binding to GMP. Dalton Trans. 14, 1796–1802 (2006).
Henning, T., Kraus, M., Brischwein, M., Otto, A. M. & Wolf, B. Relevance of tumor microenvironment for progression, therapy and drug development. Anticancer Drugs 15, 7–14 (2004).
Reisner, E., Arion, V. B., Keppler, B. K. & Pombeiro, A. J. L. Electron-transfer activated metal-based anticancer drugs. Inorg. Chim. Acta 361, 1569–1583 (2008).
Clarke, M. J., Bitier, S., Rennert, D. & Buchbinder, M. Reduction and subsequent binding of ruthenium ions catalyzed by subcellular components. J. Inorg. Biochem. 12, 79–87 (1980).
Allardyce, C. S., Dyson, P. J., Ellis, D. J. & Heath, S. L. [Ru(η6-p-cymene)Cl2(pta)] (pta=1,3,5-triaza-7-phosphatricyclo[3.3.1.1]decane): a water soluble compound that exhibits pH dependent DNA binding providing selectivity for diseased cells. Chem. Commun. 2, 1396–1397 (2001).
Scolaro, C. et al. In vitro and in vivo evaluation of ruthenium(ii)-arene PTA complexes. J. Med. Chem. 48, 4161–4171 (2005).
Bergamo, A. et al. Modulation of the metastatic progression of breast cancer with an organometallic ruthenium compound. Int. J. Oncol. 33, 1281–1289 (2008).
Aird, R. E. et al. In vitro and in vivo activity and cross resistance profiles of novel ruthenium(ii) organometallic arene complexes in human ovarian cancer. Br. J. Cancer 86, 1652–1657 (2002).
Wang, F. et al. Kinetics of aquation and anation of ruthenium(ii) arene anticancer complexes, acidity and X-ray structures of aqua adducts. Chem. Eur. J. 9, 5810–5820 (2003).
Bacac, M. et al. The hydrolysis of the anti-cancer ruthenium complex NAMI-A affects its DNA binding and antimetastatic activity: an NMR evaluation. J. Inorg. Chem. 98, 402–412 (2004).
Chatlas, J., van Eldik, R. & Keppler, B. K. Spontaneous aquation reactions of a promising tumor inhibitor trans-imidazolium-tetrachlorobis(imidazole)ruthenium(iii). trans-HIm[RuCl4(Im)2]. Inorg. Chim. Acta 233, 59–63 (1995).
Dhubhghaill, O. M. N., Hagen, W. R., Keppler, B. K., Lipponerc, K. & Sadler, P. J. Aquation of the anticancer complex trans-[RuCI4(Him)2] - (Him = imidazole). J. Chem. Soc. Dalton Trans. 0, 3305–3310 (1994).
Debreczeni, J. É. et al. Ruthenium half-sandwich complexes bound to protein kinase Pim-1. Angew. Chem. Int. Ed. 45, 1580–1585 (2006).
Reedijk, J. New clues for platinum antitumor chemistry: Kinetically controlled metal binding to DNA. Proc. Natl Acad. Sci. USA 100, 3611–3616 (2003).
Yamada, H., Koike, T. & Hurst, J. K. Water exchange rates in the diruthenium μ-oxo ion cis. cis-[(bpy)2Ru(OH2)]2O4+. J. Am. Chem. Soc. 123, 12775–12780 (2001).
Klausner, R. D. et al. Receptor-mediated endocytosis of transferrin in K562 cells. J. Am. Chem. Soc. 258, 4715–4724 (1983).
Singh, M. Transferrin as a targeting ligand for liposomes and anticancer drugs. Curr. Pharm. Des. 5, 443–451 (1999).
Alessio, E. Thirty years of the drug candidate NAMI-A and the myths in the field of ruthenium anticancer compounds: a personal perspective. Eur. J. Inorg. Chem. 12, 1549–1560 (2017).
Suss-Fink, G. Arene ruthenium complexes as anticancer agents. Dalton Trans. 39, 1673–1688 (2010).
Pongratz, M. et al. Transferrin binding and transferrin-mediated cellular uptake of the ruthenium coordination compound KP1019, studied by means of AAS, ESI-MS and CD spectroscopy. J. Anal. At. Spectrom. 19, 46–51 (2004).
Thota, S., Rodrigues, D. A., Crans, D. C. & Barreiro, E. J. Ru(ii) compounds: next-generation anticancer metallotherapeutics? J. Med. Chem. 61, 5805–5821 (2018).
Bergamo, A., Messori, L., Piccioli, F., Cochietto, M. & Sava, G. Biological role of adduct formation of the ruthenium(iii) complex NAMI-A with serum albumin and serum transferrin. Invest. New Drugs 21, 401–411 (2003).
Hartinger, C. G. et al. From bench to bedside — preclinical and early clinical development of the anticancer agent indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(iii)] (KP1019 or FFC14A). J. Inorg. Biochem. 100, 891–904 (2006).
Hartinger, G., Jakupec, M. A., Zorbas-Seifried, S. & Groessl, M. KP1019, a new redox-active anticancer agent — preclinical development and results of a clinical phase i study in tumor patients. Chem. Biodivers. 5, 2140–2155 (2008).
Heffeter, P., Atil, B., Kryeziu, K. & Groza, D. The ruthenium compound KP1339 potentiates the anticancer activity of sorafenib in vitro and in vivo. Eur. J. Cancer. 15, 3366–3375 (2013).
Bytzek, A. K., Koellensperger, G., Keppler, B. K. & Hartinger, G. Biodistribution of the novel anticancer drug sodium trans-[tetrachloridobis(1H-indazole)ruthenate(iii)] KP-1339/IT139 in nude BALB/c mice and implications on its mode of action. J. Inorg. Biochem. 160, 250–255 (2016).
Fong, J. et al. A novel class of ruthenium-based photosensitizers effectively kills in vitro cancer cells and in vivo tumors. Photochem. Photobiol. Sci. 14, 2014–2023 (2015).
Larson, N. & Ghandehari, H. Polymeric conjugates for anti-cancer drug delivery. Chem. Mater. 24, 840–853 (2012).
Zeng, L. et al. The development of anticancer ruthenium complexes: from single molecule compounds to nanomaterials. Chem. Soc. Rev. 46, 5571–5804 (2017). This article is a complete review with an overview of anticancer Ru(ii) complexes and an introduction for Ru(ii)-based nanomaterials systems.
Ulbrich, K. et al. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 116, 5338–5431 (2016).
Langer, R. & Tirrell, D. A. Designing materials for biology and medicine. Nature 428, 487–492 (2004).
Lenz, R. W. Biodegradable polymers. Adv. Polym. Sci. 107, 1–40 (1993).
Albertsson, A. C. & Karlsson, S. in Chemistry and Technology of Biodegradable Polymers (ed. Griffin, G.) 7–17 (Springer Netherlands, 1994).
Li, S. & Vert, M. in Degradable Polymers: Principles and Applications (eds Scott, G. & Gilead, D.) 43–87 (Chapman & Hall, London, 1995).
Kopeček, J. & Ulbrich, K. Biodegradation of biomedical polymers. Prog. Polym. Sci. 9, 1–58 (1983).
Albertsson, A. C. & Varma, I. K. Aliphatic polyesters: synthesis, properties and applications. Adv. Polym. Sci. 157, 1–40 (2002).
Matlaga, B. F., Yasenchak, L. P. & Salthouse, T. N. Tissue response to implanted polymers: the significance of sample shape. J. Biomed. Mater. Res. 10, 391–397 (1976).
Liechty, W. B., Kryscio, D. R., Slaughter, B. V. & Peppas, N. A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 1, 149–173 (2010).
Esser-Kahn, A. P., Odom, S. A., Sottos, N. R., White, S. R. & Moore, J. S. Triggered release from polymer capsules. Macromolecules 44, 5539–5553 (2011).
Kamaly, N., Yameen, B., Wu, J. & Farokhzad, O. C. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 116, 2602–2663 (2016).
Wong, P. T. & Choi, S. K. Mechanisms of drug release in nanotherapeutic delivery systems. Chem. Rev. 115, 3388–3432 (2015).
Holohan, C., Schaeybroeck, Van, S., Longley, D. B. & Johnston, P. G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer 13, 714–726 (2013).
Blair, J. M., Webber, M. A., Baylay, A. J., Ogbolu, D. O. & Piddock, L. J. V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 13, 42–51 (2014).
Maeda, H., Bharate, G. Y. & Daruwalla, J. Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect. Eur. J. Pharm. Biopharm. 71, 409–419 (2009).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agents Smancs. Cancer Res. 46, 6387–6392 (1986).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284 (2000).
Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 41, 189–207 (2001).
Maeda, H., Fang, J., Inutsuka, T. & Kitamoto, Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implication. Int. Immunopharmacol. 3, 319–328 (2003).
Lammers, T., Kiessling, F., Hennink, W. E. & Storm, G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J. Control. Release 161, 175–187 (2012).
Prabhakar, U., Blakey, D. C. & Maeda, H. Challenges and key considerations of the enhanced permeability and retention effect (EPR) for nanomedicine drug delivery in oncology. Cancer Res. 15, 2412–2417 (2013).
Cheng, L., Wang, C. & Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 114, 10869–10939 (2014).
Prabhu, R. H., Patravale, V. B. & Joshi, M. D. Polymeric nanoparticles for targeted treatment in oncology: current insights. Int. J. Nanomed. 10, 1001–1018 (2015).
Portney, N. G. & Ozkan, M. Nano-oncology: drug delivery, imaging, and sensing. Anal. Bioanal. Chem. 384, 620–630 (2006).
Shastri, V. P. Non-degradable biocompatible polymers in medicine: past, present and future. Curr. Pharm. Biotechnol. 4, 331–337 (2003).
Svenson, S. Dendrimers as versatile platform in drug delivery applications. Eur. J. Pharm. Biopharm. 71, 445–462 (2009).
Nair, L. S. & Laurencin, C. T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32, 762–798 (2007).
Hofmann, D., Entrialgo-Castaño, M., Kratz, K. & Lendlein, A. Knowledge-based approach towards hydrolytic degradation of polymer-based biomaterials. Adv. Mater. 21, 3237–3245 (2009).
Allison, S. D. Effect of structural relaxation on the preparation and drug release behavior of poly(lactic-co-glycolic) acid microparticle drug delivery systems. J. Pharm. Sci. 97, 2022–2035 (2008).
Thomas, C. M. & Lutz, J. F. Precision synthesis of biodegradable polymers. Angew. Chem. Int. Ed. 50, 9244–9246 (2011).
Bader, H., Ringsdorf, H. & Schmidt, B. Water soluble polymers in medicine. Macromol. Mater. Eng. 123, 457–485 (1984).
Kopeček, J. Soluble biomedical polymers. Polym. Med. 7, 191–221 (1977).
Yokoyama, M. Clinical applications of polymeric micelle carrier systems in chemotherapy and image diagnosis of solid tumors. J. Exp. Clin. Med. 3, 151–158 (2011).
Choi, H. S. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25, 1165–1170 (2007).
Douliez, J.-P., Navailles, L. & Nallet, F. Self-assembly of fatty acid-alkylboladiamine salts. Langmuir 22, 622–627 (2006).
Nishiyama, N. & Kataoka, K. Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol. Ther. 112, 630–648 (2006).
Torchilin, V. P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 73, 137–172 (2001).
Evans, F. D. & Wennerström, H. The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet 2nd edn (Wiley-Blackwell, 1999).
Nicolas, J., Mura, S., Brambilla, D., Mackiewicz, N. & Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 42, 1147–1235 (2013).
Mora-huertas, C. E., Fessi, H. & Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 385, 113–142 (2010).
Sant, V. P., Smith, D. & Leroux, J. Novel pH-sensitive supramolecular assemblies for oral delivery of poorly water soluble drugs: preparation and characterization. J. Control. Release 97, 301–312 (2004).
Hu, M., Zhu, J. & Qiu, L. Y. Polymer micelle-based combination therapy of paclitaxel and resveratrol with enhanced and selective antitumor activity. RSC Adv. 4, 64151–64161 (2014).
Tyrrell, Z. L., Shen, Y. & Radosz, M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog. Polym. Sci. 35, 1128–1143 (2010).
Newcome, G. R., Moorefield, C. N. & Vögtle, F. Dendritic Molecules: Concepts, Synthesis, Prespectives (VCH-Weinheim, 1996).
Dvornic, P. R. & Tomalia, D. A. Recent advances in dendritic polymers. Curr. Opin. Colloid Interface Sci. 1, 221–235 (1996).
Letchford, K. & Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 65, 259–269 (2007).
Slomkowski, S. Functionalized biodegradable nano- and microspheres for medical applications. Macromol. Symp. 288, 121–129 (2010).
Rabea, E. I., Badawy, M. E. T., Stevens, C. V., Smagghe, G. & Steurbaut, W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4, 1457–1465 (2003).
Muxika, A., Etxabide, A., Uranga, J., Guerrero, P. & de la Caba, K. Chitosan as a bioactive polymer: processing, properties and applications. Int. J. Biol. Macromol. 105, 1358–1368 (2017).
Tsvirko, M., Tkaczyk, S., Kozak, M. & Kalota, B. Luminescent temperature sensor based on [Ru(bpy)3]2+ incorporated into chitosan. Funct. Mater. 20, 127–132 (2013).
Tønnesen, H. H. & Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 28, 621–630 (2002).
Heathman, T. R. J. et al. The translation of cell-based therapies: clinical landscape and challenges. Regen. Med. 10, 49–64 (2015).
Mount, N. M., Ward, S. J., Kefalas, P. & Hyllner, J. Cell-based therapy technology classifications and translational challenges. Phil. Trans. R. Soc. B 370, 20150017 (2015).
Kumar, S., Kumar, R., Ratnam, A., Mishra, N. C. & Ghosh, K. Novel drug delivery system for photoinduced nitric oxide (NO) delivery. Inorg. Chem. Commun. 53, 23–25 (2015).
Soppimath, K. S., Aminabhavi, T. M., Kulkarni, A. R. & Rudzinski, W. E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 70, 1–20 (2001).
Panyam, J. & Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 55, 329–347 (2003).
Hans, M. & Lowman, A. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 6, 319–327 (2002).
Zolnik, B. S. & Burgess, D. J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 122, 338–344 (2007).
Mishra, B., Patel, B. B. & Tiwari, S. Colloidal nanocarriers: a review on formulation technology, types and applications toward targeted drug delivery. Nanomedicine 6, 9–24 (2010).
Fischer, B. et al. Poly(lactic acid) nanoparticles of the lead anticancer ruthenium compound KP1019 and its surfactant-mediated activation. Dalton Trans. 43, 1096–1104 (2014).
Kerwin, B. A., Kerwin, B. A. & Kerwin, B. A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J. Pharm. Sci. 97, 2924–2935 (2008).
Boeuf, G. et al. Encapsulated ruthenium(ii) complexes in biocompatible poly(D, L-lactide-co-glycolide) nanoparticles for application in photodynamic therapy. Chempluschem 79, 171–180 (2014).
Gomes, A. J., Barbougli, P. A., Espreafico, E. M. & Tfouni, E. Trans-[Ru(NO)(NH3)4(py)](BF4)3∙H2O encapsulated in PLGA microparticles for delivery of nitric oxide to B16-F10 cells: cytotoxicity and phototoxicity. J. Inorg. Biochem. 102, 757–766 (2008).
Gomes, A. J., Espreafico, E. M. & Tfouni, E. Trans-[Ru(NO)Cl(cyclam)](PF6)2 and [Ru(NO)(Hedta)] incorporated in PLGA nanoparticles for the delivery of nitric oxide to B16-F10 cells: cytotoxicity and phototoxicity. Mol. Pharm. 10, 3544–3554 (2013).
de Souza Oliveira, F. et al. Development of biodegradable nanoparticles containing trans-RuCl([15]ane)(NO)]2+ as nitric oxide donor. Trends Inorg. Chem. 10, 27–34 (2008).
Bohlender, C., Landfester, K., Crespy, D. & Schiller, A. Unconventional non-aqueous emulsions for the encapsulation of a phototriggerable NO-donor complex in polymer nanoparticles. Part. Part. Syst. Charact. 30, 138–142 (2013).
Bohlender, C. et al. Light-triggered NO release from a nanofibrous non-woven. J. Mater. Chem. 22, 8785–8792 (2012).
Moreno, M. J. et al. Production of singlet oxygen by Ru(dpp(SO3)2)3 incorporated in polyacrylamide PEBBLES. Sens Actuators B Chem. 90, 82–89 (2003).
Yin, H., Fang, J., Liao, L., Nakamura, H. & Maeda, H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J. Control. Release 187, 14–21 (2014).
Dickerson, M., Howerton, B., Bae, Y. & Glazer, E. Light-sensitive ruthenium complex-loaded cross-linked polymeric nanoassemblies for the treatment of cancer. J. Mater. Chem. B. 4, 394–408 (2016).
Appold, M. et al. Multi-stimuli responsive block copolymers as a smart release platform for a polypyridyl ruthenium complex. Polym. Chem. 8, 890–900 (2017).
Shachaf, Y., Gonen-Wadmany, M. & Seliktar, D. The biocompatibility of Pluronic®F127 fibrinogen-based hydrogels. Biomaterials 31, 2836–2847 (2010).
Batrakova, E. V. & Kabanov, A. V. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J. Control. Release 130, 98–106 (2008).
Riess, G. Micellization of block copolymers. Prog. Polym. Sci. 28, 1107–1170 (2003).
Khan, A. A., Fullerton-Shirey, S. K. & Howard, S. S. Easily prepared ruthenium-complex nanomicelle probes for two-photon quantitative imaging of oxygen in aqueous media. RSC Adv. 5, 291–300 (2015).
Papkovsky, D. B. & Dmitriev, R. I. Biological detection by optical oxygen sensing. Chem. Soc. Rev. 42, 8700 (2013).
Barry, N. P. E. et al. Precious metal carborane polymer nanoparticles: characterisation of micellar formulations and anticancer activity. Faraday Discuss. 175, 229–240 (2014).
Gregoriadis, G. The carrier potential of liposomes in biology and medecine. N. Engl. J. Med. 295, 765–770 (1976).
Gao, W., Hu, C.-M., Ronnie, J., Fang, H. & Zhang, L. Liposome-like nanostructures for drug delivery. J. Mater. Chem. B 1, 6569–6585 (2013).
Kasera, N. K., Sharma, P. K. & Gupta, R. Recent advancement and patents of the lipid polymer hybrid nanoparticles. Peertechz J. Med. Chem. Res. 2, 25–29 (2016).
Maranho, D. S., De Lima, R. G., Primo, F. L., Da Silva, R. S. & Tedesco, A. C. Photoinduced nitric oxide and singlet oxygen release from ZnPC liposome vehicle associated with the nitrosyl ruthenium complex: synergistic effects in photodynamic therapy application. Photochem. Photobiol. 85, 705–713 (2009).
de Lima, R. G., Tedesco, A. C., da Silva, R. S. & Lawrence, M. J. Ultradeformable liposome loaded with zinc phthalocyanine and [Ru(NH.NHq)(tpy)NO]3+ for photodynamic therapy by topical application. Photodiagnosis Photodyn. Ther. 19, 184–193 (2017).
Lim, W. H. & Lawrence, M. J. Influence of surfactant and lipid chain length on the solubilisation of phosphatidylcholine vesicles by micelles comprised of polyoxyethylene sorbitan monoesters. Colloids Surf. A Physicochem. Eng. Asp. 250, 449–457 (2004).
Simeone, L. et al. Nucleolipid nanovectors as molecular carriers for potential applications in drug delivery. Mol. Biosyst. 7, 3075–3086 (2011).
Mangiapia, G. et al. Ruthenium-based complex nanocarriers for cancer therapy. Biomaterials 33, 3770–3782 (2012).
Mangiapia, G. et al. Anticancer cationic ruthenium nanovectors: from rational molecular design to cellular uptake and bioactivity. Biomacromolecules 14, 2549–2560 (2013).
Montesarchio, D. et al. A new design for nucleolipid-based Ru(iii) complexes as anticancer agents. Dalton Trans. 42, 16697–16708 (2013). This study uses a polymer–lipid hybrid as a nanovehicle for the delivery of a Ru complex. The decoration of the surface of the ‘liposome’ is achieved by self-assembly and incorporation of an amphiphilic Ru complex in the lipid membrane.
Askes, S. H. C., Meijer, M. S., Bouwens, T., Landman, I. & Bonnet, S. Red light activation of Ru(ii) polypyridyl prodrugs via triplet–triplet annihilation upconversion: feasibility in air and through meat. Molecules 21, E1460 (2016).
Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Polym. Symp. 51, 135–153 (2007).
Fuertges, F. & Abuchowski, A. The clinical efficacy of poly(ethylene glycol)-modified proteins. J. Control. Release 11, 139–148 (1990).
Duncan, R., Lloyd, J. B. & Kopec˘ek, J. Degradation of side chains of N-(2 hydroxypropyl) methacrylamide copolymers by lysosomal enzymes. Biochem. Biophys. Res. Commun. 94, 284–290 (1980).
Duncan, R. Soluble synthetic polymers as potential drug carriers. Adv. Polym. Sci. 57, 53–100 (1984).
Hasegawa, U., Van Der Vlies, A. J., Simeoni, E., Wandrey, C. & Hubbell, J. A. Carbon monoxide-releasing micelles for immunotherapy. J. Am. Chem. Soc. 132, 18273–18280 (2010). The study presents the first example of CO delivery using a Ru-based metallopolymer.
Nguyen, D., Nguyen, T. K., Rice, S. A. & Boyer, C. CO-releasing polymers exert antimicrobial activity. Biomacromolecules 16, 2776–2786 (2015).
Nguyen, D., Adnan, N. N. M., Oliver, S. & Boyer, C. The Interaction of CORM-2 with block copolymers containing poly(4-vinylpyridine): macromolecular scaffolds for carbon monoxide delivery in biological systems. Macromol. Rapid Commun. 37, 739–744 (2016).
Halpenny, G. M., Olmstead, M. M. & Mascharak, P. K. Incorporation of a designed ruthenium nitrosyl in PolyHEMA hydrogel and light-activated delivery of NO to myoglobin. Inorg. Chem. 46, 6601–6606 (2007).
Xu, L. Q. Ruthenium(ii)-terpyridine complexes-containing glyconanoparticles for one- and two-photon excited fluorescence imaging. Eur. Polym. J. 71, 279–288 (2015).
Wang, Y. et al. Nanoparticles of chitosan conjugated to organo-ruthenium complexes. Inorg. Chem. Front. 3, 1058–1064 (2016).
Vadivel, T. & Dhamodaran, M. Synthesis, characterization and antibacterial studies of ruthenium(iii) complexes derived from chitosan schiff base. Int. J. Biol. Macromol. 90, 44–52 (2016).
Sharma, R. et al. Ruthenium tris(2-pyridylmethyl)amine as an effective photocaging group for nitriles. Inorg. Chem. 53, 3272–3274 (2014).
Li, A., Turro, C. & Kodanko, J. J. Ru(ii) polypyridyl complexes as photocages for bioactive compounds containing nitriles and aromatic heterocycles. Chem. Commun. 54, 1280–1290 (2018).
Sun, W. et al. An amphiphilic ruthenium polymetallodrug for combined photodynamic therapy and photochemotherapy in vivo. Adv. Mater. 29, 1603702 (2017).
Sun, W. et al. Ruthenium-containing block copolymer assemblies: red-light-responsive metallopolymers with tunable nanostructures for enhanced cellular uptake and anticancer phototherapy. Adv. Healthc. Mater. 5, 467–473 (2016).
Rapp, T. L., Highley, C. B., Manor, B. C., Burdick, J. A. & Dmochowski, I. J. Ruthenium-crosslinked hydrogels with rapid, visible-light degradation. Chem. Eur. J. 24, 2328–2333 (2018).
Corbin, P. S., Webb, M. P., McAlvin, J. E. & Fraser, C. L. Biocompatible polyester macroligands: New subunits for the assembly of star-shaped polymers with luminescent and cleavable metal cores. Biomacromolecules 2, 223–232 (2001).
Nawaby, A. V., Farah, A. A., Liao, X., Pietro, W. J. & Day, M. Biodegradable open cell foams of telechelic poly(ε-caprolactone) macroligand with ruthenium(ii) chromophoric subunits via sub-critical CO2 processing. Biomacromolecules 6, 2458–2461 (2005).
Valente, A. et al. First polymer ‘ruthenium-cyclopentadienyl’ complex as potential anticancer agent. J. Inorg. Biochem. 127, 79–81 (2013).
Johnson, R. M. & Fraser, C. L. Metalloinitiation routes to biocompatible poly(lactic acid) and poly(acrylic acid) stars with luminescent ruthenium tris(bipyridine) cores. Biomacromolecules 5, 580–588 (2004). This study uses a Ru(ii) complex as a metalloinitiator for the ring-opening polymerization of esters.
Neu, M., Fischer, D. & Kissel, T. Recent advances in rational gene transfer vector design based on poly(ethylene imine) and its derivatives. J. Gene Med. 7, 992–1009 (2005).
Kircheis, R., Wightman, L. & Wagner, E. Design and gene delivery activity of modified polyethylenimines. Adv. Drug Deliv. Rev. 53, 341–358 (2001).
Fiore, G. L. et al. Ruthenium(ii) tris(bipyridine)-centered poly(ethylenimine) for gene delivery. Biomacromolecules 8, 2829–2835 (2007).
Mitchell-Koch, J. T., Reed, T. M. & Borovik, A. S. Light-activated transfer of nitric oxide from a porous material. Angew. Chem. Int. Ed. 43, 2806–2809 (2004). The study provides the first example of NO photorelease from a polymer-encapsulated Ru complex.
Libera, M. et al. Amphiphilic dendritic copolymers of tert-butyl-glycidylether and glycidol as a nanocontainer for an anticancer ruthenium complex. J. Polym. Sci. A Polym. Chem. 52, 3488–3497 (2014).
Govender, P. et al. Anticancer activity of multinuclear arene ruthenium complexes coordinated to dendritic polypyridyl scaffolds. J. Organomet. Chem. 694, 3470–3476 (2009). This study presents an example of multi-Ru dendrimers and the relationship between the size and the toxicity of metallodendrimers.
Govender, P. et al. Antiproliferative activity of chelating N,O− and N,N-ruthenium(ii)arene functionalised poly(propyleneimine) dendrimer scaffolds. Dalton Trans. 40, 1158–1167 (2011).
Govender, P. et al. First- and second-generation heterometallic dendrimers containing ferrocenyl−ruthenium(ii)−arene motifs: synthesis, structure, electrochemistry, and preliminary cell proliferation studies. Organometallics 33, 5535–5545 (2014).
Govender, P. et al. The influence of RAPTA moieties on the antiproliferative activity of peripheral-functionalised poly(salicylaldiminato) metallodendrimers. Dalton Trans. 4, 1267–1277 (2013).
Ruggi, A. et al. Dendritic ruthenium(ii)-based dyes tuneable for diagnostic or therapeutic applications. Chem. Eur. J. 17, 464–467 (2011).
Benini, P. G. Z., McGarvey, B. R. & Franco, D. W. Functionalization of PAMAM dendrimers with [Ruiii(edta)(H2O)]−. Nitric Oxide 19, 245–251 (2008).
Duncan, R. & Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 57, 2215–2237 (2005).
Armspach, D., Cattalini, M., Constable, E. C., Housecroft, C. E. & Phillips, D. Boron-rich metallodendrimers — mix-and-match assembly of multifunctional metallosupramolecules. Chem. Commun. 1823–1824 (1996).
Housecroft, C. E. Icosahedral building blocks: towards dendrimers with twelve primary branches? Angew. Chem. Int. Ed. 38, 2717–2719 (1999).
Callari, M., Aldrich-Wright, J. R., De Souza, P. L. & Stenzel, M. H. Polymers with platinum drugs and other macromolecular metal complexes for cancer treatment. Prog. Polym. Sci. 39, 1614–1643 (2014).
Oberoi, H. S., Nukolova, N. V., Kabanov, A. V. & Bronicha, T. K. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv. Rev. 65, 1667–1685 (2013).
Geng, Y. et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2, 249–255 (2007).
Yang, Y., He, Q., Duan, L., Cui, Y. & Li, J. Assembled alginate/chitosan nanotubes for biological application. Biomaterials 28, 3083–3090 (2007).
Shi, Y. et al. Recent progress and development on polymeric nanomaterials for photothermal therapy: a brief overview. J. Mater. Chem. B 5, 194–206 (2017).
Komor, A. C. & Barton, J. K. The path for metal complexes to a DNA target. Chem. Commun. 49, 3617–3630 (2013).
Deweese, J. E. & Osheroff, N. The DNA cleavage reaction of topoisomerase II: wolf in sheep’s clothing. Nucleic Acids Res. 37, 738–748 (2009).
Gopal, Y. N. V., Jayaraju, D. & Kondapi, A. K. Inhibition of topoisomerase II catalytic activity by two ruthenium compounds: a ligand-dependent mode of action. Biochemistry 38, 4382–4388 (1999).
Heffeter, P., Bo, K., Ute, B. & Bernhard, J. Intracellular protein binding patterns of the anticancer ruthenium drugs KP1019 and KP1339. J. Biol. Inorg. Chem. 15, 737–748 (2010).
Heffeter, P. et al. Intrinsic and acquired forms of resistance against the anticancer ruthenium compound KP1019 [indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(iii)] (FFC14A). J. Pharm. Exp. Ther. 312, 281–289 (2005).
Pa˘unescu, E. et al. Organometallic glutathione S-transferase inhibitors. Organometallics 36, 3313–3321 (2017).
Feng, L. et al. Structurally sophisticated octahedral metal complexes as highly selective protein kinase inhibitors. J. Am. Chem. Soc. 133, 5976–5986 (2011).
Duncan, R., Dimitrijevic, S. & Evagorou, E. The role of polymer conjugates in the diagnosis and treatment of cancer. Pharm. Sci. 6, 237–263 (1996).
Farokhzad, O. C. & Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 3, 16–20 (2009).
Li, S. & Huang, L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 5, 496–504 (2008).
Noguchi, Y., Wu, J., Duncan, R., Ulbrich, K. & Akaike, T. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn J. Cancer Res. 89, 307–314 (1998).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012).
Longmire, M., Choyke, P. L. & Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 3, 703–717 (2012).
Nag, O. K. & Awasthi, V. Surface engineering of liposomes for stealth behavior. Pharmaceutics 5, 542–569 (2013).
Immordino, M. L., Dosio, F. & Cattel, L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomed. 1, 297–315 (2006).
Stolnik, S., Illum, L. & Davis, S. S. Long circulating microparticulate drug carriers. Adv. Drug Deliv. Rev. 16, 195–214 (1995).
Allen, T. M., Hansen, C. & Rutledge, J. Liposomes with prolonged circulation times: factors affecting uptake by reticuloendothelial and other tissues. Biochim. Biophys. Acta 981, 27–35 (1989).
Garay, R. P., El-Gewely, R., Armstrong, J. K., Garratty, G. & Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 9, 1319–1323 (2012).
Acknowledgements
The authors acknowledge funding from École Nationale Supérieure de Chimie de Paris (ENSCP), the Centre National de la Recherche Scientifique (CNRS), a European Research Council (ERC) Consolidator Grant PhotoMedMet GA 681679 (G.G.), the Swiss National Science Foundation Grant Sinergia CRSII5_173718 (G.G.) and the Investissements d’Avenir programme launched by the French government and implemented by the Agence Nationale de la Recherche (ANR), reference ANR-10-IDEX-0001-02 PSL (G.G.). C.M.T. thanks the Institut Universitaire de France (IUF) for financial support.
Author information
Authors and Affiliations
Contributions
E.V, C.M.T. and G.G. are the major contributors to this Review, with Y.C.O. contributing to a lesser extent.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Villemin, E., Ong, Y.C., Thomas, C.M. et al. Polymer encapsulation of ruthenium complexes for biological and medicinal applications. Nat Rev Chem 3, 261–282 (2019). https://doi.org/10.1038/s41570-019-0088-0
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-019-0088-0
This article is cited by
-
A supramolecular photosensitizer derived from an Arene-Ru(II) complex self-assembly for NIR activated photodynamic and photothermal therapy
Nature Communications (2022)
-
Recent Advances in Schiff Base Ruthenium Metal Complexes: Synthesis and Applications
Topics in Current Chemistry (2021)
-
Metal–polymer-coordinated complexes as potential nanovehicles for drug delivery
Journal of Nanostructure in Chemistry (2021)
-
Critical discussion of the applications of metal complexes for 2-photon photodynamic therapy
JBIC Journal of Biological Inorganic Chemistry (2020)