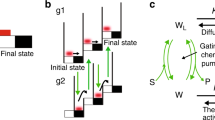
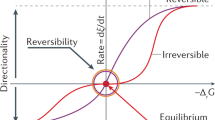
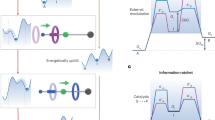
Abstract
The past few decades have seen tremendous progress in the synthesis and operation of molecular systems capable of controlled mechanical movement. Here, we review the use of molecular machines as catalysts for controlling chemical reactions. We highlight the various catalyst designs with a focus on how mechanical motion is used to control catalysis with varying degrees of success. This Review discusses the current challenges of designing effective catalysts, the scope and limitations of various systems and the future potential and aims for the field. Although it is difficult to predict which concepts will become most important, as much of the work is at the proof-of-concept level, it seems clear that molecular machines have the potential to substantially impact the field of catalysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Feynman, R. P. There's plenty of room at the bottom. Eng. Sci. 23, 22–36 (1960).
Drexler, K. E. Nanosystems: Molecular Machinery, Manufacturing, and Computation. (John Wiley & Sons, Inc., 1992).
Coskun, A., Banaszak, M., Astumian, R. D., Stoddart, J. F. & Grzybowski, B. A. Great expectations: can artificial molecular machines deliver on their promise? Chem. Soc. Rev. 41, 19–30 (2012).
Baum, R. Nanotechnology: Drexler and Smalley make the case for and against ‘molecular assemblers’. Chem. Eng. News. 81, 37–42 (2003).
Chang, K. Yes, they can! No, they can't: charges fly in nanobot debate. The New York Times http://www.nytimes.com/2003/12/09/science/yes-they-can-no-they-can-t-charges-fly-in-nanobot-debate.html (2003).
Siegel, J. Inventing the nanomolecular wheel. Science 310, 63–64 (2005).
Kassem, S. et al. Artificial molecular motors. Chem. Soc. Rev. 46, 2592–2621 (2017).
Kinbara, K. & Aida, T. Toward intelligent molecular machines: directed motions of biological and artificial molecules and assemblies. Chem. Rev. 105, 1377–1400 (2005).
Schliwa, M. & Woehlke, G. Molecular motors. Nature 422, 759–765 (2003).
Sauvage, J. P. From chemical topology to molecular machines (Nobel Lecture). Angew. Chem. Int. Ed. 56, 11080–11093 (2017).
Stoddart, J. F. Mechanically interlocked molecules (MIMs) — molecular shuttles, switches, and machines (Nobel lecture). Angew. Chem. Int. Ed. 56, 11094–11125 (2017).
Feringa, B. L. The art of building small: from molecular switches to motors (Nobel lecture). Angew. Chem. Int. Ed. 56, 11060–11078 (2017).
Schummer, J. & Baird, D. Nanotechnology Challenges: Implications for Philosophy, Ethics and Society (World Scientific Publishing, 2006).
Blanco, V., Leigh, D. A. & Marcos, V. Artificial switchable catalysts. Chem. Soc. Rev. 44, 5341–5370 (2015).
Wheeldon, I. et al. Substrate channelling as an approach to cascade reactions. Nat. Chem. 8, 299–309 (2016).
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015).
Kay, E. R. & Leigh, D. A. Rise of the molecular machines. Angew. Chem. Int. Ed. 54, 10080–10088 (2015).
Vlatkovic, M., Collins, B. S. L. & Feringa, B. L. Dynamic responsive systems for catalytic function. Chem. Eur. J. 22, 17080–17111 (2016).
Cacciapaglia, R., Di Stefano, S. & Mandolini, L. The bis-barium complex of a butterfly crown ether as a phototunable supramolecular catalyst. J. Am. Chem. Soc. 125, 2224–2227 (2003).
Imahori, T., Yamaguchi, R. & Kurihara, S. Azobenzene-tethered bis(trityl alcohol) as a photoswitchable cooperative acid catalyst for Morita–Baylis–Hillman reactions. Chem. Eur. J. 18, 10802–10807 (2012).
Samanta, M., Siva Rama Krishna, V. & Bandyopadhyay, S. A photoresponsive glycosidase mimic. Chem. Commun. 50, 10577–10579 (2014).
Würthner, F. & Rebek, J. Light-switchable catalysis in synthetic receptors. Angew. Chem. Int. Ed. Engl. 34, 446–448 (1995).
Wurthner, F. & Rebek, J. Photoresponsive synthetic receptors: binding properties and photocontrol of catalytic activity. J. Chem. Soc., Perkin Trans. 2, 1727–1734 (1995).
Conn, M. M., Deslongchamps, G., de Mendoza, J. & Rebek, J. Jr. Convergent functional groups. 13. High-affinity complexation of adenosine derivatives within induced binding pockets. J. Am. Chem. Soc. 115, 3548–3557 (1993).
Peters, M. V., Stoll, R. S., Kühn, A. & Hecht, S. Photoswitching of basicity. Angew. Chem. Int. Ed. 47, 5968–5972 (2008).
Stoll, R. S. et al. Photoswitchable catalysts: correlating structure and conformational dynamics with reactivity by a combined experimental and computational approach. J. Am. Chem. Soc. 131, 357–367 (2009).
Osorio-Planes, L., Rodríguez-Escrich, C. & Pericàs, M. A. Photoswitchable thioureas for the external manipulation of catalytic activity. Org. Lett. 16, 1704–1707 (2014).
De Bo, G., Leigh, D. A., McTernan, C. T. & Wang, S. A complementary pair of enantioselective switchable organocatalysts. Chem. Sci. 8, 7077–7081 (2017).
Huang, Z. et al. ASD: a comprehensive database of allosteric proteins and modulators. Nucleic Acids Res. 39, D663–D669 (2011).
Schmittel, M., De, S. & Pramanik, S. Reversible ON/OFF nanoswitch for organocatalysis: mimicking the locking and unlocking operation of CaMKII. Angew. Chem. Int. Ed. 51, 3832–3836 (2012).
De, S., Pramanik, S. & Schmittel, M. A. Toggle nanoswitch alternately controlling two catalytic reactions. Angew. Chem. Int. Ed. 53, 14255–14259 (2014).
Schmittel, M., Pramanik, S. & De, S. A reversible nanoswitch as an ON–OFF photocatalyst. Chem. Commun. 48, 11730–11732 (2012).
Mittal, N., Pramanik, S., Paul, I., De, S. & Schmittel, M. Networking nanoswitches for ON/OFF control of catalysis. J. Am. Chem. Soc. 139, 4270–4273 (2017).
Gaikwad, S., Goswami, A., De, S. & Schmittel, M. A. Metalloregulated four-state nanoswitch controls two-step sequential catalysis in an eleven-component system. Angew. Chem. Int. Ed. 55, 10512–10517 (2016).
Wang, J. & Feringa, B. L. Dynamic control of chiral space in a catalytic asymmetric reaction using a molecular motor. Science 331, 1429–1432 (2011).
Vlatkovic, M., Bernardi, L., Otten, E. & Feringa, B. L. Dual stereocontrol over the Henry reaction using a light- and heat-triggered organocatalyst. Chem. Commun. 50, 7773–7775 (2014).
Trost, B. M. & Vranken, D. L. V. Flexible strategy to polyfunctional cyclopentanes. a synthesis mannostatin A J. Am. Chem. Soc. 113, 6317–6318 (1991).
Trost, B. M., Breit, B., Peukert, S., Zambrano, J. & Ziller, J. W. A new platform for designing ligands for asymmetric induction in allylic alkylations. Angew. Chem. Int. Ed. 34, 2386–2388 (1995).
Zhao, D., Neubauer, T. M. & Feringa, B. L. Dynamic control of chirality in phosphine ligands for enantioselective catalysis. Nat. Commun. 6, 6652 (2015).
Sud, D., Norsten, T. B. & Branda, N. R. Photoswitching of stereoselectivity in catalysis using a copper dithienylethene complex. Angew. Chem. Int. Ed. 44, 2019–2021 (2005).
Traut, T. W. Dissociation of enzyme oligomers: a mechanism for allosteric regulation. Crit. Rev. Biochem. Mol. Biol. 29, 125–163 (1994).
Stang, P. J. & Olenyuk, B. Self-assembly, symmetry, and molecular architecture: coordination as the motif in the rational design of supramolecular metallacyclic polygons and polyhedra. Acc. Chem. Res. 30, 502–518 (1997).
Farrell, J. R., Mirkin, C. A., Guzei, I. A., Liable-Sands, L. M. & Rheingold, A. L. The weak-link approach to the synthesis of inorganic macrocycles. Angew. Chem. Int. Ed. 37, 465–467 (1998).
Gianneschi, N. C. et al. A supramolecular approach to an allosteric catalyst. J. Am. Chem. Soc. 125, 10508–10509 (2003).
Gianneschi, N. C., Nguyen, S. T. & Mirkin, C. A. Signal amplification and detection via a supramolecular allosteric catalyst. J. Am. Chem. Soc. 127, 1644–1645 (2005).
Oliveri, C. G. et al. Supramolecular allosteric cofacial porphyrin complexes. J. Am. Chem. Soc. 128, 16286–16296 (2006).
Yoon, H. J., Heo, J. & Mirkin, C. A. Allosteric regulation of phosphate diester transesterification based upon a dinuclear zinc catalyst assembled via the weak-link approach. J. Am. Chem. Soc. 129, 14182–14183 (2007).
Yoon, H. J. & Mirkin, C. A. PCR-like cascade reactions in the context of an allosteric enzyme mimic. J. Am. Chem. Soc. 130, 11590–11591 (2008).
Jeon, Y.-M., Heo, J., Brown, A. M. & Mirkin, C. A. Triple-decker complexes formed via the weak link approach. Organometallics 25, 2729–2732 (2006).
Yoon, H. J., Kuwabara, J., Kim, J.-H. & Mirkin, C. A. Allosteric supramolecular triple-layer catalysts. Science 330, 66–69 (2010).
McGuirk, C. M., Mendez-Arroyo, J., Lifschitz, A. M. & Mirkin, C. A. Allosteric regulation of supramolecular oligomerization and catalytic activity via coordination-based control of competitive hydrogen-bonding events. J. Am. Chem. Soc. 136, 16594–16601 (2014).
Leighton, J. L. & Jacobsen, E. N. Efficient synthesis of (R)-4-((trimethylsilyl)oxy)-2-cyclopentenone by enantioselective catalytic epoxide ring opening. J. Org. Chem. 61, 389–390 (1995).
Jacobsen, E. N. Asymmetric catalysis of epoxide ring-opening reactions. Acc. Chem. Res. 33, 421–431 (2000).
Hansen, K. B., Leighton, J. L. & Jacobsen, E. N. On the mechanism of asymmetric nucleophilic ring-opening of epoxides catalyzed by (salen)CrIII complexes. J. Am. Chem. Soc. 118, 10924–10925 (1996).
Breinbauer, R. & Jacobsen, E. N. Cooperative asymmetric catalysis with dendrimeric [Co(salen)] complexes. Angew. Chem. Int. Ed. 39, 3604–3607 (2000).
Gianneschi, N. C., Cho, S.-H., Nguyen, S. T. & Mirkin, C. A. Reversibly addressing an allosteric catalyst in situ: catalytic molecular tweezers. Angew. Chem. Int. Ed. 43, 5503–5507 (2004).
Ouyang, G.-H., He, Y.-M., Li, Y., Xiang, J.-F. & Fan, Q.-H. Cation-triggered switchable asymmetric catalysis with chiral aza-crownphos. Angew. Chem. Int. Ed. 54, 4334–4337 (2015).
McGuirk, C. M. et al. A concerted two-prong approach to the in situ allosteric regulation of bifunctional catalysis. Chem. Sci 7, 6674–6683 (2016).
McGuirk, C. M., Stern, C. L. & Mirkin, C. A. Small molecule regulation of self-association and catalytic activity in a supramolecular coordination complex. J. Am. Chem. Soc. 136, 4689–4696 (2014).
Ulmann, P. A. et al. Spontaneous formation of heteroligated PtII complexes with chelating hemilabile ligands. Chem. Eur. J. 13, 4529–4534 (2007).
Spokoyny, A. M., Rosen, M. S., Ulmann, P. A., Stern, C. & Mirkin, C. A. Selective formation of heteroligated Pt(II) complexes with bidentate phosphine-thioether (P,S) and phosphine-selenoether (p,se) ligands via the halide-induced ligand rearrangement reaction. Inorg. Chem. 49, 1577–1586 (2010).
Rosen, M. S. et al. Chelating effect as a driving force for the selective formation of heteroligated Pt(II) complexes with bidentate phosphino-chalcoether ligands. Inorg. Chem. 50, 1411–1419 (2011).
Rosen, M. S., Stern, C. L. & Mirkin, C. A. Heteroligated PtII weak-link approach complexes using hemilabile N-heterocyclic carbene-thioether and phosphino-thioether ligands. Chem. Sci. 4, 4193–4198 (2013).
Lifschitz, A. M. et al. An allosteric photoredox catalyst inspired by photosynthetic machinery. Nat. Commun. 6, 6541 (2015).
Xue, M., Yang, Y., Chi, X., Yan, X. & Huang, F. Development of pseudorotaxanes and rotaxanes: from synthesis to stimuli-responsive motions to applications. Chem. Rev. 115, 7398–7501 (2015).
Beswick, J. et al. Selecting reactions and reactants using a switchable rotaxane organocatalyst with two different active sites. Chem. Sci. 6, 140–143 (2015).
Blanco, V., Carlone, A., Hänni, K. D., Leigh, D. A. & Lewandowski, B. A. Rotaxane-based switchable organocatalyst. Angew. Chem. Int. Ed. 51, 5166–5169 (2012).
Martinez-Cuezva, A. et al. Photoswitchable interlocked thiodiglycolamide as a cocatalyst of a chalcogeno-Baylis–Hillman reaction. Chem. Sci. 8, 3775–3780 (2017).
Galli, M., Lewis, J. E. M. & Goldup, S. M. A. Stimuli-responsive rotaxane–gold catalyst: regulation of activity and diastereoselectivity. Angew. Chem. Int. Ed. 54, 13545–13549 (2015).
Cakmak, Y., Erbas-Cakmak, S. & Leigh, D. A. Asymmetric catalysis with a mechanically point-chiral rotaxane. J. Am. Chem. Soc. 138, 1749–1751 (2016).
Blanco, V., Leigh, D. A., Marcos, V., Morales-Serna, J. A. & Nussbaumer, A. L. A. Switchable [2]rotaxane asymmetric organocatalyst that utilizes an acyclic chiral secondary amine. J. Am. Chem. Soc. 136, 4905–4908 (2014).
Blanco, V., Leigh, D. A., Lewandowska, U., Lewandowski, B. & Marcos, V. Exploring the activation modes of a rotaxane-based switchable organocatalyst. J. Am. Chem. Soc. 136, 15775–15780 (2014).
Kwan, C.-S., Chan, A. S. C. & Leung, K. C.-F. A. Fluorescent and switchable rotaxane dual organocatalyst. Org. Lett. 18, 976–979 (2016).
Berná, J., Alajarín, M. & Orenes, R.-A. Azodicarboxamides as template binding motifs for the building of hydrogen-bonded molecular shuttles. J. Am. Chem. Soc. 132, 10741–10747 (2010).
Lewis, J. E. M., Galli, M. & Goldup, S. M. Properties and emerging applications of mechanically interlocked ligands. Chem. Commun. 53, 298–312 (2017).
Leigh, D. A., Marcos, V. & Wilson, M. R. Rotaxane catalysts. ACS Catal. 4, 4490–4497 (2014).
Pan, T. & Liu, J. Catalysts encapsulated in molecular machines. ChemPhysChem 17, 1752–1758 (2016).
Eichstaedt, K. et al. Switching between anion-binding catalysis and aminocatalysis with a rotaxane dual-function catalyst. J. Am. Chem. Soc. 139, 9376–9381 (2017).
Breyer, W. A. & Matthews, B. W. A structural basis for processivity. Protein Sci. 10, 1699–1711 (2001).
Trakselis, M. A., Alley, S. C., Abel-Santos, E. & Benkovic, S. J. Creating a dynamic picture of the sliding clamp during T4 DNA polymerase holoenzyme assembly by using fluorescence resonance energy transfer. Proc. Natl Acad. Sci. USA 98, 8368–8375 (2001).
Kovall, R. & Matthews, B. W. Toroidal structure of λ-exonuclease. Science 277, 1824–1827 (1997).
Thordarson, P., Bijsterveld, E. J. A., Rowan, A. E. & Nolte, R. J. M. Epoxidation of polybutadiene by a topologically linked catalyst. Nature 424, 915–918 (2003).
Coumans, R. G. E., Elemans, J. A. A. W., Nolte, R. J. M. & Rowan, A. E. Processive enzyme mimic: kinetics and thermodynamics of the threading and sliding process. Proc. Natl Acad. Sci. USA 103, 19647–19651 (2006).
Monnereau, C. et al. Porphyrin macrocyclic catalysts for the processive oxidation of polymer substrates. J. Am. Chem. Soc. 132, 1529–1531 (2010).
Deutman, A. B. C., Cantekin, S., Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Designing processive catalytic systems. Threading polymers through flexible macrocycle ring. J. Am. Chem. Soc. 136, 9165–9172 (2014).
Hidalgo Ramos, P., Saisaha, P., Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Conformational analysis and binding properties of a cavity containing porphyrin catalyst provided with urea functions. Eur. J. Org. Chem., 4487–4495 (2016).
De Bo, G. et al. Efficient assembly of threaded molecular machines for sequence-specific synthesis. J. Am. Chem. Soc. 136, 5811–5814 (2014).
Lewandowski, B. et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339, 189–193 (2013).
McGonigal, P. R. & Stoddart, J. F. Interlocked molecules: a molecular production line. Nat. Chem. 5, 260–262 (2013).
Bertran-Vicente, J. & Hackenberger, C. P. R. A. Supramolecular peptide synthesizer. Angew. Chem. Int. Ed. 52, 6140–6142 (2013).
Wilson, C. M., Gualandi, A. & Cozzi, P. G. A. Rotaxane turing machine for peptides. ChemBioChem 14, 1185–1187 (2013).
Miyagawa, N. et al. Successive catalytic reactions specific to Pd-based rotaxane complexes as a result of wheel translation along the axle. Chem. Commun. 46, 1920–1922 (2010).
Tachibana, Y., Kihara, N. & Takata, T. Asymmetric benzoin condensation catalyzed by chiral rotaxanes tethering a thiazolium salt moiety via the cooperation of the component: can rotaxane be an effective reaction field? J. Am. Chem. Soc. 126, 3438–3439 (2004).
Xu, K., Nakazono, K. & Takata, T. Design of rotaxane catalyst for O-acylative asymmetric desymmetrization of meso-1,2-diol utilizing the cooperative effect of the components. Chem. Lett. 45, 1274–1276 (2016).
van Dongen, S. F. M. et al. A clamp-like biohybrid catalyst for DNA oxidation. Nat. Chem. 5, 945–951 (2013).
Berná, J. et al. Macroscopic transport by synthetic molecular machines. Nat. Mater. 4, 704–710 (2005).
Liu, Y. et al. Linear artificial molecular muscles. J. Am. Chem. Soc. 127, 9745–9759 (2005).
Iamsaard, S. et al. Conversion of light into macroscopic helical motion. Nat. Chem. 6, 229–235 (2014).
Schäfer, C. et al. An artificial molecular transporter. ChemistryOpen 5, 120–124 (2016).
Kassem, S., Lee, A. T. L., Leigh, D. A., Markevicius, A. & Solà, J. Pick-up, transport and release of a molecular cargo using a small-molecule robotic arm. Nat. Chem. 8, 138–143 (2016).
Chen, J., Wezenberg, S. J. & Feringa, B. L. Intramolecular transport of small-molecule cargo in a nanoscale device operated by light. Chem. Commun. 52, 6765–6768 (2016).
Kassem, S. et al. Stereodivergent synthesis with a programmable molecular machine. Nature 549, 374–378 (2017).
Yoon, T. P. & Jacobsen, E. N. Privileged chiral catalysts. Science 299, 1691–1693 (2003).
Sigma-Aldrich. Privileged ligands. ChemFiles 8 https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Aldrich/Brochure/al_chemfile_v6_n8.pdf (2006).
Acknowledgements
The authors are grateful to the Engineering and Physical Sciences Research Council (EPSRC) Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1) for studentships, generously supported by AstraZeneca, Diamond Light Source, Defence Science and Technology Laboratory, Evotec, GlaxoSmithKline, Janssen, Novartis, Pfizer, Syngenta, Takeda, UCB and Vertex.
Author information
Authors and Affiliations
Contributions
L.v.D., M.J.T., R.S., O.A.S. and H.A.P.B. contributed equally to the preparation of the article and are listed in reverse alphabetical order. S.P.F. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Glossary
- Molecular machines
-
Systems in which a stimulus triggers the controlled motion of one molecular or submolecular component relative to another and potentially results in a net task (or work) being done.
- Molecular switches
-
Molecules that can be reversibly shifted between two or more stable states. An important distinction between molecular switches and motors is that when switches return to their original state, any mechanical work is undone.
- Allosteric regulation
-
The regulation of the structure and activity of a catalyst by the binding of a ligand at a site topologically distinct from the catalytically active site.
- Chemoselectivity
-
The preferential reaction of one functional group over another in a chemical reaction.
- Stereoselectivity
-
The preferential formation of one stereoisomer over another in a chemical reaction. If the stereoisomers are enantiomers, enantioselectivity applies (quantified by the enantiomeric excess, e.e., or enantiomeric ratio, e.r.); if they are diastereomers, diastereoselectivity applies (quantified by the diastereomeric ratio, d.r.).
- Mechanical bonding
-
This results from an interlocked molecular architecture. Mechanically interlocked molecules cannot be separated without breaking covalent bonds.
- Distributive catalysis
-
The most common mode of operation for homogeneous and heterogeneous catalysis in which conversion occurs at a single site before dissociation of the catalyst.
- Processive catalysis
-
A process in which a catalyst remains attached to the substrate and performs multiple rounds of catalysis before dissociation.
- Stereodivergent synthesis
-
A synthetic approach capable of selectively producing all the possible stereoisomers of a molecule.
Rights and permissions
About this article
Cite this article
van Dijk, L., Tilby, M., Szpera, R. et al. Molecular machines for catalysis. Nat Rev Chem 2, 0117 (2018). https://doi.org/10.1038/s41570-018-0117
Published:
DOI: https://doi.org/10.1038/s41570-018-0117
This article is cited by
-
Atomically precise photothermal nanomachines
Nature Materials (2024)
-
Ratcheting synthesis
Nature Reviews Chemistry (2023)
-
From autocatalysis to survival of the fittest in self-reproducing lipid systems
Nature Reviews Chemistry (2023)
-
Switchable aqueous catalytic systems for organic transformations
Communications Chemistry (2022)
-
Enantiodivergent epoxidation of alkenes with a photoswitchable phosphate manganese-salen complex
Nature Synthesis (2022)