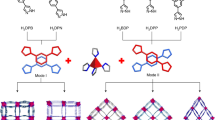
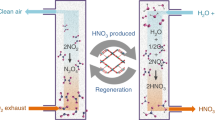
Abstract
Sulfur dioxide and nitrogen oxides generated by anthropogenic activities are air pollutants that cause serious environmental problems and pose substantial health threats. Although established methods for emission desulfurization and denitrogenation already exist, more efficient and flexible technologies are still required. In this Review, we highlight state-of-the-art examples in which metal–organic frameworks (MOFs), an emerging class of porous sorbents, have been applied to the adsorptive removal of SO2 and NO2. MOFs can simultaneously exhibit superior adsorption capacities and exceptional selectivities for SO2 and NO2 in the presence of other flue and exhaust gases while maintaining their structural integrity. The highly crystalline nature and rich chemical functionality of MOFs have enabled the elucidation of host–guest interactions at a molecular level to afford insights and new knowledge that will inspire and inform the design of new generations of adsorbents.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
European Environment Agency. Sulphur dioxide (SO2) emissions. Indicator codes: APE 001. EEA https://www.eea.europa.eu/data-and-maps/indicators/eea-32-sulphur-dioxide-so2-emissions-1 (2015).
European Environment Agency. Nitrogen dioxides (NOx) emissions. Indicator codes: APE 002. EEA https://www.eea.europa.eu/data-and-maps/indicators/eea-32-nitrogen-oxides-nox-emissions-1/assessment.2010-08-19.0140149032-3 (2018).
European Environment Agency. EEA: Air Quality in Europe-2017 report. Report no. 13/2017 (EEA, 2017).
Edwards, P. M. et al. High winter ozone pollution from carbonyl photolysis in an oil and gas basin. Nature 514, 351–354 (2014).
United States Environmental Protection Agency. Acid Rain and Related Programs: 2006 Progress Report (EPA, 2006).
United States Environmental Protection Agency. EPA base case v.4.10: chapter 5: emission control technologies. EPA https://www.epa.gov/sites/production/files/2015-07/documents/chapter_5_emission_control_technologies.pdf (2010).
Suh, M. P., Park, H. J., Prasad, T. K. & Lim, D. W. Hydrogen storage in metal organic frameworks. Chem. Rev. 112, 782–835 (2012).
Sumida, K. et al. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 112, 724–781 (2012).
European Environment Agency. The impact of international shipping on European air quality and climate forcing: Technical Report No. 4/2013 (EEA, 2013).
Aksoyoglu, S., Baltensperger, U. & Prévôt, A. S. H. Contribution of ship emissions to the concentration of air pollutants in Europe. Atoms. Chem. Phys. 16, 1895–1906 (2016).
Srivastava, R. K., Jozewicz, W. & Singer, C. SO2 scrubbing technologies: a review. Environ. Prog. 20, 219–227 (2001).
Pandey, R. A. et al. Flue gas desulfurization: physicochemial and biotechnological approaches. Crit. Rev. Environ. Sci. Technol. 35, 571–622 (2005).
Jordan, R. J. The feasibility of wet scrubbing for treating waste-to-energy flue gas. J. Air Waste Manag. Assoc. 37, 422–430 (1987).
Tomas-Alonso, F. A new perspective about recovering SO2 off-gas in coal power plants: energy saving. Part I. Regenerable wet methods. Energy Sources 27, 1035–1041 (2005).
Sporer, J. The Linde Solinox process: gypsum-free flue-gas desulphurization. Gas Sep. Purif. 6, 133–140 (1992).
United States Environmental Protection Agency. Sulfur oxides control technology series: flue gas desulfurization magnesium oxide process, summary report No. 4/1981 (EPA, 1981).
Yang, J. et al. Novel process of removal of sulfur dioxide by aqueous ammonia-fulvic acid solution with ammonia escape inhibition. Energy Fuels 30, 3205–3218 (2016).
Tomas-Alonso, F. A new perspective about recovering SO2 off-gas in coal power plants: energy saving. Part II. Regenerable dry methods. Energy Sources 27, 1043–1049 (2005).
Keener, T. C., Wang, J. & Khang, S. in Dry Scrubbing Technologies for Flue Gas Desulfurization (ed. Toole-O’Neil, B.) 607–690 (Springer, Boston, MA, 1998).
Mathieu, Y. et al. Adsorption of SOx by oxide materials: a review. Fuel Process Technol. 114, 81–100 (2013).
Bandosz, T. J. in Activated Carbon Surfaces in Environmental Remediation (ed. Bandosz, T. J.) 231–292 (Elsevier, 2006).
Ray, G. C. & Box, E. O. Adsorption of gases on activated charcoal. Ind. Eng. Chem. 42, 1315–1318 (1950).
Raymundo-Pinero, E., Cazorla-Amoros, D., de Lecea, C. S. M. & Linares-Solano, A. Factors controling the SO2 removal by porous carbons: relevance of the SO2 oxidation step. Carbon 38, 335–344 (2000).
Yang, S. et al. Irreversible network transformation in a dynamic porous host catalyzed by sulfur dioxide. J. Am. Chem. Soc. 135, 4954–4957 (2013).
Cui, X. et al. Ultrahigh and selective SO2 uptake in inorganic anion-pillared hybrid porous materials. Adv. Mater. 29, 1606929 (2017).
Carter, J. H. et al. Exceptional adsorption and binding of sulfer dioxide in a robust zirconium-based metal-organic framework. J. Am. Chem. Soc. 140, 15564–15567 (2018).
Mochida, I. & Kisamori, S. Roles of surface oxygen groups on poly(acrylonitrile)-based acitve carbon fibers in SO2 adsorption. Langmuir 10, 1241–1245 (1994).
Lizzio, A. A. & DeBarr, J. A. Mechanism of SO2 removal by carbon. Energy Fuels 11, 284–291 (1997).
Britt, D., Tranchemontagne, D. & Yaghi, O. M. Metal-organic frameworks with high capacity and selectivity for harmful gases. PNAS 105, 11623–11627 (2008).
Glover, T. G., Peterson, G. W., Schindler, B. J., Britt, D. & Yaghi, O. M. MOF-74 building unit has a direct impact on toxic gas adsorption. Chem. Eng. Sci. 66, 163–170 (2011).
DeCoste, J. B., Demasky, T. J., Katz, M. J., Farha, O. K. & Hupp, J. T. A. UiO-66 analogue with uncoordinated carboxylic acids for the broad-spectrum removal of toxic chemicals. New J. Chem. 39, 2396–2399 (2015).
Schneemann, A. et al. Flexible metal-organic frameworks. Chem. Soc. Rev. 43, 6062–6096 (2014).
Fernandez, C. A. et al. Gas-induced expansion and contraction of a fluorinated metal-organic framework. Cryst. Growth Des. 10, 1037–1039 (2010).
Thallapally, P. K., Motkuri, R. K., Fernandez, C. A., McGrail, B. P. & Behrooz, G. S. Prussian blue analogues for CO2 and SO2 capture and separation applications. Inorg. Chem. 49, 4909–4915 (2010).
Windisch Jr. C. F., Thallapally, P. K. & McGrail, B. P. Competitive adsorption study of CO2 and SO2 on CoII 3[CoIII(CN)6]2 using DRIFTS. Spectrochim. Acta A. 77, 287–291 (2010).
Easun, T. et al. Structural and dynamic studies of substrate binding in porous metal–organic frameworks. Chem. Soc. Rev. 46, 239–274 (2017).
Tan, K. et al. Mechanism of preferential adsorption of SO2 into two microporous paddle wheel frameworks M(bdc)(ted)0.5. Chem. Mater. 25, 4653–4662 (2013).
Yang, S. et al. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nat. Chem. 4, 887–894 (2012).
Savage, M. et al. Selective Adsorption of sulfur dioxide in a robust metal-organic framework material. Adv. Mater. 28, 8705–8711 (2016).
Chernikova, V. et al. Highly sensitive and selective SO2 MOF sensor: the integration of MFM-300 MOF as a sensitive layer on a capacitive interdigitated electrode. J. Mater. Chem. A 6, 5550–5554 (2018).
United States Environmental Protection Agency. Technical Bulletin: Nitrogen Oxides (NO x ), Why and How They are Controlled (EPA, 1999).
Amann, M., Klimont, Z. & Wagner, F. in Annual Review of Environment and Resources (eds Gadgil, A. & Liverman, D. M.) 31–55 (Annual Reviews, Palo Alto, 2013).
Liu, Z. M. & Woo, S. I. Recent advances in catalytic deNOx science and technology. Catal. Rev. Sci. Eng. 48, 43–89 (2006).
Ciardelli, C. et al. Reactivity of NO/NO2-NH3 SCR system for diesel exhaust aftertreatment: identification of the reaction network as a function of temperature and NO2 feed content. Appl. Catal. B 70, 80–90 (2007).
Busca, G., Lietti, L., Ramis, G. & Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: a review. Appl. Catal. B 18, 1–36 (1998).
Lesage, T., Verrier, C., Bazin, P., Saussey, J. & Daturi, M. Studying the NOx-trap mechanism over a Pt-Rh/Ba/Al2O3 catalyst by operando FT-IR spectroscopy. Phys. Chem. Chem. Phys. 5, 4435–4440 (2003).
Brandenberger, S., Krocher, O., Tissler, A. & Althoff, R. The state of the art in selective catalytic reduction of NOx by ammonia using metal-exchanged zeolite catalysts. Catal. Rev. Sci. Eng. 50, 492–531 (2008).
Neathery, J. K., Rubel, A. M. & Stencel, J. M. Uptake of NOx by activated carbons: bench-scale and pilot-plant testing. Carbon 35, 1321–1327 (1997).
Shirahama, N. et al. Mechanistic study on adsorption and reduction of NO2 over activated carbon fibers. Carbon 40, 2605–2611 (2002).
Hass, H. B., Dorsky, J. & Hodge, E. B. Nitration of propane by nitrogen dioxide. Ind. Eng. Chem. 33, 1138–1143 (1941).
Kharasch, M. S. et al. Inhibition of polymerization-laborotory and plant control of popcorn polymer growth. Ind. Eng. Chem. Res. 39, 830–837 (1947).
Shomali, M., Opie, D., Avasthi, T. & Trilling, A. Nitogen dioxide sterilization in low-resource environments: a feasibility study. PLOS ONE 10, e0130043 (2015).
Levasseur, B., Petit, C. & Bandosz, T. J. Reactive adsorption of NO2 on copper-based metal-organic framework and graphite oxide/metal-organic framework composites. ACS Appl. Mater. Interfaces 2, 3606–3613 (2010).
Petit, C., Levasseur, B., Mendoza, B. & Bandosz, T. J. Reactive adsorption of acidic gases on MOF/graphite oxide composites. Micropor. Mesopor. Mater. 154, 107–112 (2012).
Ebrahim, A. M., Levasseur, B. & Bandosz, T. J. Interactions of NO2 with Zr-based MOF: effects of the size of organic linkers on NO2 adsorption at ambient conditions. Langmuir 29, 168–174 (2013).
Ebrahim, A. M. & Bandosz, T. J. Effect of amine modification on the properties of zirconium- carboxylic acid based materials and their applications as NO2 adsorbents at ambient conditions. Micropor. Mesopor. Mater. 188, 149–162 (2014).
Peterson, G. W., Mahle, J. J., DeCoste, J. B., Gordon, W. O. & Rossin, J. A. Extraordinary NO2 removal by the metal-organic framework UiO-66-NH2. Angew. Chem. Int. Ed. 55, 6235–6238 (2016).
Han, X. et al. Reversible adsorption of nitrogen dioxide within a robust porous metal–organic framework. Nat. Mat. 17, 691–696 (2018).
Ebrahim, A. M. & Bandosz, T. J. Ce(III) doped Zr-based MOFs as excellent NO2 adsorbents at ambient conditions. ACS Appl. Mater. Interfaces 5, 10565–10573 (2013).
Kvick, Å. et al. The structure of dinitrogen tetroxide N2O4: neutron diffraction study at 100, 60, and 20 K and ab initio theoretical calculations. J. Chem. Phys. 76, 3754–3761 (1982).
Rosi, N. et al. Rod packings and metal-organic frameworks constructed from rod-shaped secondary building units. J. Am. Chem. Soc. 127, 1504–1518 (2005).
Acknowledgements
Financial support is provided by the UKRI (EPSRC — EP/I011870) and the European Research Council (ERC — AdG 742041 and ERC — PoC 665632).
Author information
Authors and Affiliations
Contributions
X.H. and S.Y. researched data for the article. All authors contributed equally to the writing and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, X., Yang, S. & Schröder, M. Porous metal–organic frameworks as emerging sorbents for clean air. Nat Rev Chem 3, 108–118 (2019). https://doi.org/10.1038/s41570-019-0073-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-019-0073-7
This article is cited by
-
A sustainable solution: mitigating sulphur dioxide emissions through adsorption on chemically modified iron oxide nanoparticles
Clean Technologies and Environmental Policy (2024)
-
Incorporation of multiple supramolecular binding sites into a robust MOF for benchmark one-step ethylene purification
Nature Communications (2023)
-
Direct prediction of gas adsorption via spatial atom interaction learning
Nature Communications (2023)
-
Direct synthesis of amorphous coordination polymers and metal–organic frameworks
Nature Reviews Chemistry (2023)
-
A robust ultra-microporous cationic aluminum-based metal-organic framework with a flexible tetra-carboxylate linker
Communications Chemistry (2023)