Abstract
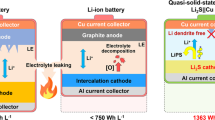
Solid sulfide electrolytes are key materials in all-solid-state lithium batteries because of their high lithium-ion conductivity and deformability, which enable the lithium-ion path to be connected between the material’s grain boundaries under pressure near room temperature. However, sulfur species are moisture-sensitive and exhibit high vapour pressures; therefore, syntheses of sulfide electrolytes need to be carefully designed. Liquid-phase reactions can be performed at low temperatures in controlled atmospheres, opening up the prospect of scalable processes for the preparation of sulfide electrolytes. Here, we review liquid-phase syntheses for the preparation of sulfide-based solid electrolytes and composites of electrolytes and electrodes, and we compare the charge–discharge performances of the all-solid-state lithium batteries using these components.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tatsumisago, M., Nagao, M. & Hayashi, A. Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries. J. Asian Ceram. Soc. 1, 17–25 (2013).
Hayashi, A., Sakuda, A. & Tatsumisago, M. Development of sulfide solid electrolytes and interface formation processes for bulk-type all-solid-state Li and Na batteries. Front. Energy Res. 4, 25 (2016).
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Gao, Z. et al. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 30, 1705702 (2018).
Park, K. H. et al. Design strategies, practical considerations, and new solution processes of sulfide solid electrolytes for all-solid-state batteries. Adv. Energy Mater. 8, 1800035 (2018).
Meesala, Y., Jena, A., Chang, H. & Liu, R.-S. Recent advancements in Li-ion conductors for all-solid-state Li-ion batteries. ACS Energy Lett. 2, 2734–2751 (2017).
Zheng, F., Kotobuki, M., Song, S., Lai, M. O. & Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 389, 198–213 (2018).
Lim, H.-D. et al. Solid electrolyte layers by solution deposition. Adv. Mater. Interfaces 5, 1701328 (2018).
Takada, K. Progress in solid electrolytes toward realizing solid-state lithium batteries. J. Power Sources 394, 74–85 (2018).
Hayashi, A., Hama, S., Morimoto, H., Tatsumisago, M. & Minami, T. Preparation of Li2S–P2S5 amorphous solid electrolytes by mechanical milling. J. Am. Ceram. Soc. 84, 477–479 (2001).
Hayashi, A., Hama, S., Minami, T. & Tatsumisago, M. Formation of superionic crystals from mechanically milled Li2S–P2S5 glasses. Electrochem. Commun. 5, 111–114 (2003).
Mizuno, F., Hayashi, A., Tadanaga, K. & Tatsumisago, M. New, highly ion-conductive crystals precipitated from Li2S–P2S5 glasses. Adv. Mater. 17, 918–921 (2005).
Hong, H.Y-P. Crystal structure and ionic conductivity of Li14Zn(GeO4)4 and other new Li+ superionic conductors Mater. Res. Bull. 13 117–124 (1978).
Kanno, R. & Murayama, M. Lithium ionic conductor thio-LISICON the Li2S-GeS2-P2S5 system. J. Electrochem. Soc. 148, A742–A746 (2001).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Deiseroth, H.-J. et al. Li6PS5X: a class of crystalline Li-rich solids with an unusually high Li+ mobility. Angew. Chem. 120, 767–770 (2008).
Boulineau, S., Courty, M., Tarascon, J.-M. & Viallet, V. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ion. 221, 1–5 (2012).
Inaguma, Y. et al. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 86, 689–693 (1993).
Aono, H., Sugimoto, E., Sadaaka, Y., Imanaka, N. & Adachi, G. Y. Ionic conductivity of the lithium titanium phosphate (Li1+XMXTi2−X(PO4)3, M=Al, Sc, Y, and La) systems. J. Electrochem. Soc. 136, 590–591 (1989).
Murugan, R., Thangadurai, V. & Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. 46, 7778–7781 (2007).
Tadanaga, K. et al. Low temperature synthesis of highly ion conductive Li7La3Zr2O12–Li3BO3 composites. Electrochem. Commun. 33, 51–54 (2013).
Nagao, M., Kitaura, H., Hayashi, A. & Tatsumisago, M. High rate performance, wide temperature operation and long cyclability of all-solid-state rechargeable lithium batteries using Mo-S Chevrel-phase compound. J. Electrochem. Soc. 160, A819–A823 (2013).
Sakuda, A., Hayashi, A., Takigawa, Y., Higashi, K. & Tatsumisago, M. Evaluation of elastic modulus of Li2S-P2S5 glassy solid electrolyte by ultrasonic sound velocity measurement and compression test. J. Ceram. Soc. Jpn 121, 946–949 (2013).
Seino, Y., Ota, T., Takada, K., Hayashi, A. & Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 7, 627–631 (2014).
Sakuda, A., Hayashi, A. & Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 3, 2261 (2013).
Ohta, N. et al. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 9, 1486–1490 (2007).
Hayashi, A., Muramatsu, H., Ohtomo, T., Hama, S. & Tatsumisago, M. Improvement of chemical stability of Li3PS4 glass electrolytes by adding MxOy (M=Fe, Zn, and Bi) nanoparticles. J. Mater. Chem. A 1, 6320 (2013).
Park, K. H. et al. Solution-processable glass LiI-Li4SnS4 superionic conductors for all-solid-state Li-Ion batteries. Adv. Mater. 28, 1874–1883 (2016).
Choi, Y. E. et al. Coatable Li4SnS4 solid electrolytes prepared from aqueous solutions for all-solid-state lithium-ion batteries. ChemSusChem 10, 2605–2611 (2017).
Muramatsu, H., Hayashi, A., Ohtomo, T., Hama, S. & Tatsumisago, M. Structural change of Li2S–P2S5 sulfide solid electrolytes in the atmosphere. Solid State Ion. 182, 116–119 (2011).
Manna, L., Scher, E. C. & Alivisatos, A. P. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 122, 12700–12706 (2000).
Peng, X. Mechanisms for the shape-control and shape-evolution of colloidal semiconductor nanocrystals. Adv. Mater. 15, 459–463 (2003).
Henkel, G. & Krebs, B. Metallothioneins: zinc, cadmium, mercury, and copper thiolates and selenolates mimicking protein active site features–structural aspects and biological implications. Chem. Rev. 104, 801–824 (2004).
Prouzet, E., Ouvrard, G., Brec, R. & Seguineau, P. Room temperature synthesis of pure amorphous nickel hexathiodiphosphate Ni2P2S6. Solid State Ion. 31, 79–90 (1988).
Huang, Z.-L., Zhao, J.-T., Mi, J.-X., Mao, S.-Y. & Zheng, L.-S. Room temperature solid state synthesis and characterization of a new chromium thiophosphate Cr4(P2S6)3. J. Solid State Chem. 144, 388–391 (1999).
Sakuda, A., Kitaura, H., Hayashi, A., Tadanaga, K. & Tatsumisago, M. Improvement of high-rate performance of all-solid-state lithium secondary batteries using LiCoO2 coated with Li2O-SiO2 glasses. Electrochem. Solid State Lett. 11, A1–A3 (2008).
Auvergniot, J. et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem. Mater. 29, 3883–3890 (2017).
Sakuda, A., Hayashi, A., Hama, S. & Tatsumisago, M. Preparation of highly lithium-ion conductive 80Li2S· 20P2S5 thin-film electrolytes using pulsed laser deposition. J. Am. Ceram. Soc. 93, 765–768 (2010).
Sakuda, A., Hayashi, A., Ohtomo, T., Hama, S. & Tatsumisago, M. All-solid-state lithium secondary batteries using LiCoO2 particles with pulsed laser deposition coatings of Li2S–P2S5 solid electrolytes. J. Power Sources 196, 6735–6741 (2011).
Ito, Y., Otoyama, M., Hayashi, A., Ohtomo, T. & Tatsumisago, M. Electrochemical and structural evaluation for bulk-type all-solid-state batteries using Li4GeS4-Li3PS4 electrolyte coating on LiCoO2 particles. J. Power Sources 360, 328–335 (2017).
Phuc, N. H. H., Yamamoto, T., Muto, H. & Matsuda, A. Fast synthesis of Li2S–P2S5–LiI solid electrolyte precursors. Inorg. Chem. Front. 4, 1660–1664 (2017).
Lim, H.-D. et al. Designing solution chemistries for low-temperature synthesis of sulfide-based solid electrolytes. J. Mater. Chem. A 6, 7370–7374 (2018).
Wang, Y. et al. Mechanism of formation of Li7P3S11 solid electrolytes through liquid phase synthesis. Chem. Mater. 30, 990–997 (2018).
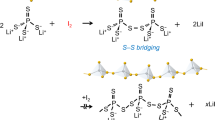
Liu, Z. et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 135, 975–978 (2013). This paper describes the suspension synthesis of β-Li 3 PS 4 and proposes the formation of a Li 3 PS 4 ·3THF complex as part of the reaction mechanism.
Homma, K. et al. Crystal structure and phase transitions of the lithium ionic conductor Li3PS4. Solid State Ion. 182, 53–58 (2011).
Rangasamy, E. et al. An iodide-based Li7P2S8I superionic conductor. J. Am. Chem. Soc. 137, 1384 (2015).
Ito, S., Nakakita, M., Aihara, Y., Uehara, T. & Machida, N. A synthesis of crystalline Li7P3S11 solid electrolyte from 1,2-dimethoxyethane solvent. J. Power Sources 271, 342–345 (2014). This paper describes the suspension synthesis of Li 7 P 3 S 11.
Xu, R. C. et al. Preparation of Li7P3S11 glass-ceramic electrolyte by dissolution-evaporation method for all-solid-state lithium ion batteries. Electrochim. Acta 219, 235–240 (2016).
Yao, X. et al. High-energy all-solid-state lithium batteries with ultralong cycle life. Nano Lett. 16, 7148–7154 (2016).
Phuc, N. H. H., Totani, M., Morikawa, K., Muto, H. & Matsuda, A. Preparation of Li3PS4 solid electrolyte using ethyl acetate as synthetic medium. Solid State Ion. 288, 240–243 (2016).
Wang, H., Hood, Z. D., Xia, Y. & Liang, C. Fabrication of ultrathin solid electrolyte membranes of β-Li3PS4 nanoflakes by evaporation-induced self-assembly for all-solid-state batteries. J. Mater. Chem. A 4, 8091–8096 (2016).
Sedlmaier, S. J. et al. Li4PS4I: a Li+ superionic conductor synthesized by a solvent-based soft chemistry approach. Chem. Mater. 29, 1830–1835 (2017).
Phuc, N. H. H., Morikawa, K., Totani, M., Muto, H. & Matsuda, A. Chemical synthesis of Li3PS4 precursor suspension by liquid-phase shaking. Solid State Ion. 285, 2–5 (2016).
Phuc, N. H. H., Hirahara, E., Morikawa, K., Muto, H. & Matsuda, A. One-pot liquid phase synthesis of (100−x)Li3PS4–xLiI solid electrolytes. J. Power Sources 365, 7–11 (2017).
Calpa, M., Rosero-Navarro, N. C., Miura, A. & Tadanaga, K. Instantaneous preparation of high lithium-ion conducting sulfide solid electrolyte Li7P3S11 by a liquid phase process. RSC Adv. 7, 46499–46504 (2017).
Calpa, M., Rosero-Navarro, N. C., Miura, A. & Tadanaga, K. Preparation of sulfide solid electrolytes in the Li2S–P2S5 system by a liquid phase process. Inorg. Chem. Front. 5, 501–508 (2018).
Wang, Y., Liu, Z., Zhu, X., Tang, Y. & Huang, F. Highly lithium-ion conductive thio-LISICON thin film processed by low-temperature solution method. J. Power Sources 224, 225–229 (2013). This is the first report of a liquid-phase reaction as well as dissolution–precipitation for sulfide electrolytes.
Choi, S., Lee, S., Park, J., Nichols, W. T. & Shin, D. Facile synthesis of Li2S–P2S5 glass-ceramics electrolyte with micron range particles for all-solid-state batteries via a low-temperature solution technique (LTST). Appl. Surf. Sci. 444, 10–14 (2018).
Azuma, S. et al. Colloidal processing of Li2S–P2S5 films fabricated via electrophoretic deposition methods and their characterization as a solid electrolyte for all solid state lithium ion batteries. J. Ceram. Soc. Jpn 125, 287–292 (2017).
Teragawa, S., Aso, K., Tadanaga, K., Hayashi, A. & Tatsumisago, M. Preparation of Li2S–P2S5 solid electrolyte from N-methylformamide solution and application for all-solid-state lithium battery. J. Power Sources 248, 939–942 (2014).
Teragawa, S., Aso, K., Tadanaga, K., Hayashi, A. & Tatsumisago, M. Liquid-phase synthesis of a Li3PS4 solid electrolyte using N-methylformamide for all-solid-state lithium batteries. J. Mater. Chem. A 2, 5095–5099 (2014).
Yubuchi, S. et al. Preparation of high lithium-ion conducting Li6PS5Cl solid electrolyte from ethanol solution for all-solid-state lithium batteries. J. Power Sources 293, 941–945 (2015). This paper describes the dissolution–precipitation of Li 6 PS 5 Cl through a Li 6 PS 5 Cl–ethanol solution and its application to all-solid-state lithium batteries.
Rosero-Navarro, N. C., Kinoshita, T., Miura, A., Higuchi, M. & Tadanaga, K. Effect of the binder content on the electrochemical performance of composite cathode using Li6PS5Cl precursor solution in an all-solid-state lithium battery. Ionics 23, 1619–1624 (2017).
Yubuchi, S., Uematsu, M., Deguchi, M., Hayashi, A. & Tatsumisago, M. Lithium-ion-conducting argyrodite-type Li6PS5X (X=Cl, Br, I) solid electrolytes prepared by a liquid-phase technique using ethanol as a solvent. ACS Appl. Energy Mater. 1, 3622–3629 (2018).
Rosero-Navarro, N. C., Miura, A. & Tadanaga, K. Composite cathode prepared by argyrodite precursor solution assisted by dispersant agents for bulk-type all-solid-state batteries. J. Power Sources 396, 33–40 (2018). The morphology of precipitated Li 6 PS 5 Cl particles is controlled by a dispersant in a homogeneous solution.
Chida, S. et al. Liquid-phase synthesis of Li6PS5Br using ultrasonication and application to cathode composite electrodes in all-solid-state batteries. Ceram. Int. 44, 742–746 (2018).
Yubuchi, S. et al. An argyrodite sulfide-based superionic conductor synthesized by a liquid-phase technique with tetrahydrofuran and ethanol. J. Mater. Chem. A 7, 558–566 (2019).
Phuc, N. H. H., Morikawa, K., Mitsuhiro, T., Muto, H. & Matsuda, A. Synthesis of plate-like Li3PS4 solid electrolyte via liquid-phase shaking for all-solid-state lithium batteries. Ionics 23, 2061–2067 (2017). This paper describes the suspension process for the composite positive electrode of an all-solid-state lithium battery.
Oh, D. Y. et al. Single-step wet-chemical fabrication of sheet-type electrodes from solid-electrolyte precursors for all-solid-state lithium-ion batteries. J. Mater. Chem. A 5, 20771–20779 (2017).
Zhang, Q. et al. Fe3S4@Li7P3S11 nanocomposites as cathode materials for all-solid-state lithium batteries with improved energy density and low cost. J. Mater. Chem. A 5, 23919–23925 (2017).
Xu, R. C. et al. Rational coating of Li7P3S11 solid electrolyte on MoS2 electrode for all-solid-state lithium ion batteries. J. Power Sources 374, 107–112 (2018).
Rosero-Navarro, C., Miura, A. & Tadanaga, K. Preparation of lithium ion conductive Li6PS5Cl solid electrolyte from solution for the fabrication of composite cathode of all-solid-state lithium battery. J. Sol-Gel Sci. Technol. 89, 303–309 (2019).
Kim, D. H. et al. Infiltration of solution-processable solid electrolytes into conventional Li-ion-battery electrodes for all-solid-state Li-ion batteries. Nano Lett. 17, 3013–3020 (2017). This paper describes sheet-type all-solid-state batteries fabricated by a dissolution–precipitation process.
Yubuchi, S., Hayashi, A. & Tatsumisago, M. Sodium-ion conducting Na3PS4 electrolyte synthesized via a liquid-phase process using N-methylformamide. Chem. Lett. 44, 884–886 (2015).
Banerjee, A. et al. Na3SbS4: a solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew. Chem. 55, 9634–9638 (2016).
Uematsu, M. et al. Preparation of Na3PS4 electrolyte by liquid-phase process using ether. Solid State Ion. 320, 33–37 (2018).
Kim, T. W., Park, K. H., Choi, Y. E., Lee, J. Y. & Jung, Y. S. Aqueous-solution synthesis of Na3SbS4 solid electrolytes for all-solid-state Na-ion batteries. J. Mater. Chem. A 6, 840–844 (2018).
Xu, X. et al. Li7P3S11 solid electrolyte coating silicon for high-performance lithium-ion batteries. Electrochim. Acta 276, 325–332 (2018).
Momma, K. & Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. J. Appl. Crystallogr. 41, (653–658 (2008).
Yamane, H. et al. Crystal structure of a superionic conductor, Li7P3S11. Solid State Ion. 178, 1163–1167 (2007).
Acknowledgements
This Review includes research achievements partially supported by the Japan Science and Technology Agency (JST), the Advanced Low Carbon Technology Research and Development Program (ALCA) and the Specially Promoted Research for Innovative Next Generation Batteries (SPRING) project.
Author information
Authors and Affiliations
Contributions
A.Mi., N.C.R.N., A.S. and K.T. wrote the draft. N.H.H.P., A.Ma., N.M., A.H. and M.T. discussed the Review. All the authors agreed upon the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Miura, A., Rosero-Navarro, N.C., Sakuda, A. et al. Liquid-phase syntheses of sulfide electrolytes for all-solid-state lithium battery. Nat Rev Chem 3, 189–198 (2019). https://doi.org/10.1038/s41570-019-0078-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-019-0078-2
This article is cited by
-
Recycling of solid-state batteries
Nature Energy (2024)
-
Self-assembled hydrated copper coordination compounds as ionic conductors for room temperature solid-state batteries
Nature Communications (2024)
-
Advances in All-Solid-State Lithium–Sulfur Batteries for Commercialization
Nano-Micro Letters (2024)
-
Self-organized hetero-nanodomains actuating super Li+ conduction in glass ceramics
Nature Communications (2023)
-
Non-flammable solvent-free liquid polymer electrolyte for lithium metal batteries
Nature Communications (2023)