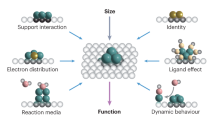
Abstract
Single-atom catalysis has arguably become the most active new frontier in heterogeneous catalysis. Aided by recent advances in practical synthetic methodologies, characterization techniques and computational modelling, we now have a large number of single-atom catalysts (SACs) that exhibit distinctive performances for a wide variety of chemical reactions. This Perspective summarizes recent experimental and computational efforts aimed at understanding the bonding in SACs and how this relates to catalytic performance. The examples described here illustrate the utility of SACs in a broad scope of industrially important reactions and highlight the advantages these catalysts have over those presently used. SACs have well-defined active centres, such that unique opportunities exist for the rational design of new catalysts with high activities, selectivities and stabilities. Indeed, given a certain practical application, we can often design a suitable SAC; thus, the field has developed very rapidly and afforded promising catalyst leads. Moreover, the control we have over certain SAC structures paves the way for designing base metal catalysts with the activities of noble metal catalysts. It appears that we are entering a new era of heterogeneous catalysis in which we have control over well-dispersed single-atom active sites whose properties we can readily tune.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ye, R., Hurlburt, T. J., Sabyrov, K., Alayoglu, S. & Somorjai, G. A. Molecular catalysis science: perspective on unifying the fields of catalysis. Proc. Natl Acad. Sci. USA 113, 5159–5166 (2016).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeO x . Nat. Chem. 3, 634–641 (2011).
Taylor, H. S. A theory of the catalytic surface. Proc. R. Soc. Lond. A 108, 105–111 (1925).
Rooney, J. J. & Webb, G. The importance of π-bonded intermediates in hydrocarbon reactions on transition metal catalysts. J. Catal. 3, 488–501 (1964).
Patterson, W. R. & Rooney, J. J. Single atom sites and hydrocarbon reaction mechanisms. Catal. Today 12, 113–129 (1992).
Böhme, D. K. & Schwarz, H. Gas-phase catalysis by atomic and cluster metal ions: the ultimate single-site catalysts. Angew. Chem. Int. Ed. 44, 2336–2354 (2005).
Kirlin, P. S. & Gates, B. C. Activation of the C–C bond provides a molecular basis for structure sensitivity in metal catalysis. Nature 325, 38–40 (1987).
Vidal, V., Théolier, A., Thivolle-Cazat, J. & Basset, J.-M. Metathesis of alkanes catalyzed by silica-supported transition metal hydrides. Science 276, 99–102 (1997).
Copéret, C., Chabanas, M., Saint-Arroman, R. P. & Basset, J.-M. Homogeneous and heterogeneous catalysis: bridging the gap through surface organometallic chemistry. Angew. Chem. Int. Ed. 42, 156–181 (2003).
Serna, P. & Gates, B. C. Molecular metal catalysts on supports: organometallic chemistry meets surface science. Acc. Chem. Res. 47, 2612–2620 (2014).
Bayram, E. et al. Agglomerative sintering of an atomically dispersed Ir-1/Zeolite Y catalyst: compelling evidence against Ostwald ripening but for bimolecular and autocatalytic agglomeration catalyst sintering steps. ACS Catal. 5, 3514–3527 (2015).
Serna, P., Yardimci, D., Kistler, J. D. & Gates, B. C. Formation of supported rhodium clusters from mononuclear rhodium complexes controlled by the support and ligands on rhodium. Phys. Chem. Chem. Phys. 16, 1262–1270 (2014).
Serna, P. & Gates, B. C. A bifunctional mechanism for ethene dimerization: catalysis by rhodium complexes on Zeolite HY in the absence of halides. Angew. Chem. Int. Ed. 50, 5528–5531 (2011).
Asakura, K., Nagahiro, H., Ichikuni, N. & Iwasawa, Y. Structure and catalytic combustion activity of atomically dispersed Pt species at MgO surface. Appl. Catal. A Gen. 188, 313–324 (1999).
Li, Z. Y. et al. Three-dimensional atomic-scale structure of size-selected gold nanoclusters. Nature 451, 46–48 (2008).
Kaden, W. E., Wu, T., Kunkel, W. A. & Anderson, S. L. Electronic structure controls reactivity of size-selected Pd clusters adsorbed on TiO2 surfaces. Science 326, 826–829 (2009).
Vajda, S. et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 8, 213–216 (2009).
Abbet, S. et al. Acetylene cyclotrimerization on supported size-selected Pd n clusters (1 ≤ n ≤ 30): one atom is enough. J. Am. Chem. Soc. 122, 3453–3457 (2000).
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301, 935–938 (2003).
Zhang, X., Shi, H. & Xu, B.-Q. Catalysis by gold: isolated surface Au3+ ions are active sites for selective hydrogenation of 1,3-butadiene over Au/ZrO2 catalysts. Angew. Chem. Int. Ed. 44, 7132–7135 (2005).
Hackett, S. F. J. et al. High-activity, single-site mesoporous Pd/Al2O3 catalysts for selective aerobic oxidation of allylic alcohols. Angew. Chem. Int. Ed. 46, 8593–8596 (2007).
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Thomas, J. M., Raja, R. & Lewis, D. W. Single-site heterogeneous catalysts. Angew. Chem. Int. Ed. 44, 6456–6482 (2005).
Liu, J. Aberration-corrected scanning transmission electron microscopy in single-atom catalysis: probing the catalytically active centers. Chin. J. Catal. 38, 1460–1472 (2017).
Ogino, I. X-Ray absorption spectroscopy for single-atom catalysts: critical importance and persistent challenges. Chin. J. Catal. 38, 1481–1488 (2017).
Asokan, C., DeRita, L. & Christopher, P. Using probe molecule FTIR spectroscopy to identify and characterize Pt-group metal based single atom catalysts. Chin. J. Catal. 38, 1473–1480 (2017).
Parkinson, G. S. Unravelling single atom catalysis: the surface science approach. Chin. J. Catal. 38, 1454–1459 (2017).
Hutchings, G. J. Vapor phase hydrochlorination of acetylene: correlation of catalytic activity of supported metal chloride catalysts. J. Catal. 96, 292–295 (1985).
Malta, G. et al. Identification of single-site gold catalysis in acetylene hydrochlorination. Science 355, 1399–1403 (2017).
Wei, H. et al. FeO x -supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 5, 5634 (2014).
Zhou, H. et al. PdZn intermetallic nanostructure with Pd−Zn−Pd ensembles for highly active and chemoselective semi-hydrogenation of acetylene. ACS Catal. 6, 1054–1061 (2016).
Matsubu, J. C., Yang, V. N. & Christopher, P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 137, 3076–3084 (2015).
Yang, S., Kim, J., Tak, Y. J., Soon, A. & Lee, H. Single-atom catalyst of platinum supported on titanium nitride for selective electrochemical reactions. Angew. Chem. Int. Ed. 55, 2058–2062 (2016).
Qiao, B. et al. Highly efficient catalysis of preferential oxidation of CO in H2-rich stream by gold single-atom catalysts. ACS Catal. 5, 6249–6254 (2015).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Zhang, Z. et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation. Nat. Commun. 8, 16100 (2017).
Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335, 1209–1212 (2012).
Liu, W. et al. Single-atom dispersed Co–N–C catalyst: structure identification and performance for hydrogenative coupling of nitroarenes. Chem. Sci. 7, 5758–5764 (2016).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Wei, H. et al. Iced photochemical reduction to synthesize atomically dispersed metals by suppressing nanocrystal growth. Nat. Commun. 8, 1490 (2017).
Yan, H. et al. Single-atom Pd1/graphene catalyst achieved by atomic layer deposition: remarkable performance in selective hydrogenation of 1,3-butadiene. J. Am. Chem. Soc. 137, 10484–10487 (2015).
Yin, P. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem. Int. Ed. 55, 10800–10805 (2016).
Liu, J. Catalysis by supported single metal atoms. ACS Catal. 7, 34–59 (2017).
Schwarz, H. Ménage-à-trois: single-atom catalysis, mass spectrometry, and computational chemistry. Catal. Sci. Technol. 7, 4302–4314 (2017).
Flytzani-Stephanopoulos, M. Supported metal catalysts at the single-atom limit — a viewpoint.Chin. J. Catal. 38, 1432–1442 (2017).
Kim, J., Kim, H.-E. & Lee, H. Single-atom catalysts of precious metals for electrochemical reactions. ChemSusChem 11, 104–113 (2018).
Zhu, C., Fu, S., Shi, Q., Du, D. & Lin, Y. Single-atom electrocatalysts. Angew. Chem. Int. Ed. 56, 13944–13960 (2017).
Qiao, B. T. et al. Ultrastable single-atom gold catalysts with strong covalent metal–support interaction (CMSI). Nano Res. 8, 2913–2924 (2015).
Lin, J. et al. Remarkable performance of Ir1/FeO x single-atom catalyst in water gas shift reaction. J. Am. Chem. Soc. 135, 15314–15317 (2013).
Lin, J. et al. Little do more: a highly effective Pt1/FeO x single-atom catalyst for the reduction of NO by H2. Chem. Commun. 51, 7911–7914 (2015).
Liang, J.-X. et al. Theoretical and experimental investigations on single-atom catalysis: Ir1/FeO x for CO oxidation. J. Phys. Chem. C 118, 21945–21951 (2014).
Novotný, Z. et al. Ordered array of single adatoms with remarkable thermal stability: Au/Fe3O4(001). Phys. Rev. Lett. 108, 216103 (2012).
Parkinson, G. S. et al. Carbon monoxide-induced adatom sintering in a Pd–Fe3O4 model catalyst. Nat. Mater. 12, 724–728 (2013).
Bliem, R. et al. Cluster nucleation and growth from a highly supersaturated adatom phase: silver on magnetite. ACS Nano 8, 7531–7537 (2014).
Bliem, R. et al. Adsorption and incorporation of transition metals at the magnetite Fe3O4(001) surface. Phys. Rev. B 92, 075440 (2015).
Bliem, R. et al. Subsurface cation vacancy stabilization of the magnetite (001) surface. Science 346, 1215–1218 (2014).
Zhang, S. et al. Catalysis on singly dispersed bimetallic sites. Nat. Commun. 6, 7938 (2015).
Ma, X.-L., Liu, J.-C., Xiao, H. & Li, J. Surface single-cluster catalyst for N2-to-NH3 thermal conversion. J. Am. Chem. Soc. 140, 46–49 (2018).
Bruix, A. et al. Maximum noble-metal efficiency in catalytic materials: atomically dispersed surface platinum. Angew. Chem. Int. Ed. 53, 10525–10530 (2014).
Neitzel, A. et al. Atomically dispersed Pd, Ni, and Pt species in ceria-based catalysts: principal differences in stability and reactivity. J. Phys. Chem. C 120, 9852–9862 (2016).
Dvorák, F. et al. Creating single-atom Pt–ceria catalysts by surface step decoration. Nat. Commun. 7, 10801 (2016).
Zhang, S. et al. Solid frustrated-Lewis-pair catalysts constructed by regulations on surface defects of porous nanorods of CeO2. Nat. Commun. 8, 15266 (2017).
Zhang, B. et al. Stabilizing a platinum1 single-atom catalyst on supported phosphomolybdic acid without compromising hydrogenation activity. Angew. Chem. Int. Ed. 55, 8319–8323 (2016).
Huang, W. et al. Low-temperature transformation of methane to methanol on Pd1O4 single sites anchored on the internal surface of microporous silicate. Angew. Chem. Int. Ed. 55, 13441–13445 (2016).
Cotton, A. G. W. F., Murillo, C. A. & Bochmann, M. Advanced Inorganic Chemistry 6th edn (Wiley, New York, 1999).
Peterson, E. J. et al. Low-temperature carbon monoxide oxidation catalysed by regenerable atomically dispersed palladium on alumina. Nat. Commun. 5, 4885 (2014).
Kwak, J. H. et al. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on γ-Al2O3. Science 325, 1670–1673 (2009).
Bulushev, D. A. et al. Single atoms of Pt-group metals stabilized by N-doped carbon nanofibers for efficient hydrogen production from formic acid. ACS Catal. 6, 3442–3451 (2016).
Vilé, G. et al. A stable single-site palladium catalyst for hydrogenations. Angew. Chem. Int. Ed. 54, 11265–11269 (2015).
Li, X. et al. Single-atom Pt as co-catalyst for enhanced photocatalytic H2 evolution. Adv. Mater. 28, 2427–2431 (2016).
Gao, G., Jiao, Y., Waclawik, E. R. & Du, A. J. Single atom (Pd/Pt) supported on graphitic carbon nitride as an efficient photocatalyst for visible-light reduction of carbon dioxide. J. Am. Chem. Soc. 138, 6292–6297 (2016).
Li, F. Y., Li, Y. F., Zeng, X. C. & Chen, Z. F. Exploration of high-performance single-atom catalysts on support M1/FeO x for CO oxidation via computational study. ACS Catal. 5, 544–552 (2015).
Choi, C. H. et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun. 7, 10922 (2016).
Choi, M., Wu, Z. & Iglesia, E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation. J. Am. Chem. Soc. 132, 9129–9137 (2010).
Fei, H. et al. Atomic cobalt on nitrogen-doped graphene for hydrogen generation. Nat. Commun. 6, 8668 (2015).
Wu, H. et al. Highly doped and exposed Cu(i)–N active sites within graphene towards efficient oxygen reduction for zinc–air batteries. Energy Environ. Sci. 9, 3736–3745 (2016).
Qiu, H.-J. et al. Nanoporous graphene with single-atom nickel dopants: an efficient and stable catalyst for electrochemical hydrogen production. Angew. Chem. Int. Ed. 54, 14031–14035 (2015).
Cui, X. et al. A graphene composite material with single cobalt active sites: a highly efficient counter electrode for dye-sensitized solar cells. Angew. Chem. Int. Ed. 55, 6708–6712 (2016).
Chen, Y. et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 56, 6937–6941 (2017).
Chen, P. et al. Atomically dispersed iron–nitrogen species as electrocatalysts for bifunctional oxygen evolution and reduction reactions. Angew. Chem. Int. Ed. 56, 610–614 (2017).
Zitolo, A. et al. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 14, 937–942 (2015).
Zitolo, A. et al. Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 8, 957 (2017).
Zhang, L. et al. Co−N−C catalyst for C–C coupling reactions: on the catalytic performance and active sites. ACS Catal. 5, 6563–6572 (2015).
Liu, W. et al. Discriminating catalytically active FeN x species of atomically dispersed Fe–N–C catalyst for selective oxidation of C–H bond. J. Am. Chem. Soc. 139, 10790–10798 (2017).
Yang, M. et al. Catalytically active Au-O(OH) x species stabilized by alkali ions on zeolites and mesoporous oxides. Science 346, 1498–1501 (2014).
Yang, M. et al. A common single-site Pt(ii)−O(OH) x − species stabilized by sodium on “active” and “inert” supports catalyzes the water–gas shift reaction. J. Am. Chem. Soc. 137, 3470–3473 (2015).
Wei, H. et al. Remarkable effect of alkalis on the chemoselective hydrogenation of functionalized nitroarenes over high-loading Pt/FeO x catalysts. Chem. Sci. 8, 5126–5131 (2017).
Zhang, L. et al. Efficient and durable Au alloyed Pd single-atom catalyst for the Ullmann reaction of aryl chlorides in water. ACS Catal. 4, 1546–1553 (2014).
Lucci, F. R. et al. Controlling hydrogen activation, spillover, and desorption with Pd–Au single-atom alloys. J. Phys. Chem. Lett. 7, 480–485 (2016).
Pei, G. X. et al. Ag alloyed Pd single-atom catalysts for efficient selective hydrogenation of acetylene to ethylene in excess ethylene. ACS Catal. 5, 3717–3725 (2015).
Pei, G. X. et al. Performance of Cu-alloyed Pd single-atom catalyst for semihydrogenation of acetylene under simulated front-end conditions. ACS Catal. 7, 1491–1500 (2017).
Feng, Q. et al. Isolated single-atom Pd sites in intermetallic nanostructures: high catalytic selectivity for semihydrogenation of alkynes. J. Am. Chem. Soc. 139, 7294–7301 (2017).
Lucci, F. R. et al. Selective hydrogenation of 1,3-butadiene on platinum–copper alloys at the single-atom limit. Nat. Commun. 6, 8550 (2015).
Liu, J. et al. Tackling CO poisoning with single-atom alloy catalysts. J. Am. Chem. Soc. 138, 6396–6399 (2016).
Yang, M., Allard, L. F. & Flytzani-Stephanopoulos, M. Atomically dispersed Au−(OH) x species bound on titania catalyze the low-temperature water-gas shift reaction. J. Am. Chem. Soc. 135, 3768–3771 (2013).
Wang, C., Yang, M. & Flytzani-Stephanopoulos, M. Single gold atoms stabilized on nanoscale metal oxide supports are catalytic active centers for various reactions. AIChE J. 62, 429–439 (2016).
Guan, H. et al. Enhanced performance of Rh1/TiO2 catalyst without methanation in water–gas shift reaction. AIChE J. 63, 2081–2088 (2017).
Ding, K. et al. Identification of active sites in CO oxidation and water–gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Guo, L.-W. et al. Contributions of distinct gold species to catalytic reactivity for carbon monoxide oxidation. Nat. Commun. 7, 13481 (2016).
Guan, H. et al. Catalytically active Rh sub-nanoclusters on TiO2 for CO oxidation at cryogenic temperatures. Angew. Chem. Int. Ed. 55, 2820–2824 (2016).
Therrien, A. J. et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 1, 192–198 (2018).
Wang, C. et al. Water-mediated Mars–Van Krevelen mechanism for CO oxidation on ceria-supported single-atom Pt1 catalyst. ACS Catal. 7, 887–891 (2017).
Li, L. et al. Origin of the high activity of Au/FeO x for low-temperature CO oxidation: direct evidence for a redox mechanism. J. Catal. 299, 90–100 (2013).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Yi, N., Si, R., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active gold species on cerium oxide nanoshapes for methanol steam reforming and the water gas shift reactions. Energy Environ. Sci. 3, 831–837 (2010).
Gu, X.-K. et al. Supported single Pt1/Au1 atoms for methanol steam reforming. ACS Catal. 4, 3886–3890 (2014).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Bayatsarmadi, B., Zheng, Y., Vasileff, A. & Qiao, S.-Z. Recent advances in atomic metal doping of carbon-based nanomaterials for energy conversion. Small 13, 1700191 (2017).
Wu, G. & Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 46, 1878–1889 (2013).
Cheng, N. et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun. 7, 13638 (2016).
Liu, J. et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun. 8, 15938 (2017).
Deng, J. et al. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 8, 1594–1601 (2015).
Zhang, S. et al. High catalytic activity and chemoselectivity of sub-nanometric Pd clusters on porous nanorods of CeO2 for hydrogenation of nitroarenes. J. Am. Chem. Soc. 138, 2629–2637 (2016).
Porosoff, M. D., Yan, B. & Chen, J. G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: challenges and opportunities. Energy Environ. Sci. 9, 62–73 (2016).
Kwak, J. H., Kovarik, L. & Szanyi, J. Heterogeneous catalysis on atomically dispersed supported metals: CO2 reduction on multifunctional Pd catalysts. ACS Catal. 3, 2094–2100 (2013).
Kwak, J. H., Kovarik, L. & Szanyi, J. CO2 reduction on supported Ru/Al2O3 catalysts: cluster size dependence of product selectivity. ACS Catal. 3, 2449–2455 (2013).
Li, S. et al. Tuning the selectivity of catalytic carbon dioxide hydrogenation over iridium/cerium oxide catalysts with a strong metal–support interaction. Angew. Chem. Int. Ed. 56, 10761–10765 (2017).
Cheng, M.-J., Clark, E. L., Pham, H. H., Bell, A. T. & Head-Gordon, M. Quantum mechanical screening of single-atom bimetallic alloys for the selective reduction of CO2 to C1 hydrocarbons. ACS Catal. 6, 7769–7777 (2016).
Back, S., Lim, J., Kim, N.-Y., Kim, Y.-H. & Jung, Y. Single-atom catalysts for CO2 electroreduction with significant activity and selectivity improvements. Chem. Sci. 8, 1090–1096 (2017).
Back, S. & Jung, Y. TiC- and TiN-supported single-atom catalysts for dramatic improvements in CO2 electrochemical reduction to CH4. ACS Energy Lett. 2, 969–975 (2017).
Sarfraz, S., Garcia-Esparza, A. T., Jedidi, A., Cavallo, L. & Takanabe, K. Cu–Sn bimetallic catalyst for selective aqueous electroreduction of CO2 to CO. ACS Catal. 6, 2842–2851 (2016).
Zhao, C. et al. Ionic exchange of metal–organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 139, 8078–8081 (2017).
Yang, H. B. et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Genovese, C. et al. Operando spectroscopy study of the carbon dioxide electro-reduction by iron species on nitrogen-doped carbon. Nat. Commun. 9, 935 (2018).
Yamanaka, I., Onizawa, T., Takenaka, S. & Otsuka, K. Direct and continuous production of hydrogen peroxide with 93% selectivity using a fuel-cell system. Angew. Chem. Int. Ed. 42, 3653–3655 (2003).
Siahrostami, S. et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 12, 1137–1143 (2013).
Verdaguer-Casadevall, A. et al. Trends in the electrochemical synthesis of H2O2: enhancing activity and selectivity by electrocatalytic site engineering. Nano Lett. 14, 1603–1608 (2014).
Liu, G. et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 9, 810–816 (2017).
Wang, Y.-G., Yoon, Y., Glezakou, V.-A., Li, J. & Rousseau, R. The role of reducible oxide−metal cluster charge transfer in catalytic processes: new insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics. J. Am. Chem. Soc. 135, 10673–10683 (2013).
Wang, Y.-G., Mei, D. H., Glezakou, V. A., Li, J. & Rousseau, R. Dynamic formation of single-atom catalytic active sites on ceria-supported gold nanoparticles. Nat. Commun. 6, 6511 (2015).
Liu, J.-C., Wang, Y.-G. & Li, J. Toward rational design of oxide-supported single-atom catalysts: atomic dispersion of gold on ceria. J. Am. Chem. Soc. 139, 6190–6199 (2017).
Wang, J. et al. Formation, migration, and reactivity of Au–CO complexes on gold surfaces. J. Am. Chem. Soc. 138, 1518–1526 (2016).
Eren, B. et al. Activation of Cu(111) surface by decomposition into nanoclusters driven by CO adsorption. Science 351, 475–478 (2016).
Bliem, R. et al. Dual role of CO in the stability of subnano Pt clusters at the Fe3O4(001) surface. Proc. Natl Acad. Sci. USA 113, 8921–8926 (2016).
Horch, S. et al. Enhancement of surface self-diffusion of platinum atoms by adsorbed hydrogen. Nature 398, 134–136 (1999).
Matsubu, J. C. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017).
Li, C. et al. Single atom dispersed Rh-biphephos&PPh3@porous organic copolymers: highly efficient catalysts for continuous fixed-bed hydroformylation of propene. Green Chem. 18, 2995–3005 (2016).
Lang, R. et al. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl(PPh3)3. Angew. Chem. Int. Ed. 55, 16054–16058 (2016).
Wang, L. et al. Atomic-level insights in optimizing reaction paths for hydroformylation reaction over Rh/CoO single-atom catalyst. Nat. Commun. 7, 14036 (2016).
Sahu, S. & Goldberg, D. P. Activation of dioxygen by iron and manganese complexes: a heme and nonheme perspective. J. Am. Chem. Soc. 138, 11410–11428 (2016).
He, L., Weniger, F., Neumann, H. & Beller, M. Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: catalysis beyond electrochemistry. Angew. Chem. Int. Ed. 55, 12582–12594 (2016).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017).
Liu, J. C. et al. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 9, 1610 (2018).
Yan, H. et al. Bottom-up precise synthesis of stable platinum dimers on graphene. Nat. Commun. 8, 1070 (2017).
Ji, S. et al. Confined pyrolysis within metal−organic frameworks to form uniform Ru3 clusters for efficient oxidation of alcohols. J. Am. Chem. Soc. 139, 9795–9798 (2017).
Shan, J., Li, M., Allard, L. F., Lee, S. & Flytzani-Stephanopoulos, M. Mild oxidation of methane to methanol or acetic acid on supported isolated rhodium catalysts. Nature 551, 605–608 (2017).
Tang, Y. et al. Single rhodium atoms anchored in micropores for efficient transformation of methane under mild conditions. Nat. Commun. 9, 1231 (2018).
Jasion, V. S. & Poulos, T. L. Leishmania major peroxidase is a cytochrome c peroxidase. Biochemistry 51, 2453–2460 (2012).
Acknowledgements
The authors thank J. Liu, B. Qiao, Y.-G. Wang, X.-F. Yang and R. Rousseau for fruitful discussions. This work is supported by the National Key Projects for Fundamental Research and Development of China (2016YFA0202801), National Natural Science Foundation of China (21690080, 21690084, 21721004, 21673228, 21522608, 21503219, 21672210, 21590792 and 91645203), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB17000000 and 17020100). The authors thank Y. Ren, S. Niu, W. Liu, M. Zhou, J.-C. Liu and X. Yang for assisting in the preparation of some of the figures.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat Rev Chem 2, 65–81 (2018). https://doi.org/10.1038/s41570-018-0010-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0010-1
This article is cited by
-
Electrochemical hydrogenation and oxidation of organic species involving water
Nature Reviews Chemistry (2024)
-
Photochemical tuning of dynamic defects for high-performance atomically dispersed catalysts
Nature Materials (2024)
-
Co-catalytic metal–support interactions in single-atom electrocatalysts
Nature Reviews Materials (2024)
-
Constructing sulfur and oxygen super-coordinated main-group electrocatalysts for selective and cumulative H2O2 production
Nature Communications (2024)
-
One-dimensional single atom arrays on ferroelectric nanosheets for enhanced CO2 photoreduction
Nature Communications (2024)