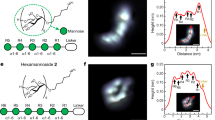
Abstract
Attempts to elucidate the roles of carbohydrate-associated structures in biology have led to the distinct field of glycobiology research. The focus of this field has been in understanding the evolution, biosynthesis and interactions of glycans, both individually and as components of larger biomolecules. However, as most approaches for studying glycans (including mass spectrometry and various binding assays) use ensemble measurements, they lack the precision required to uncover the discrete roles of glycoconjugates, which are often heterogeneous, in biomolecular processes. Single-molecule techniques can examine individual events within challenging mixtures, and they are beginning to be applied to glycobiology. For example, single-molecule force spectroscopy (SMFS) by atomic force microscopy (AFM) has enabled the molecular interactions of sugars to be studied, single-molecule fluorescence microscopy and spectroscopy have led to insight into the role of sugars in biological processes and nanopores have revealed interactions between polysaccharides and their transporters. Thus, single-molecule technology is becoming a valuable tool in glycoscience.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schnaar, R. L. Glycobiology simplified: diverse roles of glycan recognition in inflammation. J. Leukoc. Biol. 99, 825–838 (2016).
Dwek, R. A. Glycobiology: toward understanding the function of sugars. Chem. Rev. 96, 683–720 (1996).
Varki, A. Biological roles of glycans. Glycobiology 27, 3–49 (2017).
Seeberger, P. H. Chemical glycobiology: why now? Nat. Chem. Biol. 5, 368–372 (2009).
Imperiali, B. The chemistry–glycobiology frontier. J. Am. Chem. Soc. 134, 17835–17839 (2012).
Wang, L.-X. & Davis, B. G. Realizing the promise of chemical glycobiology. Chem. Sci. 4, 3381–3394 (2013).
Bertozzi, C. R. & Kiessling, L. L. Chemical glycobiology. Science 291, 2357 (2001).
Krishnamoorthy, L. & Mahal, L. K. Glycomic analysis: an array of technologies. ACS Chem. Biol. 4, 715–732 (2009).
Dube, D. H., Champasa, K. & Wang, B. Chemical tools to discover and target bacterial glycoproteins. Chem. Comm. 47, 87–101 (2011).
Grice, I. D. & Wilson, J. C. in Microbial Glycobiology (eds Brennan, J. P., von Itzstein, V. & Holst, O.) 233–252 (Academic Press, 2010).
Mulloy, B., Hart, G. W. & Stanley, P. in Essentials of Glycobiology 2nd edn (eds Varki, A. et al.) 661–678 (Cold Spring Harbor Laboratory Press, 2009).
Gray, C. J. et al. Applications of ion mobility mass spectrometry for high throughput, high resolution glycan analysis. Biochim. Biophys. Acta 1860, 1688–1709 (2016).
Hofmann, J., Hahm, H. S., Seeberger, P. H. & Pagel, K. Identification of carbohydrate anomers using ion mobility-mass spectrometry. Nature 526, 241–244 (2015). This is a striking proof of principle that ion mobility mass spectrometry can rapidly identify different glycan regioisomers and stereoisomers (here with an emphasis on synthetic products), even at low proportions.
Goldberg, D. et al. Automated N-glycopeptide identification using a combination of single- and tandem-MS. J. Proteome Res. 6, 3995–4005 (2007).
Novotny, M. V., Alley, W. R. & Mann, B. F. Analytical glycobiology at high sensitivity: current approaches and directions. Glycoconj. J. 30, 89–117 (2013).
Gaunitz, S., Nagy, G., Pohl, N. L. B. & Novotny, M. V. Recent advances in the analysis of complex glycoproteins. Anal. Chem. 89, 389–413 (2017).
Both, P. et al. Discrimination of epimeric glycans and glycopeptides using IM–MS and its potential for carbohydrate sequencing. Nat. Chem. 6, 65–74 (2014). This study complements reference 13 as it explores the application of ion mobility mass spectrometry to model glycopeptides and oligosaccharides (with an emphasis on future ‘sequencing’ of naturally derived samples).
Nagy, G. & Pohl, N. L. B. Monosaccharide identification as a first step toward de novo carbohydrate sequencing: mass spectrometry strategy for the identification and differentiation of diastereomeric and enantiomeric pentose isomers. Anal. Chem. 87, 4566–4571 (2015).
Nagy, G. & Pohl, N. L. B. Complete hexose isomer identification with mass spectrometry. J. Am. Soc. Mass Spectrom. 26, 677–685 (2015).
Schindler, B. et al. Anomeric memory of the glycosidic bond upon fragmentation and its consequences for carbohydrate sequencing. Nat. Commun. 8, 973 (2017).
Rees, D. A. & Welsh, E. J. Secondary and tertiary structure of polysaccharides in solutions and gels. Angew. Chem. Int. Ed. Engl. 16, 214–224 (1977).
Yuriev, E., Farrugia, W., Scott, A. M. & Ramsland, P. A. Three-dimensional structures of carbohydrate determinants of Lewis system antigens: implications for effective antibody targeting of cancer. Immunol. Cell Biol. 83, 709 (2005).
Yaffe, N. R., Almond, A. & Blanch, E. W. A. New route to carbohydrate secondary and tertiary structure using raman spectroscopy and raman optical activity. J. Am. Chem. Soc. 132, 10654–10655 (2010).
Johannessen, C., Pendrill, R., Widmalm, G., Hecht, L. & Barron, L. D. Glycan structure of a high-mannose glycoprotein from raman optical activity. Angew. Chem. Int. Ed. 50, 5349–5351 (2011).
Barry, C. S. et al. ‘Naked’ and hydrated conformers of the conserved core pentasaccharide of N-linked glycoproteins and its building blocks. J. Am. Chem. Soc. 135, 16895–16903 (2013).
Seeberger, P. H. The logic of automated glycan assembly. Acc. Chem. Res. 48, 1450–1463 (2015).
Sletten, E. M. & Bertozzi, C. R. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed 48, 6974–6998 (2009).
Griffin, M. E. & Hsieh-Wilson, L. C. Synthetic probes of glycosaminoglycan function. Curr. Opin. Chem. Biol. 17, 1014–1022 (2013).
Griffin, M. E. & Hsieh-Wilson, L. C. Glycan engineering for cell and developmental biology. Cell Chem. Biol. 23, 108–121 (2016).
Krasnova, L. & Wong, C.-H. Understanding the chemistry and biology of glycosylation with glycan synthesis. Ann. Rev. Biochem. 85, 599–630 (2016).
Berti, F. & Adamo, R. Recent mechanistic insights on glycoconjugate vaccines and future perspectives. ACS Chem. Biol. 8, 1653–1663 (2013).
Bernardi, A. et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 42, 4709–4727 (2013).
Astronomo, R. D. & Burton, D. R. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 9, 308–324 (2010).
Lin, W., Du, Y., Zhu, Y. & Chen, X. A. cis-Membrane FRET-based method for protein-specific imaging of cell-surface glycans. J. Am. Chem. Soc. 136, 679–687 (2014).
Belardi, B., O’Donoghue, G. P., Smith, A. W., Groves, J. T. & Bertozzi, C. R. Investigating cell surface galectin-mediated cross-linking on glycoengineered cells. J. Am. Chem. Soc. 134, 9549–9552 (2012).
Adibekian, A. et al. Comparative bioinformatics analysis of the mammalian and bacterial glycomes. Chem. Sci. 2, 337–344 (2011).
Laine, R. A. Invited commentary: a calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 1012 structures for a reducing hexasaccharide: the isomer barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 4, 759–767 (1994).
Mammen, M., Choi, S.-K. & Whitesides, G. M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. 37, 2754–2794 (1998).
Lundquist, J. J. & Toone, E. J. The cluster glycoside effect. Chem. Rev. 102, 555–578 (2002).
Hunter, C. A. & Anderson, H. L. What is cooperativity? Angew. Chem. Int. Ed. 48, 7488–7499 (2009).
Geissner, A. & Seeberger, P. H. Glycan arrays: from basic biochemical research to bioanalytical and biomedical applications. Ann. Rev. Anal. Chem. 9, 223–247 (2016).
Laurent, N., Voglmeir, J. & Flitsch, S. L. Glycoarrays-tools for determining protein-carbohydrate interactions and glycoenzyme specificity. Chem. Commun. 37, 4400–4412 (2008).
Fais, M. et al. Surface plasmon resonance imaging of glycoarrays identifies novel and unnatural carbohydrate-based ligands for potential ricin sensor development. Chem. Sci. 2, 1952–1959 (2011).
Dam, T. K. & Brewer, C. F. Thermodynamic studies of lectin–carbohydrate interactions by isothermal titration calorimetry. Chem. Rev. 102, 387–430 (2002).
Gestwicki, J. E., Cairo, C. W., Strong, L. E., Oetjen, K. A. & Kiessling, L. L. Influencing receptor–ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 124, 14922–14933 (2002).
Kitov, P. I. & Bundle, D. R. On the nature of the multivalency effect: a thermodynamic model. J. Am. Chem. Soc. 125, 16271–16284 (2003). This is a prescient and unduly underappreciated analysis of the contribution that degeneracy of states could make in the thermodynamics behind the multivalent effect; such detailed analyses (and the experiments to test them) remain rare.
Mulder, A., Huskens, J. & Reinhoudt, D. N. Multivalency in supramolecular chemistry and nanofabrication. Org. Biomol. Chem. 2, 3409–3424 (2004).
Tinoco, I. & Gonzalez, R. L. Biological mechanisms, one molecule at a time. Genes Dev. 25, 1205–1231 (2011).
Sauer, M. M. et al. Catch-bond mechanism of the bacterial adhesin FimH. Nat. Commun. 7, 10738 (2016). This is a multi-technique study that combines structural biology and molecular dynamics with binding kinetic analyses to propose the basis of a negatively regulated, lectin-containing assembly used by pathogenic bacteria. Such molecularly detailed mechanistic proposals remain rare in glycobiology.
Pejchal, R. et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334, 1097–1103 (2011).
Scholl, Z. N., Li, Q. & Marszalek, P. E. Single molecule mechanical manipulation for studying biological properties of proteins, DNA, and sugars. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 6, 211–229 (2014).
Marszalek, P. E. & Dufrene, Y. F. Stretching single polysaccharides and proteins using atomic force microscopy. Chem. Soc. Rev. 41, 3523–3534 (2012).
Zhao, W., Cai, M., Xu, H., Jiang, J. & Wang, H. A single-molecule force spectroscopy study of the interactions between lectins and carbohydrates on cancer and normal cells. Nanoscale 5, 3226–3229 (2013).
Li, Y. et al. Molecular recognition force spectroscopy study of the specific lectin and carbohydrate interaction in a living cell. Chem. Phys. Chem. 12, 909–912 (2011).
Mazmanian, S. K. & Kasper, D. L. The love–hate relationship between bacterial polysaccharides and the host immune system. 6, 849 (2006).
Hecht, M.-L., Stallforth, P., Silva, D. V., Adibekian, A. & Seeberger, P. H. Recent advances in carbohydrate-based vaccines. Curr. Opin. Chem. Biol. 13, 354–359 (2009).
Anish, C., Schumann, B., Pereira, C. L. & Seeberger, P. H. Chemical biology approaches to designing defined carbohydrate vaccines. Chem. Biol. 21, 38–50 (2014).
Joyce, J. G. et al. An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions. Proc. Nat. Acad. Sci. USA 105, 15684–15689 (2008).
Pashov, A., Garimalla, S., Monzavi-Karbassi, B. & Kieber-Emmons, T. Carbohydrate targets in HIV vaccine research: lessons from failures. Immunotherapy 1, 777–794 (2009).
Martines, E., García, I., Marradi, M., Padro, D. & Penadés, S. Dissecting the carbohydrate specificity of the Anti-HIV-1 2G12 antibody by single-molecule force spectroscopy. Langmuir 28, 17726–17732 (2012).
Hearty, S., Conroy, P. J., Ayyar, B. V., Byrne, B. & O’Kennedy, R. Surface plasmon resonance for vaccine design and efficacy studies: recent applications and future trends. Expert Rev. Vaccines 9, 645–664 (2010).
Lynch, H. E., Stewart, S. M., Kepler, T. B., Sempowski, G. D. & Alam, S. M. Surface plasmon resonance measurements of plasma antibody avidity during primary and secondary responses to anthrax protective antigen. J. Immunol. Methods 404, 1–12 (2014).
Haugstad, K. E. et al. Enhanced self-association of mucins possessing the T and Tn carbohydrate cancer antigens at the single-molecule level. Biomacromolecules 13, 1400–1409 (2012).
Garcia-Manyes, S. et al. Proteoglycan mechanics studied by single-molecule force spectroscopy of allotypic cell adhesion glycans. J. Biol. Chem. 281, 5992–5999 (2006).
Vilanova, E. et al. Carbohydrate–carbohydrate interactions mediated by sulfate esters and calcium provide the cell adhesion required for the emergence of early metazoans. J. Biol. Chem. 291, 9425–9437 (2016).
Beaussart, A. et al. Molecular mapping of the cell wall polysaccharides of the human pathogen Streptococcus agalactiae. Nanoscale 6, 14820–14827 (2014).
El-Kirat-Chatel, S. et al. Single-molecule analysis of the major glycopolymers of pathogenic and non-pathogenic yeast cells. Nanoscale 5, 4855–4863 (2013).
Zhang, M., Wang, B. & Xu, B. Measurements of single molecular affinity interactions between carbohydrate-binding modules and crystalline cellulose fibrils. Phys. Chem. Chem. Phys. 15, 6508–6515 (2013).
Zhang, M., Wang, B. & Xu, B. Mapping single molecular binding kinetics of carbohydrate-binding module with crystalline cellulose by atomic force microscopy recognition imaging. J. Phys. Chem. B 118, 6714–6720 (2014).
Zhang, M., Wu, S.-C., Zhou, W. & Xu, B. Imaging and measuring single-molecule interaction between a carbohydrate-binding module and natural plant cell wall cellulose. J. Phys. Chem. B 116, 9949–9956 (2012).
Lee, S., Mandic, J. & Van Vliet, K. J. Chemomechanical mapping of ligand–receptor binding kinetics on cells. Proc. Nat. Acad. Sci. USA 104, 9609–9614 (2007).
Zhang, Q. & Marszalek, P. E. Identification of sugar isomers by single-moleculef force spectroscopy. J. Am. Chem. Soc. 128, 5596–5597 (2006).
Neuman, K. C. & Nagy, A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 5, 491 (2008).
Björnham, O., Bugaytsova, J., Borén, T. & Schedin, S. Dynamic force spectroscopy of the Helicobacter pylori BabA–Lewis b Binding. Biophys. Chem. 143, 102–105 (2009).
Choi, Y. et al. Single-molecule dynamics of lysozyme processing distinguishes linear and cross-linked peptidoglycan substrates. J. Am. Chem. Soc. 134, 2032–2035 (2012).
La Clair, J. J. Selective detection of the carbohydrate-bound state of concanvalin A at the single molecule level. J. Am. Chem. Soc. 119, 7676–7684 (1997).
Dagel, D. J. et al. In situ imaging of single carbohydrate-binding modules on cellulose microfibrils. J. Phys. Chem. B 115, 635–641 (2011).
Li, G.-W. & Xie, X. S. Central dogma at the single-molecule level in living cells. Nature 475, 308–315 (2011).
Yu, J., Xiao, J., Ren, X., Lao, K. & Xie, X. S. Probing gene expression in live cells, one protein molecule at a time. Science 311, 1600–1603 (2006).
Choi, P. J., Cai, L., Frieda, K. & Xie, X. S. A stochastic single-molecule event triggers phenotype switching of a bacterial cell. Science 322, 442–446 (2008).
Majumdar, D. S. et al. Single-molecule FRET reveals sugar-induced conformational dynamics in LacY. Proc. Nat. Acad. Sci. USA 104, 12640–12645 (2007).
Abramson, J. et al. The lactose permease of Escherichia coli: overall structure, the sugar-binding site and the alternating access model for transport. FEBS Lett. 555, 96–101 (2003).
Abramson, J. et al. Structure and mechanism of the lactose permease of Escherichia coli. Science 301, 610–615 (2003).
Komura, N. et al. Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat. Chem. Biol. 12, 402–410 (2016). This paper highlights that the synthesis of appropriately labelled glycans (here gangliosides) that retain function (creating ‘rules’ that inform) can support single-molecule (TIRF in this study) methods.
Spiegel, S., Kassis, S., Wilchek, M. & Fishman, P. H. Direct visualization of redistribution and capping of fluorescent gangliosides on lymphocytes. J. Cell Biol. 99, 1575–1581 (1984).
Möckl, L., Lindhorst, T. K. & Bräuchle, C. Artificial formation and tuning of glycoprotein networks on live cell membranes: a single-molecule tracking study. ChemPhysChem 17, 829–835 (2016).
Zhao, W. et al. Studying the nucleated mammalian cell membrane by single molecule approaches. PLOS ONE 9, e91595 (2014).
Chen, J. et al. Systemic localization of seven major types of carbohydrates on cell membranes by dSTORM imaging. Sci. Rep. 6, 30247 (2016).
Letschert, S. et al. Super-resolution imaging of plasma membrane glycans. Angew. Chem. Int. Ed. 53, 10921–10924 (2014).
Jiang, H., English, B. P., Hazan, R. B., Wu, P. & Ovryn, B. Tracking surface glycans on live cancer cells with single-molecule sensitivity. Angew. Chem. Int. Ed. 54, 1765–1769 (2015).
Fox, J. M. et al. A single-molecule analysis reveals morphological targets for cellulase synergy. Nat. Chem. Biol. 9, 356–361 (2013). Using photoactivatable mEos2–CBM fusions, this study describes how PALM enabled the derivation of order parameters (using principal component analysis of super-resolution images) that quantified CBM organization on different cellulosic substrates. The findings move beyond the current crystalline versus amorphous classifications.
Howorka, S., Nam, J., Bayley, H. & Kahne, D. Stochastic detection of monovalent and bivalent protein–ligand interactions. Angew. Chem. Int. Ed. 43, 842–846 (2004). This study describes a prescient model system that allowed the observation of lectin–sugar binding events to a glycan-modified nanopore.
Kong, L., Almond, A., Bayley, H. & Davis, B. G. Chemical polyglycosylation and nanolitre detection enables single-molecule recapitulation of bacterial sugar export. Nat. Chem. 8, 461–469 (2016).
Kong, L. et al. Single-molecule interrogation of a bacterial sugar transporter allows the discovery of an extracellular inhibitor. Nat. Chem. 5, 651–659 (2013).
Kong, L. et al. An antibacterial vaccination strategy based on a glycoconjugate containing the core lipopolysaccharide tetrasaccharide Hep2Kdo2. Nat. Chem. 8, 242–249 (2016).
Suginta, W. & Smith, M. F. Single-molecule trapping dynamics of sugar-uptake channels in marine bacteria. Phys. Rev. Lett. 110, 238102 (2013).
Wang, Q., Goldsmith, R. H., Jiang, Y., Bockenhauer, S. D. & Moerner, W. E. Probing single biomolecules in solution using the anti-brownian electrokinetic (ABEL) trap. Acc. Chem. Res. 45, 1955–1964 (2012).
Im, J. et al. Electronic single-molecule identification of carbohydrate isomers by recognition tunnelling. Nat. Commun. 7, 13868 (2016). This study demonstrates that the creation of a tunnel junction that is functionalized with molecules that may interact with glycans (here the 2-carboxamide of imidazole) leads to current fluctuations that may be analysed with principal component analysis to distinguish configurational isomers. This study also highlights that even quite nonspecific ‘binders’ may be used in principle to dissect glycan composition.
Dufrene, Y. F. et al. Five challenges to bringing single-molecule force spectroscopy into living cells. Nat. Methods 8, 123–127 (2011).
Varki, A. et al. Symbol nomenclature for graphical representations of glycans. Glycobiology 25, 1323–1324 (2015).
Li, Y. et al. Molecular recognition force spectroscopy of a specific lectin–carbohydrate interaction at single-molecule level. J. Struct. Biol. 176, 46–51 (2011).
Acknowledgements
The authors thank S. Faulkner, M. Bilyard and K. Wals for proofreading and useful comments.
Reviewer information
Nature Reviews Chemistry thanks Xing Chen, Isabelle Compagnon, Lara Mahal and Bingqian Xu for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.L., M.R. and B.G.D. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
B.G.D. is named as an inventor on patents that describe new antibiotics based on some of the Wza science described in this Review. If licensed, these would afford B.G.D. royalties in line with normal university practice.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Force–distance cycles
-
The repeated measurement of force as a function of distance in atomic force microscopy.
- Total internal reflection fluorescence
-
(TIRF). Microscopy examining a thin region of a specimen (~100 nm) that uses internal reflection at a surface–sample interface for the selective excitation of surface-associated fluorophores, thereby removing contributions from the background.
- Stochastic optical reconstruction microscopy
-
(STORM). Dilute fluorophores are switched on and off through switching or bleaching, and the ‘centres’ of their location are ascertained using point-spread of photon emission based on a widefield image. After several rounds, a ‘super-resolution’ image is assembled by plotting the measured positions of these centres of the fluorescent probes.
- Photoactivated localization microscopy
-
(PALM). Similar to STORM but has typically used photoactivation and then bleaching to switch fluorophores on and off. It is more often associated with the use of protein fluorophores such as photoactivatable fluorescent proteins.
Rights and permissions
About this article
Cite this article
Lakshminarayanan, A., Richard, M. & Davis, B.G. Studying glycobiology at the single-molecule level. Nat Rev Chem 2, 148–159 (2018). https://doi.org/10.1038/s41570-018-0019-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0019-5
This article is cited by
-
Cell surface glycan engineering reveals that matriglycan alone can recapitulate dystroglycan binding and function
Nature Communications (2022)
-
Soft condensed matter physics of foods and macronutrients
Nature Reviews Physics (2019)