Abstract
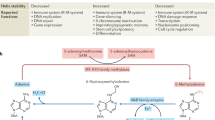
Chemically modified bases exist naturally in genomic DNA. Research into these bases has been invigorated by the discovery of several modified bases in the mammalian genome, in particular 5-methylcytosine and its oxidized derivatives, such as 5-(hydroxymethyl)cytosine and 5-formylcytosine, as well as the enzymes that form and process them, such as the DNA methyltransferases and the ten-eleven translocation enzymes. In this Review, we provide an overview of natural modified bases that have been reported in DNA, our current knowledge of their roles, and the techniques that have enabled us to probe their functions. Analytical methods have been invaluable in helping to advance this field. For example, chemical and enzymatic methods have provided the means to detect and decode modified bases, giving rise to an expanding array of sequencing approaches. Advanced liquid chromatography and tandem mass spectrometry have provided the means to detect and quantify modified bases with very high sensitivity, increasing the prospect of discovering unknown modifications. It is already evident that natural modified DNA bases and their associated enzymology are of fundamental importance to normal biology and to disease. The next decade promises to yield more insights, discoveries and applications from this burgeoning field of research.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Watson, J. D. & Crick, F. H. Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid. Nature 171, 737–738 (1953). The first report on the double-helical structure of DNA and the pairing of GC and AT bases.
Klug, A. The discovery of zinc fingers and their development for practical applications in gene regulation and genome manipulation. Q. Rev. Biophys. 43, 1–21 (2010).
Dervan, P. B. Molecular recognition of DNA by small molecules. Bioorg. Med. Chem. 9, 2215–2235 (2001).
Ruppel, W. G. Zur chemie der tuberkelbacillen [German]. Z. Physiol. Chem. 26, 218–232 (1898).
Gommers-Ampt, J. H. & Borst, P. Hypermodified bases in DNA. FASEB J. 9, 1034–1042 (1995).
Johnson, T. B. & Coghill, R. D. Researches on pyrimidines. C111. The discovery of 5-methyl-cytosine in tuberculinic acid, the nucleic acid of the Tubercle bacillus. J. Am. Chem. Soc. 47, 2838–2844 (1925).
Okano, M., Xie, S. & Li, E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 19, 219–220 (1998).
Jeltsch, A. Molecular enzymology of mammalian DNA methyltransferases. Curr. Top. Microbiol. Immunol. 301, 203–225 (2006).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Barau, J. et al. The DNA methyltransferase DNMT3C protects male germ cells from transposon activity. Science 354, 909–912 (2016).
Wyatt, G. R. Occurrence of 5-methyl-cytosine in nucleic acids. Nature 166, 237–238 (1950).
Bird, A. DNA methylation patterns and epigenetic memory DNA methylation patterns and epigenetic memory. Genes Dev. 16, 6–21 (2002).
Deaton, A. & Bird, A. CpG islands and the regulation of transcription. Genes Dev. 25, 1010–1022 (2011). A comprehensive review explaining the concept and importance of CGIs as regulatory features of the genome.
Jones, P. A. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 13, 484–492 (2012).
Du, J., Johnson, L. M., Jacobsen, S. E. & Patel, D. J. DNA methylation pathways and their crosstalk with histone methylation. Nat. Rev. Mol. Cell Biol. 16, 519–532 (2015).
Wachter, E. et al. Synthetic CpG islands reveal DNA sequence determinants of chromatin structure. eLife 3, e03397 (2014).
Krebs, A. R., Dessus-Babus, S., Burger, L. & Schubeler, D. High-throughput engineering of a mammalian genome reveals building principles of methylation states at CG rich regions. eLife 3, e04094 (2014).
Bogdanović, O. et al. Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat. Genet. 48, 417–426 (2016).
Kohli, R. M. & Zhang, Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 502, 472–479 (2013).
Cortázar, D. et al. Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature 470, 419–423 (2011).
Dawlaty, M. M. et al. Loss of Tet enzymes compromises proper differentiation of embryonic stem cells. Dev. Cell 29, 102–111 (2014).
Hahn, M. A., Szabo, P. E. & Pfeifer, G. P. 5-Hydroxymethylcytosine: a stable or transient DNA modification? Genomics 104, 314–323 (2014).
Pfaffeneder, T. et al. The discovery of 5-formylcytosine in embryonic stem cell DNA. Angew Chem. Int. Ed. 50, 7008–7012 (2011). Provides the first evidence of the existence of 5fC in ES cells.
Booth, M. J., Marsico, G., Bachman, M., Beraldi, D. & Balasubramanian, S. Quantitative sequencing of 5-formylcytosine in DNA at single-base resolution. Nat. Chem. 6, 435–440 (2014).
Su, M. et al. 5-Formylcytosine could be a semipermanent base in specific genome sites. Angew. Chem. Int. Ed. 55, 11797–11800 (2016).
McInroy, G. R. et al. in Epigenetic Mechanisms in Cellular Reprogramming (eds Meissner, A. & Walter, J. ) 167–191 (Springer, 2015).
Iurlaro, M. et al. In vivo genome-wide profiling reveals a tissue-specific role for 5-formylcytosine. Genome Biol. 17, 141 (2016).
Shen, L. et al. Genome-wide analysis reveals TET- and TDG-dependent 5-methylcytosine oxidation dynamics. Cell 153, 692–706 (2013).
Bachman, M. et al. 5-Hydroxymethylcytosine is a predominantly stable DNA modification. Nat. Chem. 6, 1049–1055 (2014). Describes the timing of the formation and metabolism of 5mC and 5hmC in genomic DNA using LC–MS/MS and stable isotopes.
Bachman, M. et al. 5-Formylcytosine can be a stable DNA modification in mammals. Nat. Chem. Biol. 11, 555–557 (2015).
Iurlaro, M. et al. A screen for hydroxymethylcytosine and formylcytosine binding proteins suggests functions in transcription and chromatin regulation. Genome Biol. 14, R119 (2013).
Spruijt, C. G. et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 152, 1146–1159 (2013).
Wang, D. et al. MAX is an epigenetic sensor of 5-carboxylcytosine and is altered in multiple myeloma. Nucleic Acids Res. 45, 2396–2407 (2016).
Sato, K., Kawamoto, K., Shimamura, S., Ichikawa, S. & Matsuda, A. An oligodeoxyribonucleotide containing 5-formyl-2′-deoxycytidine (fC) at the CpG site forms a covalent complex with DNA cytosine-5 methyltransferases (DNMTs). Bioorg. Med. Chem. Lett. 26, 5395–5398 (2016).
Thalhammer, A., Hansen, A. S., El-Sagheer, A. H., Brown, T. & Schofield, C. J. Hydroxylation of methylated CpG dinucleotides reverses stabilisation of DNA duplexes by cytosine 5-methylation. Chem. Commun. 47, 5325–5327 (2011).
Raiber, E.-A. et al. 5-Formylcytosine alters the structure of the DNA double helix. Nat. Struct. Mol. Biol. 22, 44–49 (2015).
Hardwick, J. S. et al. 5-Formylcytosine does not change the global structure of DNA. Nat. Struct. Mol. Biol. 24, 544–552 (2017).
Ngo, T. T. M. et al. Effects of cytosine modifications on DNA flexibility and nucleosome mechanical stability. Nat. Commun. 7, 10813 (2016).
Choy, J. S. et al. DNA methylation increases nucleosome compaction and rigidity. J. Am. Chem. Soc. 132, 1782–1783 (2010).
Lee, J. Y. & Lee, T.-H. Effects of DNA methylation on the structure of nucleosomes. J. Am. Chem. Soc. 134, 173–175 (2012).
Mendonca, A., Chang, E. H., Liu, W. & Yuan, C. Hydroxymethylation of DNA influences nucleosomal conformation and stability in vitro. Biochim. Biophys. Acta 1839, 1323–1329 (2014).
Collings, C. K., Waddell, P. J. & Anderson, J. N. Effects of DNA methylation on nucleosome stability. Nucleic Acids Res. 41, 2918–2931 (2013).
Galashevskaya, A. et al. A robust, sensitive assay for genomic uracil determination by LC/MS/MS reveals lower levels than previously reported. DNA Repair (Amst.) 12, 699–706 (2013).
Krokan, H. E., Drabløs, F. & Slupphaug, G. Uracil in DNA — occurrence, consequences and repair. Oncogene 21, 8935–8948 (2002).
Conticello, S. G. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 9, 229 (2008).
Santos, F. et al. Active demethylation in mouse zygotes involves cytosine deamination and base excision repair. Epigenetics Chromatin 6, 39 (2013).
Pfaffeneder, T. et al. Tet oxidizes thymine to 5-hydroxymethyluracil in mouse embryonic stem cell DNA. Nat. Chem. Biol. 10, 574–581 (2014). Details the use of stable isotopes and LC–MS/MS to elucidate the mechanism for 5hmU formation in DNA.
Masaoka, A. et al. Mammalian 5-formyluracil-DNA glycosylase. 2. Role of SMUG1 uracil-DNA glycosylase in repair of 5-formyluracil and other oxidized and deaminated base lesions. Biochemistry 42, 5003–5012 (2003).
Bauer, N. C., Corbett, A. H. & Doetsch, P. W. The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res. 43, 10083–10101 (2015).
Guo, J. U., Su, Y., Zhong, C., Ming, G. & Song, H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145, 423–434 (2011).
Nabel, C. S. et al. AID/APOBEC deaminases disfavor modified cytosines implicated in DNA demethylation. Nat. Chem. Biol. 8, 751–758 (2012).
Pais, J. E. et al. Biochemical characterization of a Naegleria TET-like oxygenase and its application in single molecule sequencing of 5-methylcytosine. Proc. Natl Acad. Sci. USA 112, 4316–4321 (2015).
Liu, S. et al. Quantitative mass spectrometry-based analysis of β-d-glucosyl-5-hydroxymethyluracil in genomic DNA of Trypanosoma brucei. J. Am. Soc. Mass Spectrom. 25, 1763–1770 (2014).
Bullard, W., Lopes Da Rosa-Spiegler, J., Liu, S., Wang, Y. & Sabatini, R. Identification of the glucosyltransferase that converts hydroxymethyluracil to base J in the trypanosomatid genome. J. Biol. Chem. 289, 20273–20282 (2014).
Kawasaki, F. et al. Genome-wide mapping of 5-hydroxymethyluracil in the eukaryote parasite Leishmania. Genome Biol. 18, 23 (2017).
Cliffe, L. J., Siegel, T. N., Marshall, M., Cross, G. A. M. & Sabatini, R. Two thymidine hydroxylases differentially regulate the formation of glucosylated DNA at regions flanking polymerase II polycistronic transcription units throughout the genome of Trypanosoma brucei. Nucleic Acids Res. 38, 3923–3935 (2010).
van Luenen, H. G. A. M. et al. Glucosylated hydroxymethyluracil, DNA base J, prevents transcriptional readthrough in Leishmania. Cell 150, 909–921 (2012).
Hazelbaker, D. Z. & Buratowski, S. Base J: blocking RNA polymerase's way. Curr. Biol. 22, R960–R962 (2012).
Reynolds, D. et al. Histone H3 variant regulates RNA polymerase II transcription termination and dual strand transcription of siRNA loci in Trypanosoma brucei. PLoS Genet. 12, e1005758 (2016).
Engel, J. D. & von Hippel, P. H. Effects of methylation on the stability of nucleic acid conformations. Studies at the polymer level. J. Biol. Chem. 253, 927–934 (1978).
Ratel, D., Ravanat, J.-L., Berger, F. & Wion, D. N6-methyladenine: the other methylated base of DNA. Bioessays 28, 309–315 (2006).
Rae, P. M. M. & Steele, R. E. Modified bases in the DNAs of unicellular eukaryotes: an examination of distributions and possible roles, with emphasis on hydroxymethyluracil in dinoflagellates. Biosystems 10, 37–53 (1978).
Greer, E. L. et al. DNA methylation on N6-adenine in C. elegans. Cell 161, 868–878 (2015). Describes the discovery, quantitation and mapping of 6mA in the model animal C. elegans.
Zhang, G. et al. N6-methyladenine DNA modification in Drosophila. Cell 161, 893–906 (2017). Reports the discovery, quantitation and mapping of 6mA in the model animal D. melanogaster.
Koziol, M. J. et al. Identification of methylated deoxyadenosines in vertebrates reveals diversity in DNA modifications. Nat. Struct. Mol. Biol. 23, 24–30 (2016).
Wu, T. P. et al. DNA methylation on N6-adenine in mammalian embryonic stem cells. Nature 532, 329–333 (2016).
Fu, Y. et al. N6-Methyldeoxyadenosine marks active transcription start sites in Chlamydomonas. Cell 161, 879–892 (2017).
Liu, F. et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell 167, 816–828 (2016).
Kawarada, L. et al. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 45, 7401–7415 (2017).
Müller, T. A., Tobar, M. A., Perian, M. N. & Hausinger, R. P. Biochemical characterization of AP lyase and m6A demethylase activities of human AlkB homologue 1 (ALKBH1). Biochemistry 56, 1899–1910 (2017).
Liu, J. et al. Abundant DNA 6 mA methylation during early embryogenesis of zebrafish and pig. Nat. Commun. 7, 13052 (2016).
Schiffers, S. et al. Quantitative LC–MS provides no evidence for m6dA or m4dC in the genome of mouse embryonic stem cells and tissues. Angew. Chem. Int. Ed. 56, 1–5 (2017).
Booth, M. J., Raiber, E.-A. & Balasubramanian, S. Chemical methods for decoding cytosine modifications in DNA. Chem. Rev. 115, 2240–2254 (2015).
Sun, Z. et al. High-resolution enzymatic mapping of genomic 5-hydroxymethylcytosine in mouse embryonic stem cells. Cell Rep. 3, 567–576 (2013).
Horton, J. R. et al. Structure of 5-hydroxymethylcytosine-specific restriction enzyme, AbaSI, in complex with DNA. Nucleic Acids Res. 42, 7947–7959 (2014).
Frommer, M. et al. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl Acad. Sci. USA 89, 1827–1831 (1992).
Huang, Y. et al. The behaviour of 5-hydroxymethylcytosine in bisulfite sequencing. PLoS ONE 5, e8888 (2010).
Booth, M. J. et al. Quantitative sequencing of 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution. Science 336, 934–937 (2012). Describes the development of a sequencing method that, for the first time, enabled the study of the dynamics and sequence context of 5hmC in mES cells at single-base resolution.
Yu, M. et al. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell 149, 1368–1380 (2012).
Song, C.-X. et al. Genome-wide profiling of 5-formylcytosine reveals its roles in epigenetic priming. Cell 153, 678–691 (2013).
Tanaka, K. & Okamoto, A. Degradation of DNA by bisulfite treatment. Bioorg. Med. Chem. Lett. 17, 1912–1915 (2007).
McInroy, G. R. et al. Enhanced methylation analysis by recovery of unsequenceable fragments. PLoS ONE 11, e0152322 (2016).
Miura, F., Enomoto, Y., Dairiki, R. & Ito, T. Amplification-free whole-genome bisulfite sequencing by post-bisulfite adaptor tagging. Nucleic Acids Res. 40, e136 (2012).
Smallwood, S. A. et al. Single-cell genome-wide bisulfite sequencing for assessing epigenetic heterogeneity. Nat. Methods 11, 817–820 (2014). Details the adaptation of the BS-seq method to single cells and reveals 5mC heterogeneity within cell populations.
Hayashi, G. et al. Base-resolution analysis of 5-hydroxymethylcytosine by one-pot bisulfite-free chemical conversion with peroxotungstate. J. Am. Chem. Soc. 138, 14178–14181 (2016).
Xia, B. et al. Bisulfite-free, base-resolution analysis of 5-formylcytosine at the genome scale. Nat. Methods 12, 1047–1050 (2015).
Feng, Z. et al. Detecting DNA modifications from SMRT sequencing data by modeling sequence context dependence of polymerase kinetic. PLoS Comput. Biol. 9, e1002935 (2013).
Song, C.-X. et al. Sensitive and specific single-molecule sequencing of 5-hydroxymethylcytosine. Nat. Methods 9, 75–77 (2012).
Chavez, L. et al. Simultaneous sequencing of oxidized methylcytosines produced by TET/JBP dioxygenases in Coprinopsis cinerea. Proc. Natl Acad. Sci. USA 111, E5149–E5158 (2014).
Genest, P. A. et al. Defining the sequence requirements for the positioning of base J in DNA using SMRT sequencing. Nucleic Acids Res. 43, 2102–2115 (2015).
Wanunu, M. et al. Discrimination of methylcytosine from hydroxymethylcytosine in DNA molecules. J. Am. Chem. Soc. 133, 486–492 (2011).
Laszlo, A. H. et al. Detection and mapping of 5-methylcytosine and 5-hydroxymethylcytosine with nanopore MspA. Proc. Natl Acad. Sci. USA 110, 18904–18909 (2013).
Ross, M. G. et al. Characterizing and measuring bias in sequence data. Genome Biol. 14, R51 (2013).
Simpson, J. T. et al. Detecting DNA cytosine methylation using nanopore sequencing. Nat. Methods 14, 407–410 (2017).
Hoenen, T. et al. Nanopore sequencing as a rapidly deployable Ebola outbreak tool. Emerg. Infect. Dis. 22, 331–334 (2016).
Quick, J. et al. Real-time, portable genome sequencing for Ebola surveillance. Nature 530, 228–232 (2016).
Polak, P. et al. Cell-of-origin chromatin organization shapes the mutational landscape of cancer. Nature 518, 360–364 (2015).
Schuster-Böckler, B. & Lehner, B. Chromatin organization is a major influence on regional mutation rates in human cancer cells. Nature 488, 504–507 (2012).
Liu, L., De, S. & Michor, F. DNA replication timing and higher-order nuclear organization determine single-nucleotide substitution patterns in cancer genomes. Nat. Commun. 4, 1502 (2013).
Raiber, E.-A. et al. Base resolution maps reveal the importance of 5-hydroxymethylcytosine in a human glioblastoma. Genomic Med. 2, 6 (2017).
Tomkova, M., McClellan, M., Kriaucionis, S. & Schuster-Boeckler, B. 5-Hydroxymethylcytosine marks regions with reduced mutation frequency in human DNA. eLife 5, e17082 (2016).
Esteller, M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 8, 286–298 (2007).
Baylin, S. B. et al. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum. Mol. Genet. 10, 687–692 (2001).
Yoo, C. B. & Jones, P. A. Epigenetic therapy of cancer: past, present and future. Nat. Rev. Drug Discov. 5, 37–50 (2006).
Portela, A. & Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 28, 1057–1068 (2010).
Pfeifer, G. P., Xiong, W., Hahn, M. A. & Jin, S.-G. The role of 5-hydroxymethylcytosine in human cancer. Cell Tissue Res. 356, 631–641 (2014).
Mou, H., Kennedy, Z., Anderson, D. G., Yin, H. & Xue, W. Precision cancer mouse models through genome editing with CRISPR–Cas9. Genome Med. 7, 53 (2015).
Kim, Y.-H. et al. TET2 promoter methylation in low-grade diffuse gliomas lacking IDH1/2 mutations. J. Clin. Pathol. 64, 850–852 (2011).
Imperiale, T. F. et al. Multitarget stool DNA testing for colorectal-cancer screening. N. Engl. J. Med. 370, 1287–1297 (2014).
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003 (2005).
Klungland, A. et al. 5-Formyluracil and its nucleoside derivatives confer toxicity and mutagenicity to mammalian cells by interfering with normal RNA and DNA metabolism. Toxicol. Lett. 119, 71–78 (2001).
Djuric, Z. et al. Levels of 5-hydroxymethyl-2′-deoxyuridine in DNA from blood as a marker of breast cancer. Cancer 77, 691–696 (1996).
Djuric, Z. et al. Levels of 5-hydroxymethyl-2′-deoxyuridine in DNA from blood of women scheduled for breast biopsy. Cancer Epidemiol. Biomarkers Prev. 10, 147–149 (2001).
Rebhandl, S., Huemer, M., Greil, R. & Geisberger, R. AID/APOBEC deaminases and cancer. Oncoscience 2, 320–333 (2015).
Pfeifer, G. P. Mutagenesis at methylated CpG sequences. Curr. Top. Microbiol. Immunol. 301, 259–281 (2006).
Stresemann, C. & Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 123, 8–13 (2008).
Stein, E. et al. Clinical safety and activity in a phase I trial of AG-221, a first in class, potent inhibitor of the IDH2-mutant protein, in patients with IDH2 mutant positive advanced hematologic malignancies [abstract]. Cancer Res. 74 (Suppl.), CT103 (2014).
Kang, J. S., Meier, J. L. & Dervan, P. B. Design of sequence-specific DNA binding molecules for DNA methyltransferase inhibition. J. Am. Chem. Soc. 136, 3687–3694 (2014).
Kubik, G. & Summerer, D. TALEored epigenetics: a DNA-binding scaffold for programmable epigenome editing and analysis. ChemBioChem 17, 975–980 (2016).
Xu, X. et al. A CRISPR-based approach for targeted DNA demethylation. Cell Discov. 2, 16009 (2016).
Borst, P. & Sabatini, R. Base J: discovery, biosynthesis, and possible functions. Annu. Rev. Microbiol. 62, 235–251 (2008).
Iyer, L. M., Tahiliani, M., Rao, A. & Aravind, L. Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids. Cell Cycle 8, 1698–1710 (2009).
Tahiliani, M. et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935 (2009). The first report on the formation of 5hmC in mammalian genomes from 5mC by the TET family of enzymes.
Heather, J. M. & Chain, B. The sequence of sequencers: the history of sequencing DNA. Genomics 107, 1–8 (2016).
Head, S. R. et al. Library construction for next-generation sequencing: overviews and challenges. Biotechniques 56, 61–64 (2014).
Balasubramanian, S. Sequencing nucleic acids: from chemistry to medicine. Chem. Commun. 47, 7281–7286 (2011).
Bentley, D. R. et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456, 53–59 (2008).
Taghizadeh, K. et al. Quantification of DNA damage products resulting from deamination, oxidation and reaction with products of lipid peroxidation by liquid chromatography isotope dilution tandem mass spectrometry. Nat. Protoc. 3, 1287–1298 (2008).
Globisch, D. et al. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS ONE 5, e15367 (2010).
Gackowski, D. et al. Accurate, direct, and high-throughput analyses of a broad spectrum of endogenously generated DNA base modifications with isotope-dilution two-dimensional ultraperformance liquid chromatography with tandem mass spectrometry: possible clinical implication. Anal. Chem. 88, 12128–12136 (2016).
Hong, H. & Wang, Y. Derivatization with Girard reagent T combined with LC-MS/MS for the sensitive detection of 5-formyl-2′-deoxyuridine in cellular DNA. Anal. Chem. 79, 322–326 (2007).
Munzel, M. et al. Quantification of the sixth DNA base hydroxymethylcytosine in the brain. Angew. Chem. Int. Ed. 49, 5375–5377 (2010).
Kraus, T. F. J. et al. Low values of 5-hydroxymethylcytosine (5hmC), the ‘sixth base,’ are associated with anaplasia in human brain tumors. Int. J. Cancer 131, 1577–1590 (2012).
Wagner, M. et al. Age-dependent levels of 5-methyl-, 5-hydroxymethyl-, and 5-formylcytosine in human and mouse brain tissues. Angew. Chem. Int. Ed. 54, 12511–12514 (2015).
Weber, M. et al. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat. Genet. 37, 853–862 (2005).
Pastor, W. A. et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature 473, 394–397 (2011).
Song, C.-X. et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat. Biotechnol. 29, 68–72 (2011).
Ficz, G. et al. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature 473, 398–402 (2011).
Robertson, A. B., Dahl, J. A., Ougland, R. & Klungland, A. Pull-down of 5-hydroxymethylcytosine DNA using JBP1-coated magnetic beads. Nat. Protoc. 7, 340–350 (2012).
Sérandour, A. A. et al. Single-CpG resolution mapping of 5-hydroxymethylcytosine by chemical labeling and exonuclease digestion identifies evolutionarily unconserved CpGs as TET targets. Genome Biol. 17, 56 (2016).
Sun, Z. et al. A sensitive approach to map genome-wide 5-hydroxymethylcytosine and 5-formylcytosine at single-base resolution. Mol Cell. 57, 750–761 (2015).
Raiber, E.-A. et al. Genome-wide distribution of 5-formylcytosine in embryonic stem cells is associated with transcription and depends on thymine DNA glycosylase. Genome Biol. 13, R69 (2012). Provides the first genome-wide map of 5fC in ES cells and demonstrates that the 5fC pattern is TDG-dependent.
Wu, H., Wu, X., Shen, L. & Zhang, Y. Single-base resolution analysis of active DNA demethylation using methylase-assisted bisulfite sequencing. Nat. Biotechnol. 32, 1231–1240 (2014).
Zhu, C. et al. Single-cell 5-formylcytosine landscapes of mammalian early embryos and ESCs at single-base resolution. Cell Stem Cell 20, 720–731.e5 (2017).
Lu, X. et al. Base-resolution maps of 5-formylcytosine and 5-carboxylcytosine reveal genome-wide DNA demethylation dynamics. Cell Res. 25, 386–389 (2015).
Acknowledgements
The Balasubramanian laboratory is supported by core funding from Cancer Research UK (C14303/A17197). S.B. is a senior investigator of the Wellcome Trust (Grant No. 099232/z/12/z).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Glossary
- Transcription factors
-
Proteins that bind to a specific DNA sequence to control the transcription of the genetic information from DNA to RNA.
- Restriction methylation
-
DNA methylation that protects bacteria from restriction endonuclease enzymes, providing a defence mechanism against invasion by bacteriophages and viruses.
- Promoters
-
Regions of DNA that are located near to transcription start sites and control transcription initiation.
- Retrotransposons
-
Genetic elements that are transcribed into RNA, then reverse-transcribed back into DNA and inserted into the genome.
- Genomic imprinting
-
An epigenetic marking of one copy of the gene (from the mother or father) that ensures gene expression in a parent-of-origin-specific manner.
- Transposon silencing
-
Gene silencing of transposons by epigenetic mechanisms, including DNA methylation and the effect of small non-coding RNAs, which prevents transcription and ensures genome stability.
- Histone modifications
-
Post-translational chemical modifications of amino acid residues on a histone.
- Chromatin-remodelling proteins
-
Proteins that control access to the genetic information by either inducing histone modifications or using energy to alter histone–DNA interactions.
- CpG islands
-
(CGIs). Regions with high cytosine-phosphate-guanine (CpG) dinucleotide density.
- Pluripotent stem cells
-
Cells that can differentiate into any other tissue of the body.
- Enhancer regions
-
Regulatory regions of the genome that are marked by histone modifications and enhance the transcription of their associated genes when bound to transcription factors.
- Base excision repair
-
(BER). A cellular mechanism that removes small base lesions, caused by mismatched or modified DNA bases, from the DNA.
- Histone octamer
-
An eight-protein complex of two histone H2A–H2B dimers and two histone H3–H4 dimers that together form the core of the nucleosome.
- B cells
-
A type of white blood cell that is fundamental to the adaptive immune system.
- Class-switch recombination
-
A process whereby B cells rearrange parts of the immunoglobulin heavy chain locus to generate antibodies with different properties.
- Telomeric regions
-
Repetitive nucleotide sequences that protect the ends of chromosomes.
- Repetitive elements
-
Sequences that occur multiple times throughout the genome.
- RNA polymerase II
-
An enzyme that catalyses the transcription of DNA to RNA.
- Transfer RNA
-
An adaptor RNA and amino acid carrier that helps to decode mRNA for translation into the synthesis of proteins.
- Restriction endonucleases
-
Enzymes that cut DNA at endogenous phosphodiester bonds.
- β-Elimination
-
DNA cleavage at the phosphodiester bond resulting in the elimination of the 3′-phosphate residue.
- δ-Elimination
-
DNA cleavage at the phosphodiester bond resulting in the elimination of the 5′-phosphate residue.
- Clustered regularly interspaced short palindromic repeats (CRISPR) systems
-
Genome-editing systems, such as CRISPR–Cas9, that rely on a bacterial virus-defence mechanism involving repetitive DNA sequences that contain snippets of viral DNA. By manipulating CRISPR systems, DNA can be cut at a desired location, allowing genes to be removed or added.
- Transcription activator-like effectors
-
(TALEs). Proteins that can be programmed to target specific DNA sequences in the genome.
Rights and permissions
About this article
Cite this article
Raiber, EA., Hardisty, R., van Delft, P. et al. Mapping and elucidating the function of modified bases in DNA. Nat Rev Chem 1, 0069 (2017). https://doi.org/10.1038/s41570-017-0069
Published:
DOI: https://doi.org/10.1038/s41570-017-0069
This article is cited by
-
Absolute quantification of single-base m6A methylation in the mammalian transcriptome using GLORI
Nature Biotechnology (2023)
-
N4-acetyldeoxycytosine DNA modification marks euchromatin regions in Arabidopsis thaliana
Genome Biology (2022)
-
Discriminating protein tags on a dsDNA construct using a Dual Nanopore Device
Scientific Reports (2022)
-
Genomic enhancers in cardiac development and disease
Nature Reviews Cardiology (2022)
-
Quantitative detection of CpG methylation level on G-quadruplex and i-motif-forming DNA by recombinase polymerase amplification
Analytical and Bioanalytical Chemistry (2022)