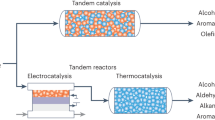
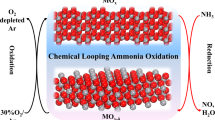
Abstract
Chemical looping offers a versatile platform to convert fuels and oxidizers in a clean and efficient manner. Central to this technology are metal oxide materials that can oxidize fuels, affording a reduced material that can be reoxidized to close the loop. Recent years have seen substantial advances in the design, formulation and manufacture of these oxygen carrier materials and their incorporation into chemical looping reactors for the production of various chemicals. This Review describes the mechanisms by which oxygen carriers undergo redox reactions and how these carriers can be incorporated into robust chemical looping reactors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Fan, L.-S. Chemical Looping Systems for Fossil Energy Conversions (Wiley, New York, 2010).
Lyngfelt, A. & Linderholm, C. Chemical-looping combustion of solid fuels — status and recent progress. Energy Procedia 114, 371–386 (2017).
Boot-Handford, M. E. et al. Carbon capture and storage update. Energy Environ. Sci. 7, 130–189 (2014).
Zhao, X. et al. Biomass-based chemical looping technologies: the good, the bad and the future. Energy Environ. Sci. 10, 1885–1910 (2017).
Adanez, J., Abad, A., Garcia-Labiano, F., Gayan, P. & de Diego, L. F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 38, 215–282 (2012).
Thursfield, A., Murugan, A., Franca, R. & Metcalfe, I. S. Chemical looping and oxygen permeable ceramic membranes for hydrogen production — a review. Energy Environ. Sci. 5, 7421–7459 (2012).
Luo, S., Zeng, L. & Fan, L.-S. Chemical looping technology: oxygen carrier characteristics. Annu. Rev. Chem. Biomol. Eng. 6, 53–75 (2015).
Buelens, L. C., Galvita, V. V., Poelman, H., Detavernier, C. & Marin, G. B. Super-dry reforming of methane intensifies CO2 utilization via Le Chatelier’s principle. Science 354, 449–452 (2016). This work demonstrates that oxygen carriers can be used in combination with other carrier materials to enable broader applications of chemical looping technology.
Hua, X. & Wang, W. Chemical looping combustion: a new low-dioxin energy conversion technology. J. Environ. Sci. 32, 135–145 (2015).
Song, T. et al. Nitrogen transfer of fuel-N in chemical looping combustion. Combust. Flame 159, 1286–1295 (2012).
Ishida, M., Zheng, D. & Akehata, T. Evaluation of a chemical-looping–combustion power-generation system by graphic exergy analysis. Energy 12, 147–154 (1987).
Dennis, J. S., Müller, C. R. & Scott, S. A. In situ gasification and CO2 separation using chemical looping with a Cu-based oxygen carrier: performance with bituminous coals. Fuel 89, 2353–2364 (2010).
Song, T. & Shen, L. Review of reactor for chemical looping combustion of solid fuels. Int. J. Greenhouse Gas Control 76, 92–110 (2018).
Fan, L.-S. Chemical Looping Partial Oxidation: Gasification, Reforming, and Chemical Syntheses (Cambridge Univ. Press, Cambridge, 2017). This book includes comprehensive descriptions of various partial oxidation processes involving chemical looping schemes.
Mattisson, T., Lyngfelt, A. & Leion, H. Chemical-looping with oxygen uncoupling for combustion of solid fuels. Int. J. Greenhouse Gas Control 3, 11–19 (2009).
Luo, S. et al. Shale gas-to-syngas chemical looping process for stable shale gas conversion to high purity syngas with a H2: CO ratio of 2: 1. Energy Environ. Sci. 7, 4104–4117 (2014).
Chen, S., Zeng, L., Tian, H., Li, X. & Gong, J. Enhanced lattice oxygen reactivity over Ni modified WO3-based redox catalysts for chemical looping partial oxidation of methane. ACS Catal. 7, 3548–3559 (2017). This work shows how bulk phase doping and surface modifications of mixed oxides can improve the performances of these oxygen-carrying materials.
Mihai, O., Chen, D. & Holmen, A. Chemical looping methane partial oxidation: the effect of the crystal size and O content of LaFeO3. J. Catal. 293, 175–185 (2012).
He, F., Galinsky, N. & Li, F. Chemical looping gasification of solid fuels using bimetallic oxygen carrier particles — feasibility assessment and process simulations. Int. J. Hydrogen Energy 38, 7839–7854 (2013).
Galvita, V. V., Poelman, H. & Marin, G. B. Hydrogen production from methane and carbon dioxide by catalyst-assisted chemical looping. Top. Catal. 54, 907–913 (2011).
Bhavsar, S., Najera, M., Solunke, R. & Veser, G. Chemical looping: to combustion and beyond. Catal. Today 228, 96–105 (2014).
Voitic, G. & Hacker, V. Recent advancements in chemical looping water splitting for the production of hydrogen. RSC Adv. 6, 98267–98296 (2016).
Jiang, Q. et al. Catalytic function of IrOx in the two-step thermochemical CO2-splitting reaction at high temperatures. ACS Catal. 6, 1172–1180 (2016).
Muhich, C. L. et al. Efficient generation of H2 by splitting water with an isothermal redox cycle. Science 341, 540–542 (2013).
Zhang, J., Haribal, V. & Li, F. Perovskite nanocomposites as effective CO2-splitting agents in a cyclic redox scheme. Sci. Adv. 3, e1701184 (2017).
Contractor, R. M. Dupont’s CFB technology for maleic anhydride. Chem. Eng. Sci. 54, 5627–5632 (1999).
Patience, G. S. & Bockrath, R. E. Butane oxidation process development in a circulating fluidized bed. Appl. Catal., A 376, 4–12 (2010).
Naeem, M. A. et al. Optimization of the structural characteristics of CaO and its effective stabilization yield high-capacity CO2 sorbents. Nat. Comm. 9, 2408 (2018).
Brunet, S., Mey, D., Pérot, G., Bouchy, C. & Diehl, F. On the hydrodesulfurization of FCC gasoline: a review. Appl. Catal., A 278, 143–172 (2005).
Michalsky, R., Avram, A. M., Peterson, B. A., Pfromm, P. H. & Peterson, A. A. Chemical looping of metal nitride catalysts: low-pressure ammonia synthesis for energy storage. Chem. Sci. 6, 3965–3974 (2015).
Laassiri, S., Zeinalipour-Yazdi, C. D., Catlow, C. R. A. & Hargreaves, J. S. J. The potential of manganese nitride based materials as nitrogen transfer reagents for nitrogen chemical looping. Appl. Catal., B 223, 60–66 (2018).
Hepworth, T. C. Oxygen for limelight. Nature 47, 176–177 (1892).
Hardie, D. W. F. & Pratt, J. D. A History of the Modern British Chemical Industry (Pergamon Press, Oxford, 1966).
Bergmann, F. J. Process for the production of calcium carbide in blast furnaces. German patent 29384 (1897).
Lewis, W. K. & Gilliland, E. R. Conversion of hydrocarbons with suspended catalyst. US Patent 2498088 (1950).
Lewis, W. K. & Gilliland, E. R. Production of pure carbon dioxide. US Patent 2665971 (1954).
Institute of Gas Technology. Development of the Steam-Iron Process for Hydrogen Production (US Department Energy, 1979).
Richter, H. J. & Knoche, K. F. Reversibility of combustion processes. ACS Symp. Ser. 235, 71–85 (1983).
Ströhle, J., Orth, M. & Epple, B. Design and operation of a 1MWth chemical looping plant. Appl. Energy 113, 1490–1495 (2014).
Whitty, K., Lighty, J. & Fry, A. Development and Scale-Up of Copper-Based Chemical Looping with Oxygen Uncoupling. Presented at the 4th International Conference on Chemical Looping (Nanjing, China, 2016).
Langorgen, O., Saanum, I. & Haugen, N. E. L. Performance of a 150 kW Chemical Looping Combustion Reactor System for Gaseous Fuels Using a Copper-Based Oxygen Carrier. Presented at the 4th International Conference on Chemical Looping (Nanjing, China, 2016).
Andrus, H. E., Brautsch, A. & Beal, C. Alstom’s chemical looping coal-fired power plant development program. Proc. Int. Tech. Conf. Coal Util. Fuel Syst. 2, 993–1004 (2007).
Fan, L.-S. Chemical Looping Technology for Combustion, Gasification, Reforming, and Chemical Syntheses: Redox Reaction Mechanism and Technology Commercialization Readiness. Presented at the 255th National Meeting and Exposition of the American-Chemical-Society (New Orleans, USA, 2018).
Corcoran, A., Knutsson, P., Lind, F. & Thunman, H. Comparing the structural development of sand and rock ilmenite during long-term exposure in a biomass fired 12MWth CFB-boiler. Fuel Process. Technol. 171, 39–44 (2018).
Shen, L. H. 3MWth Pilot Demonstration of Chemical Looping Combustion and Gasification of Coal in China. Presented at the 6th International Conference on CO2 Emission Control and Utilization (Hangzhou, China, 2018).
Li, Z. S. Demonstration of CLC Technology in Semi-Industrial Scale via a Chinese-European Collaboration. Presented at the 6th International Conference on CO2 Emission Control and Utilization (Hangzhou, China, 2018).
Lane, H. Process of producing hydrogen. US Patent 1078686 (1913).
Reed, H. C. & Berg, C. H. Hydrogen. US Patent 2635947 (1953).
Dobbyn, R. C. et al. Evaluation of the Performance of Materials and Components Used in the CO2 Acceptor Process Gasification Pilot Plant (US Department Energy, 1978).
Rao, C. N. R. & Dey, S. Solar thermochemical splitting of water to generate hydrogen. Proc. Natl Acad. Sci. USA 114, 13385–13393 (2017).
Muhich, C. L. et al. A review and perspective of efficient hydrogen generation via solar thermal water splitting. WIREs Energy Environ. 5, 261–287 (2016).
Haribal, V. P., He, F., Mishra, A. & Li, F. Iron-doped BaMnO3 for hybrid water splitting and syngas generation. ChemSusChem 10, 3402–3408 (2017).
Otsuka, K., Wang, Y., Sunada, E. & Yamanaka, I. Direct partial oxidation of methane to synthesis gas by cerium oxide. J. Catal. 175, 152–160 (1998).
Steinfeld, A., Kuhn, P. & Karni, J. High-temperature solar thermochemistry: production of iron and synthesis gas by Fe3O4-reduction with methane. Energy 18, 239–249 (1993).
Zheng, Y. et al. Designed oxygen carriers from macroporous LaFeO3 supported CeO2 for chemical-looping reforming of methane. Appl. Catal., B 202, 51–63 (2017).
Zhao, K. et al. Exploration of the mechanism of chemical looping steam methane reforming using double perovskite-type oxides La1.6Sr0.4FeCoO6. Appl. Catal., B 219, 672–682 (2017).
Kulkarni, P. et al. Fuel-Flexible Gasification-Combustion Technology for Production of H2 and Sequestration-Ready CO2 (US Department Energy, 2008).
Jia, Z. et al. Design Basis of 1 MWth Calcium Looping Gasification Pilot Unit. Presented at the 2018 International Pittsburgh Coal Conference (Xuzhou, China, 2018).
Chung, C., Qin, L., Shah, V. & Fan, L.-S. Chemically and physically robust, commercially-viable iron-based composite oxygen carriers sustainable over 3000 redox cycles at high temperatures for chemical looping applications. Energy Environ. Sci. 10, 2318–2323 (2017).
Fan, L.-S., Zeng, L., Wang, W. & Luo, S. Chemical looping processes for CO2 capture and carbonaceous fuel conversion — prospect and opportunity. Energy Environ. Sci. 5, 7254–7280 (2012).
Zeng, L., Kathe, M. V., Chung, E. Y. & Fan, L.-S. Some remarks on direct solid fuel combustion using chemical looping processes. Curr. Opin. Chem. Eng. 1, 290–295 (2012).
Qin, L. et al. Nanostructure formation mechanism and ion diffusion in iron–titanium composite materials with chemical looping redox reactions. J. Mater. Chem. A 3, 11302–11312 (2015). This paper details ionic diffusion in a mixed-oxide system and how this is related to morphology changes during chemical looping processes.
Cavani, F. & Trifirò, F. The oxidative dehydrogenation of ethane and propane as an alternative way for the production of light olefins. Catal. Today 24, 307–313 (1995).
Keller, G. E. & Bhasin, M. M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts. J. Catal. 73, 9–19 (1982).
Neal, L. M., Yusuf, S., Sofranko, J. A. & Li, F. Oxidative dehydrogenation of ethane: a chemical looping approach. Energy Technol. 4, 1200–1208 (2016).
Chung, E. Y. et al. Catalytic oxygen carriers and process systems for oxidative coupling of methane using the chemical looping technology. Ind. Eng. Chem. Res. 55, 12750–12764 (2016).
Brady, C., Murphy, B. & Xu, B. Enhanced methane dehydroaromatization via coupling with chemical looping. ACS Catal. 7, 3924–3928 (2017).
Sushkevich, V. L., Palagin, D., Ranocchiari, M. & van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356, 523–527 (2017). This work describes the potential application of chemical looping processes in the direct syntheses of chemicals from simple feedstocks.
Grasselli, R. K., Stern, D. L. & Tsikoyiannis, J. G. Catalytic dehydrogenation (DH) of light paraffins combined with selective hydrogen combustion (SHC): I. DH SHC DH catalysts in series (co-fed process mode). Appl. Catal., A 189, 1–8 (1999).
Grasselli, R. K., Stern, D. L. & Tsikoyiannis, J. G. Catalytic dehydrogenation (DH) of light paraffins combined with selective hydrogen combustion (SHC): II. DH+ SHC catalysts physically mixed (redox process mode). Appl. Catal., A 189, 9–14 (1999).
Mamedov, E. A. & Cortés Corberán, V. Oxidative dehydrogenation of lower alkanes on vanadium oxide-based catalysts. The present state of the art and outlooks. Appl. Catal., A 127, 1–40 (1995).
Al-Ghamdi, S. A. & de Lasa, H. I. Propylene production via propane oxidative dehydrogenation over VOx/γ-Al2O3 catalyst. Fuel 128, 120–140 (2014).
Gao, Y., Neal, L. M. & Li, F. Li-promoted LaxSr2−xFeO4−δ core–shell redox catalysts for oxidative dehydrogenation of ethane under a cyclic redox scheme. ACS Catal. 6, 7293–7302 (2016).
Yusuf, S., Neal, L. M. & Li, F. Effect of promoters on manganese-containing mixed metal oxides for oxidative dehydrogenation of ethane via a cyclic redox scheme. ACS Catal. 7, 5163–5173 (2017).
Yusuf, S. et al. Manganese silicate based redox catalysts for greener ethylene production via chemical looping — oxidative dehydrogenation of ethane. Appl. Catal., B 232, 77–85 (2018).
Novotný, P., Yusuf, S., Li, F. & Lamb, H. H. Oxidative dehydrogenation of ethane using MoO3/Fe2O3 catalysts in a cyclic redox mode. Catal. Today. https://doi.org/10.1016/j.cattod.2018.02.046 (2018).
Gao, Y., Haeri, F., He, F. & Li, F. Alkali metal-promoted LaxSr2−xFeO4−δ redox catalysts for chemical looping oxidative dehydrogenation of ethane. ACS Catal. 8, 1757–1766 (2018).
Chan, M. S. C., Marek, E., Scott, S. A. & Dennis, J. S. Chemical looping epoxidation. J. Catal. 359, 1–7 (2018).
Chan, M. S. C. et al. Improving hydrogen yields, and hydrogen:steam ratio in the chemical looping production of hydrogen using Ca2Fe2O5. Chem. Eng. J. 296, 406–411 (2016).
Zhao, K. et al. Perovskite-type oxides LaFe1−xCoxO3 for chemical looping steam methane reforming to syngas and hydrogen co-production. Appl. Energy 168, 193–203 (2016).
Rydén, M. et al. Novel oxygen-carrier materials for chemical-looping combustion and chemical-looping reforming; LaxSr1−xFeyCo1−yO3−δ perovskites and mixed-metal oxides of NiO, Fe2O3 and Mn3O4. Int. J. Greenhouse Gas Control 2, 21–36 (2008).
Fan, L.-S., Tong, A. & Zeng, L. in Multiphase Reactor Engineering for Clean and Low–Carbon Energy Applications (eds Cheng, Y., Wei, F. & Jin, Y.) 377–400 (Wiley, 2017).
Imtiaz, Q., Hosseini, D. & Müller, C. R. Review of oxygen carriers for chemical looping with oxygen uncoupling (CLOU): thermodynamics, material development, and synthesis. Energy Technol. 1, 633–647 (2013).
Lau, C. Y., Dunstan, M. T., Hu, W., Grey, C. P. & Scott, S. A. Large scale in silico screening of materials for carbon capture through chemical looping. Energy Environ. Sci. 10, 818–831 (2017). This paper describes how advanced computational tools can be used for the screening of new potential oxygen carriers.
Adánez, J. et al. Selection of oxygen carriers for chemical-looping combustion. Energy Fuels 18, 371–377 (2004).
Andersson, D. A., Simak, S. I., Skorodumova, N. V., Abrikosov, I. A. & Johansson, B. Optimization of ionic conductivity in doped ceria. Proc. Natl Acad. Sci. USA 103, 3518–3521 (2006).
Kümmerle, E. A. & Heger, G. The Structures of C–Ce2O3+δ, Ce7O12, and Ce11O20. J. Solid State Chem. 147, 485–500 (1999).
Yang, Z., Woo, T. K., Baudin, M. & Hermansson, K. Atomic and electronic structure of unreduced and reduced CeO2 surfaces: a first-principles study. J. Chem. Phys. 120, 7741–7749 (2004).
Nolan, M., Parker, S. C. & Watson, G. W. The electronic structure of oxygen vacancy defects at the low index surfaces of ceria. Surf. Sci. 595, 223–232 (2005).
Cheng, Z., Sherman, B. J. & Lo, C. S. Carbon dioxide adsorption and activation on ceria (110): a density functional theory study. J. Chem. Phys. 138, 014702 (2013).
Paier, J., Penschke, C. & Sauer, J. Oxygen defects and surface chemistry of ceria: quantum chemical studies compared to experiment. Chem. Rev. 113, 3949–3985 (2013).
Mahato, N., Banerjee, A., Gupta, A., Omar, S. & Balani, K. Progress in material selection for solid oxide fuel cell technology: a review. Prog. Mater. Sci. 72, 141–337 (2015).
Dai, X. P., Li, R. J., Yu, C. C. & Hao, Z. P. Unsteady-state direct partial oxidation of methane to synthesis gas in a fixed-bed reactor using AFeO3 (A = La, Nd, Eu) perovskite-type oxides as oxygen storage. J. Phys. Chem. B 110, 22525–22531 (2006).
Mihai, O., Chen, D. & Holmen, A. Catalytic consequence of oxygen of lanthanum ferrite perovskite in chemical looping reforming of methane. Ind. Eng. Chem. Res. 50, 2613–2621 (2011).
Nalbandian, L., Evdou, A. & Zaspalis, V. La1−xSrxMyFe1−yO3−δ perovskites as oxygen-carrier materials for chemical-looping reforming. Int. J. Hydrogen Energy 36, 6657–6670 (2011).
Pineau, A., Kanari, N. & Gaballah, I. Kinetics of reduction of iron oxides by H2: part I: low temperature reduction of hematite. Thermochim. Acta 447, 89–100 (2006).
Pineau, A., Kanari, N. & Gaballah, I. Kinetics of reduction of iron oxides by H2: part II. Low temperature reduction of magnetite. Thermochim. Acta 456, 75–88 (2007).
Thomas, T., Fan, L.-S., Gupta, P. & Velazquez-Vargas, L. G. Combustion looping using composite oxygen carriers. US Patent 7767191 (2003).
Tong, A., Zeng, L., Kathe, M. V., Sridhar, D. & Fan, L.-S. Application of the moving-bed chemical looping process for high methane conversion. Energy Fuels 27, 4119–4128 (2013).
Cheng, Z. et al. Methane adsorption and dissociation on iron oxide oxygen carriers: the role of oxygen vacancies. Phys. Chem. Chem. Phys. 18, 16423–16435 (2016).
Hsia, C., Pierre, G. R. St., Raghunathan, K. & Fan, L.-S. Diffusion through CaSO4 formed during the reaction of CaO with SO2 and O2. AIChE J. 39, 698–700 (1993).
Hsia, C., Pierre, G. R. S. & Fan, L.-S. Isotope study on diffusion in CaSO4 formed during sorbent-flue-gas reaction. AIChE J. 41, 2337–2340 (1995).
Ando, K. & Oishi, Y. Self-diffusion coefficients of oxygen ion in single crystals of MgO·nAl2O3 spinels. J. Chem. Phys. 61, 625–629 (1974).
Oishi, Y. & Ando, K. Self-diffusion of oxygen in polycrystalline MgAl2O4. J. Chem. Phys. 63, 376–378 (1975).
Reddy, K. P. R. & Cooper, A. R. Oxygen diffusion in MgO and α-Fe2O3. J. Am. Ceram. Soc. 66, 664–666 (1983).
Uberuaga, B. P. et al. Defect kinetics in spinels: long-time simulations of MgAl2O4, MgGa2O4, and MgIn2O4. Phys. Rev. B 75, 104116 (2007).
Wilson, C. et al. Structure and properties of ilmenite from first principles. Phys. Rev. B 71, 075202 (2005).
Sun, Z., Luo, S. & Fan, L.-S. Ionic transfer mechanism of COS reaction with CaO: inert marker experiment and density functional theory (DFT) calculation. AIChE J. 58, 2617–2620 (2012).
Nakamura, R., Tokozakura, D., Nakajima, H., Lee, J. G. & Mori, H. Hollow oxide formation by oxidation of Al and Cu nanoparticles. J. Appl. Phys. 101, 074303 (2007).
Qin, L., Majumder, A., Fan, J. A., Kopechek, D. & Fan, L.-S. Evolution of nanoscale morphology in single and binary metal oxide microparticles during reduction and oxidation processes. J. Mater. Chem. A 2, 17511–17520 (2014).
Fu, Y., Chen, J. & Zhang, H. Synthesis of Fe2O3 nanowires by oxidation of iron. Chem. Phys. Lett. 350, 491–494 (2001).
Wen, X., Wang, S., Ding, Y., Wang, Z. L. & Yang, S. Controlled growth of large-area, uniform, vertically aligned arrays of α-Fe2O3 nanobelts and nanowires. J. Phys. Chem. B 109, 215–220 (2005).
Dang, H. Y., Wang, J. & Fan, S. S. The synthesis of metal oxide nanowires by directly heating metal samples in appropriate oxygen atmospheres. Nanotechnology 14, 738 (2003).
He, F., Wei, Y., Li, H. & Wang, H. Synthesis gas generation by chemical-looping reforming using Ce-based oxygen carriers modified with Fe, Cu, and Mn oxides. Energy Fuels 23, 2095–2102 (2009).
Li, F. et al. Syngas chemical looping gasification process: oxygen carrier particle selection and performance. Energy Fuels 23, 4182–4189 (2009).
Azimi, G., Mattisson, T., Leion, H., Rydén, M. & Lyngfelt, A. Comprehensive study of Mn–Fe–Al oxygen-carriers for chemical-looping with oxygen uncoupling (CLOU). Int. J. Greenhouse Gas Control 34, 12–24 (2015).
de Diego, L. F. et al. Synthesis gas generation by chemical-looping reforming in a batch fluidized bed reactor using Ni-based oxygen carriers. Chem. Eng. J. 144, 289–298 (2008).
Qin, L., Cheng, Z., Guo, M., Fan, J. A. & Fan, L.-S. Morphology evolution and nanostructure of chemical looping transition metal oxide materials upon redox processes. Acta Mater. 124, 568–578 (2017).
Shafiefarhood, A., Zhang, J., Neal, L. M. & Li, F. Rh-promoted mixed oxides for “low-temperature” methane partial oxidation in the absence of gaseous oxidants. J. Mater. Chem. A 5, 11930–11939 (2017).
Jin, Y., Sun, C. & Su, S. Experimental and theoretical study of the oxidation of ventilation air methane over Fe2O3 and CuO. Phys. Chem. Chem. Phys. 17, 16277–16284 (2015).
Cheng, Z. et al. Oxygen vacancy promoted methane partial oxidation over iron oxide oxygen carriers in the chemical looping process. Phys. Chem. Chem. Phys. 18, 32418–32428 (2016).
Neal, L. M., Shafiefarhood, A. & Li, F. Dynamic methane partial oxidation using a Fe2O3@La0.8Sr0.2FeO3−δ core–shell redox catalyst in the absence of gaseous oxygen. ACS Catal. 4, 3560–3569 (2014). This work describes a new core–shell catalytic oxygen carrier and the mechanism by which it converts methane into syngas.
Shafiefarhood, A., Galinsky, N., Huang, Y., Chen, Y. & Li, F. Fe2O3@LaxSr1−xFeO3 core–shell redox catalyst for methane partial oxidation. ChemCatChem 6, 790–799 (2014).
Liss, W. E. Impacts of shale gas advancements on natural gas utilization in the United States. Energy Technol. 2, 953–967 (2014).
Fleischer, V. et al. Investigation of the role of the Na2WO4/Mn/SiO2 catalyst composition in the oxidative coupling of methane by chemical looping experiments. J. Catal. 360, 102–117 (2018).
Borchert, H. & Baerns, M. The effect of oxygen-anion conductivity of metal–oxide doped lanthanum oxide catalysts on hydrocarbon selectivity in the oxidative coupling of methane. J. Catal. 168, 315–320 (1997).
Cheng, Z. & Lo, C. S. Effect of support structure and composition on the catalytic activity of Pt nanoclusters for methane dehydrogenation. Ind. Eng. Chem. Res. 52, 15447–15454 (2013).
Liu, S., Tan, X., Li, K. & Hughes, R. Methane coupling using catalytic membrane reactors. Catal. Rev. 43, 147–198 (2001).
Voskresenskaya, E. N., Roguleva, V. G. & Anshits, A. G. Oxidant activation over structural defects of oxide catalysts in oxidative methane coupling. Catal. Rev. 37, 101–143 (1995).
Malekzadeh, A. et al. Correlation of electrical properties and performance of OCM MOx/Na2WO4/SiO2 catalysts. Catal. Commun. 2, 241–247 (2001).
Greish, A. A. et al. Oxidative coupling of methane in the redox cyclic mode over the catalysts on the basis of CeO2 and La2O3. Mendeleev Commun. 20, 28–30 (2010).
Sung, J. S. et al. Peculiarities of oxidative coupling of methane in redox cyclic mode over Ag–La2O3/SiO2 catalysts. Appl. Catal., A 380, 28–32 (2010).
Huang, K., Zhan, X.-L., Chen, F.-Q. & Lü, D.-W. Catalyst design for methane oxidative coupling by using artificial neural network and hybrid genetic algorithm. Chem. Eng. Sci. 58, 81–87 (2003).
Chua, Y. T., Mohamed, A. R. & Bhatia, S. Oxidative coupling of methane for the production of ethylene over sodium-tungsten-manganese-supported-silica catalyst (Na-W-Mn/SiO2). Appl. Catal., A 343, 142–148 (2008).
Hou, Y.-H., Han, W.-C., Xia, W.-S. & Wan, H.-L. Structure sensitivity of La2O2CO3 catalysts in the oxidative coupling of methane. ACS Catal. 5, 1663–1674 (2015).
Cheng, Z. et al. C2 selectivity enhancement in chemical looping oxidative coupling of methane over a Mg–Mn composite oxygen carrier by Li-doping-induced oxygen vacancies. ACS Energy Lett. 3, 1730–1736 (2018).
Cao, Y., Sit, S. P. & Pan, W.-P. Preparation and characterization of lanthanum-promoted copper-based oxygen carriers for chemical looping combustion process. Aerosol Air Qual. Res. 14, 572–584 (2014).
Cao, Y., Zhao, H.-Y., Sit, S. P. & Pan, W.-P. Lanthanum-promoted copper-based oxygen carriers for chemical looping combustion process. J. Therm. Anal. Calorim. 116, 1257–1266 (2014).
Liu, L. & Zachariah, M. R. Enhanced performance of alkali metal doped Fe2O3 and Fe2O3/Al2O3 composites as oxygen carrier material in chemical looping combustion. Energy Fuels 27, 4977–4983 (2013).
Mohamed, S. A., Quddus, M. R., Razzak, S. A., Hossain, M. M. & de Lasa, H. I. Fluidizable NiO/Ce-γAl2O3 oxygen carrier for chemical looping combustion. Energy Fuels 29, 6095–6103 (2015).
Wang, M., Liu, J., Hu, J. & Liu, F. O2–CO2 mixed gas production using a Zr-doped Cu-based oxygen carrier. Ind. Eng. Chem. Res. 54, 9805–9812 (2015).
Imtiaz, Q., Kurlov, A., Rupp, J. L. M. & Müller, C. R. Highly efficient oxygen-storage material with intrinsic coke resistance for chemical looping combustion-based CO2 capture. ChemSusChem 8, 2055–2065 (2015).
Qin, L. et al. Impact of 1% lanthanum dopant on carbonaceous fuel redox reactions with an iron-based oxygen carrier in chemical looping processes. ACS Energy Lett. 2, 70–74 (2017). This article discloses how very low concentrations of a metal oxide dopant can lower the energy barrier for C–H bond activation.
Qin, L. et al. Enhanced methane conversion in chemical looping partial oxidation systems using a copper doping modification. Appl. Catal., B 235, 143–149 (2018).
Chroneos, A., Yildiz, B., Tarancón, A., Parfitt, D. & Kilner, J. A. Oxygen diffusion in solid oxide fuel cell cathode and electrolyte materials: mechanistic insights from atomistic simulations. Energy Environ. Sci. 4, 2774–2789 (2011).
Acknowledgements
L.Z. and J.G. thank the National Key R&D Program of China (2016YFB0600901), the National Natural Science Foundation of China (Grants 21525626 and U1663224) and the Program of Introducing Talents of Discipline to Universities of China (Grant B06006). J.A.F. acknowledges support from the US National Science Foundation (Grant 1804224) and the Packard Fellowship Foundation. Z.C. and L.-S.F. thank the US National Science Foundation (Grant 1236467), Ohio State University (funding from the C. John Easton Professorship in Engineering) and the Ohio Supercomputer Center.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, L., Cheng, Z., Fan, J.A. et al. Metal oxide redox chemistry for chemical looping processes. Nat Rev Chem 2, 349–364 (2018). https://doi.org/10.1038/s41570-018-0046-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0046-2
This article is cited by
-
Achieving surface and bulk rate matching for chemical looping partial oxidation of methane by modulating oxygen transport
Science China Materials (2024)
-
Concerted oxygen diffusion across heterogeneous oxide interfaces for intensified propane dehydrogenation
Nature Communications (2023)
-
Lithium carbonate-promoted mixed rare earth oxides as a generalized strategy for oxidative coupling of methane with exceptional yields
Nature Communications (2023)
-
Chemical looping: a technology platform for upcycling low-grade industrial resources
Discover Chemical Engineering (2023)
-
Chemical looping approaches to decarbonization via CO2 repurposing
Discover Chemical Engineering (2023)