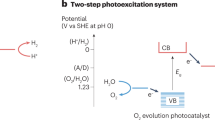
Abstract
Sunlight is by far the most plentiful renewable energy resource, providing Earth with enough power to meet all of humanity's needs several hundred times over. However, it is both diffuse and intermittent, which presents problems regarding how best to harvest this energy and store it for times when the sun is not shining. Devices that use sunlight to split water into hydrogen and oxygen could be one solution to these problems, because hydrogen is an excellent fuel. However, if such devices are to become widely adopted, they must be cheap to produce and operate. Therefore, the development of electrocatalysts for water splitting that comprise only inexpensive, earth-abundant elements is critical. In this Review, we investigate progress towards such electrocatalysts, with special emphasis on how they might be incorporated into photoelectrocatalytic water-splitting systems and the challenges that remain in developing these devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Dunn, B., Kamath, H. & Tarascon, J.-M. Electrical energy storage for the grid: a battery of choices. Science 334, 928–935 (2011).
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006). As powerful today as when it was written 10 years ago, this article by two of the leaders in the field explains why renewable energy storage is so important.
Blankenship, R. E. et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011).
Armaroli, N. & Balzani, V. Solar electricity and solar fuels: status and perspectives in the context of the energy transition. Chem. Eur. J. 22, 32–57 (2016). A well-argued and up-to-date summary of our options for solar fuels generation and the broader techno-economic factors affecting the field.
Styring, S. Artificial photosynthesis for solar fuels. Faraday Discuss. 155, 357–376 (2012).
Le Formal, F., Bourée, W. S., Prévot, M. S. & Sivula, K. Challenges towards economic fuel generation from renewable electricity: the need for efficient electro-catalysis. Chimia 69, 789–798 (2015).
Joya, K. S., Joya, Y. F., Ocakoglu, K. & van de Krol, R. Water-splitting catalysis and solar fuel devices: artificial leaves on the move. Angew. Chem. Int. Ed. 52, 10426–10437 (2013).
Tachibana, Y., Vayssieres, L. & Durrant, J. R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 6, 511–518 (2012).
McCrory, C. C. L. et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015). An article exploring how different electrocatalysts can be compared for the OER and HER in the context of solar-to-fuels devices.
Carmo, M., Fritz, D. L., Mergel, J. & Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 38, 4901–4934 (2013).
Faber, M. S. & Jin, S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci. 7, 3519–3542 (2014).
Nocera, D. G. The artificial leaf. Acc. Chem. Res. 45, 767–776 (2012).
Zou, X. & Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 44, 5148–5180 (2015).
Zeng, M. & Li, Y. Recent advances in heterogeneous electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 3, 14942–14962 (2015).
Kang, D. et al. Electrochemical synthesis of photoelectrodes and catalysts for use in solar water splitting. Chem. Rev. 115, 12839–12887 (2015).
Roger, I. & Symes, M. D. First row transition metal catalysts for solar-driven water oxidation produced by electrodeposition. J. Mater. Chem. A 4, 6724–6741 (2016).
Fukuzumi, S., Yamada, Y., Suenobu, T., Ohkubo, K. & Kotani, H. Catalytic mechanisms of hydrogen evolution with homogeneous and heterogeneous catalysts. Energy Environ. Sci. 4, 2754–2766 (2011).
Ismail, A. A. & Bahnemann, D. W. Photochemical splitting of water for hydrogen production by photocatalysis: a review. Sol. Energy Mater Sol. Cells 128, 85–101 (2014).
Parent, A. R. & Sakai, K. Progress in base-metal water oxidation catalysis. ChemSusChem 7, 2070–2080 (2014).
Fan, C., Piron, D. L., Sleb, A. & Paradis, P. Study of electrodeposited nickel–molybdenum, nickel–tungsten, cobalt–molybdenum, and cobalt–tungsten as hydrogen electrodes in alkaline water electrolysis. J. Electrochem. Soc. 141, 382–387 (1994).
Arul Raj, I. & Vasu, K. I. Transition metal-based hydrogen electrodes in alkaline solution — electrocatalysis on nickel based binary alloy coatings. J. Appl. Electrochem. 20, 32–38 (1990).
McKone, J. R., Sadtler, B. F., Werlang, C. A., Lewis, N. S. & Gray, H. B. Ni–Mo nanopowders for efficient electrochemical hydrogen evolution. ACS Catal. 3, 166–169 (2013).
Wang, Y. et al. A 3D nanoporous Ni–Mo electrocatalyst with negligible overpotential for alkaline hydrogen evolution. ChemElectroChem. 1, 1138–1144 (2014).
Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. 39, 163–184 (1972).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23–J26 (2005).
Sheng, W., Myint, M., Chen, J. G. & Yan, Y. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 6, 1509–1512 (2013).
Hinnemann, B. et al. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 127, 5308–5309 (2005). This paper showed how computational methods could be used to identify new HER catalysts (in this case MoS 2 ) and then demonstrated the same experimentally.
Jaegermann, W. & Tributsch, H. Interfacial properties of semiconducting transition metal chalcogenides. Prog. Surf. Sci. 29, 1–167 (1988).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Kibsgaard, J., Chen, Z., Reinecke, B. N. & Jaramillo, T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 11, 963–969 (2012).
Merki, D., Fierro, S., Vrubel, H. & Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2, 1262–1267 (2011).
Li, Y. et al. MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 133, 7296–7299 (2011).
Li, H. et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 15, 48–53 (2016).
Faber, M. S. et al. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. J. Am. Chem. Soc. 136, 10053–10061 (2014).
Sun, Y. et al. Electrodeposited cobalt-sulfide catalyst for electrochemical and photoelectrochemical hydrogen generation from water. J. Am. Chem. Soc. 135, 17699–17702 (2013).
Tran, P. D. et al. Novel cobalt/nickel–tungsten-sulfide catalysts for electrocatalytic hydrogen generation from water. Energy Environ. Sci. 6, 2452–2459 (2013).
Voiry, D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850–855 (2013).
Xu, Y.-F., Gao, M.-R., Zheng, Y.-R., Jiang, J. & Yu, S.-H. Nickel/nickel(II) oxide nanoparticles anchored onto cobalt(IV) diselenide nanobelts for the electrochemical production of hydrogen. Angew. Chem. Int. Ed. 52, 8546–8550 (2013).
Kiran, V., Mukherjee, D., Jenjeti, R. N. & Sampath, S. Active guests in the MoS2/MoSe2 host lattice: efficient hydrogen evolution using few-layer alloys of MoS2(1 − x) Se2x . Nanoscale 6, 12856–12863 (2014).
Zhou, H. et al. One-step synthesis of self-supported porous NiSe2/Ni hybrid foam: an efficient 3D electrode for hydrogen evolution reaction. Nano Energy 20, 29–36 (2016).
Popczun, E. J. et al. Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J. Am. Chem. Soc. 135, 9267–9270 (2013).
Popczun, E. J., Read, C. G., Roske, C. W., Lewis, N. S. & Schaak, R. E. Highly Active electrocatalysis of the hydrogen evolution reaction by cobalt phosphide nanoparticles. Angew. Chem. Int. Ed. 53, 5427–5430 (2014).
Jiang, P. et al. A cost-effective 3D hydrogen evolution cathode with high catalytic activity: fep nanowire array as the active phase. Angew. Chem. Int. Ed. 53, 12855–12859 (2014).
Kibsgaard, J. & Jaramillo, T. F. Molybdenum phosphosulfide: an active, acid-stable, earth-abundant catalyst for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 53, 14433–14437 (2014).
Liang, H.-W. et al. Molecular metal–Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 6, 7992 (2015).
Chen, W.-F. et al. Hydrogen-evolution catalysts based on non-noble metal nickel–molybdenum nitride nanosheets. Angew. Chem. Int. Ed. 51, 6131–6135 (2012).
Cao, B., Veith, G. M., Neuefeind, J. C., Adzic, R. R. & Khalifah, P. G. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 135, 19186–19192 (2013).
Vrubel, H. & Hu, X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions. Angew. Chem. Int. Ed. 51, 12703–12706 (2012).
Liao, L. et al. A nanoporous molybdenum carbide nanowire as an electrocatalyst for hydrogen evolution reaction. Energy Environ. Sci. 7, 387–392 (2014).
Shi, Z. et al. Porous nanoMoC@graphite shell derived from a MOFs-directed strategy: an efficient electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 4, 6006–6013 (2016).
Fan, L. et al. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis. Nat. Commun. 7, 10667 (2016).
Lu, Q. et al. Highly porous non-precious bimetallic electrocatalysts for efficient hydrogen evolution. Nat. Commun. 6, 6567 (2015).
Merrill, M. D. & Dougherty, R. C. Metal Oxide Catalysts for the Evolution of O2 from H2O. J. Phys. Chem. C 112, 3655–3666 (2008).
Lu, X. & Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 6, 6616 (2015).
Gong, M. et al. An advanced Ni–Fe layered double hydroxide electrocatalyst for water oxidation. J. Am. Chem. Soc. 135, 8452–8455 (2013).
Li, X., Walsh, F. C. & Pletcher, D. Nickel based electrocatalysts for oxygen evolution in high current density, alkaline water electrolysers. Phys. Chem. Chem. Phys. 13, 1162–1167 (2011).
Corrigan, D. A. The catalysis of the oxygen evolution reaction by iron impurities in thin film nickel oxide electrodes. J. Electrochem. Soc. 134, 377–384 (1987).
Louie, M. W. & Bell, A. T. An investigation of thin-film Ni–Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 135, 12329–12337 (2013).
Friebel, D. et al. Identification of highly active Fe sites in (Ni, Fe)OOH for electrocatalytic water splitting. J. Am. Chem. Soc. 137, 1305–1313 (2015).
Lambert, T. N. et al. Electrodeposited NixCo3 − xO4 nanostructured films as bifunctional oxygen electrocatalysts. Chem. Commun. 51, 9511–9514 (2015).
Tian, J. et al. Self-supported NiMo hollow nanorod array: an efficient 3D bifunctional catalytic electrode for overall water splitting. J. Mater. Chem. A 3, 20056–20059 (2015).
Trotochaud, L., Young, S. L., Ranney, J. K. & Boettcher, S. W. Nickel–iron oxyhydroxide oxygen-evolution electrocatalysts: the role of intentional and incidental iron incorporation. J. Am. Chem. Soc. 136, 6744–6753 (2014).
Smith, A. M., Trotochaud, L., Burke, M. S. & Boettcher, S. W. Contributions to activity enhancement via Fe incorporation in Ni-(oxy)hydroxide/borate catalysts for near-neutral pH oxygen evolution. Chem. Commun. 51, 5261–5263 (2015).
Burke, M. S., Kast, M. G., Trotochaud, L., Smith, A. M. & Boettcher, S. W. Cobalt–iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 137, 3638 –3648 (2015).
Roger, I. & Symes, M. D. Efficient electrocatalytic water oxidation at neutral and high pH by adventitious nickel at nanomolar concentrations. J. Am. Chem. Soc. 137, 13980–13988 (2015).
Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016).
Suntivich, J., May, K. J., Gasteiger, H. A., Goodenough, J. B. & Shao-Horn, Y. A. Perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011).
Haber, J. A. et al. Discovering Ce-rich oxygen evolution catalysts, from high throughput screening to water electrolysis. Energy Environ. Sci. 7, 682–688 (2014).
Smith, R. D. L. et al. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 340, 60–63 (2013).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008). The paper that ignited the field in terms of oxygen evolution electrocatalysis under mild conditions.
Lutterman, D. A., Surendranath, Y. & Nocera, D. G. A. Self-healing oxygen-evolving catalyst. J. Am. Chem. Soc. 131, 3838–3839 (2009).
Surendranath, Y., Dinca, M. & Nocera, D. G. Electrolyte-dependent electrosynthesis and activity of cobalt-based water oxidation catalysts. J. Am. Chem. Soc. 131, 2615–2620 (2009).
Risch, M. et al. Cobalt–oxo core of a water-oxidizing catalyst film. J. Am. Chem. Soc. 131, 6936–6937 (2009).
Kanan, M. W. et al. Structure and valency of a cobalt-phosphate water oxidation catalyst determined by in situ X-ray spectroscopy. J. Am. Chem. Soc. 132, 13692–13701 (2010).
McAlpin, J. G. et al. EPR evidence for Co(iv) species produced during water oxidation at neutral pH. J. Am. Chem. Soc. 132, 6882–6883 (2010).
Surendranath, Y., Kanan, M. W. & Nocera, D. G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010).
Esswein, A. J., Surendranath, Y., Reece, S. Y. & Nocera, D. G. Highly active cobalt phosphate and borate based oxygen evolving catalysts operating in neutral and natural waters. Energy Environ. Sci. 4, 499–504 (2011).
Koza, J. A., He, Z., Miller, A. S. & Switzer, J. A. Electrodeposition of crystalline Co3O4 — a catalyst for the oxygen evolution reaction. Chem. Mater. 24, 3567–3573 (2012).
Gerken, J. B., Landis, E. C., Hamers, R. J. & Stahl, S. S. Fluoride-modulated cobalt catalysts for electrochemical oxidation of water under non-alkaline conditions. ChemSusChem 3, 1176–1179 (2010).
Gerken, J. B. et al. Electrochemical water oxidation with cobalt-based electrocatalysts from pH 0–14: the thermodynamic basis for catalyst structure, stability, and activity. J. Am. Chem. Soc. 133, 14431–14442 (2011).
Bloor, L. G., Molina, P. I., Symes, M. D. & Cronin, L. Low pH electrolytic water splitting using earth-abundant metastable catalysts that self-assemble in situ. J. Am. Chem. Soc. 136, 3304–3311 (2014).
Jiang, N., You, B., Sheng, M. & Sun, Y. Electrodeposited cobalt-phosphorous-derived films as competent bifunctional catalysts for overall water splitting. Angew. Chem. Int. Ed. 54, 6251–6254 (2015).
Li, D., Baydoun, H., Verani, C. N. & Brock, S. L. Efficient water oxidation using CoMnP nanoparticles. J. Am. Chem. Soc. 138, 4006–4009 (2016).
Dinca, M., Surendranath, Y. & Nocera, D. G. Nickel–borate oxygen-evolving catalyst that functions under benign conditions. Proc. Natl Acad. Sci. USA 107, 10337–10341 (2010).
Bediako, D. K., Surendranath, Y. & Nocera, D. G. Mechanistic studies of the oxygen evolution reaction mediated by a nickel–borate thin film electrocatalyst. J. Am. Chem. Soc. 135, 3662–3674 (2013).
Bediako, D. K. et al. Structure–activity correlations in a nickel–borate oxygen evolution catalyst. J. Am. Chem. Soc. 134, 6801–6809 (2012).
Wu, L.-K. Hu, J.-M. Zhang, J.-Q. & Cao, C.-N. A silica co-electrodeposition route to highly active Ni-based film electrodes. J. Mater. Chem. A 1, 12885–12892 (2013).
Zhao, Y. et al. Ultrafine NiO nanosheets stabilized by TiO2 from monolayer NiTi-LDH precursors: an active water oxidation electrocatalyst. J. Am. Chem. Soc. 138, 6517–6524 (2016).
Barber, J. Crystal structure of the oxygen-evolving complex of photosystem II. Inorg. Chem. 47, 1700–1710 (2008).
Gorlin, Y. & Jaramillo, T. F. A. Bifunctional nonprecious metal catalyst for oxygen reduction and water oxidation. J. Am. Chem. Soc. 132, 13612–13614 (2010).
Ramírez, A. et al. Evaluation of MnOx, Mn2O3, and Mn3O4 electrodeposited films for the oxygen evolution reaction of water. J. Phys. Chem. C 118, 14073–14081 (2014).
Zhou, F. et al. Improvement of catalytic water oxidation on MnOx films by heat treatment. ChemSusChem 6, 643–651 (2013).
Zaharieva, I. et al. Electrosynthesis, functional, and structural characterization of a water-oxidizing manganese oxide. Energy Environ. Sci. 5, 7081–7089 (2012).
Mattioli, G., Zaharieva, I., Dau, H. & Guidoni, L. Atomistic texture of amorphous manganese oxides for electrochemical water splitting revealed by ab initio calculations combined with X-ray spectroscopy. J. Am. Chem. Soc. 137, 10254 –10267 (2015).
Gorlin, Y. et al. In situ X-ray absorption spectroscopy investigation of a bifunctional manganese oxide catalyst with high activity for electrochemical water oxidation and oxygen reduction. J. Am. Chem. Soc. 135, 8525–8534 (2013).
Zhou, F. et al. Electrodeposited MnOx films from ionic liquid for electrocatalytic water oxidation. Adv. Energy Mater. 2, 1013–1021 (2012).
Takashima, T., Hashimoto, K. & Nakamura, R. Mechanisms of pH-dependent activity for water oxidation to molecular oxygen by MnO2 electrocatalysts. J. Am. Chem. Soc. 134, 1519–1527 (2012).
Takashima, T., Hashimoto, K. & Nakamura, R. Inhibition of charge disproportionation of MnO2 electrocatalysts for efficient water oxidation under neutral conditions. J. Am. Chem. Soc. 134, 18153–18156 (2012).
Huynh, M., Bediako, D. K., Liu, Y. & Nocera, D. G. Nucleation and growth mechanisms of an electrodeposited manganese oxide oxygen evolution catalyst. J. Phys. Chem. C 118, 17142–17152 (2014).
Huynh, M., Bediako, D. K. & Nocera, D. G. A. Functionally stable manganese oxide oxygen evolution catalyst in acid. J. Am. Chem. Soc. 136, 6002–6010 (2014).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Hou, Y. et al. Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution. Nat. Mater. 10, 434–438 (2011).
Berglund, S. P. et al. p-Si/W2C and p-Si/W2C/Pt photocathodes for the hydrogen evolution reaction. J. Am. Chem. Soc. 136, 1535–1544 (2014).
Ding, Q. et al. Efficient photoelectrochemical hydrogen generation using heterostructures of Si and chemically exfoliated metallic MoS2 . J. Am. Chem. Soc. 136, 8504–8507 (2014).
Cabán-Acevedo, M. et al. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide. Nat. Mater. 14, 1245–1251 (2015).
Downes, C. A. & Marinescu, S. C. Efficient electrochemical and photoelectrochemical H2 production from water by a cobalt dithiolene one-dimensional metal–organic surface. J. Am. Chem. Soc. 137, 13740–13743 (2015).
McKone, J. R. et al. Evaluation of Pt, Ni, and Ni–Mo electrocatalysts for hydrogen evolution on crystalline Si electrodes. Energy Environ. Sci. 4, 3573–3583 (2011).
Morales-Guio, C. G. et al. Solar hydrogen production by amorphous silicon photocathodes coated with a magnetron sputter deposited Mo2C catalyst. J. Am. Chem. Soc. 137, 7035–7038 (2015).
Lang, D., Cheng, F. & Xiang, Q. Enhancement of photocatalytic H2 production activity of CdS nanorods by cobalt-based cocatalyst modification. Catal. Sci. Technol. 6, 6207–6216 (2016).
Meng, P., Wang, M., Yang, Y., Zhang, S. & Sun, L. CdSe quantum dots/molecular cobalt catalyst co-grafted open porous NiO film as a photocathode for visible light driven H2 evolution from neutral water. J. Mater. Chem. A 3, 18852–18859 (2015).
Lin, C.-Y., Lai, Y.-H., Mersch, D. & Reisner, E. Cu2O |NiOx nanocomposite as an inexpensive photocathode in photoelectrochemical water splitting. Chem. Sci. 3, 3482–3487 (2012).
Yang, C. et al. Engineering a Cu2O/NiO/Cu2MoS4 hybrid photocathode for H2 generation in water. Nanoscale 6, 6506–6510 (2014).
Dubale, A. A. et al. Heterostructured Cu2O/CuO decorated with nickel as a highly efficient photocathode for photoelectrochemical water reduction. J. Mater. Chem. A 3, 12482–12499 (2015).
Morales-Guio, C. G., Tilley, S. D., Vrubel, H., Grätzel, M. & Hu, X. Hydrogen evolution from a copper(i) oxide photocathode coated with an amorphous molybdenum sulphide catalyst. Nat. Commun. 5, 3059 (2014).
Paracchino, A., Laporte, V., Sivula, K., Grätzel, M. & Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 10, 456–461 (2011).
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972). The first photoelectrochemical cell for water splitting.
Hodes, G., Cahen, D. & Manassen, J. Tungsten trioxide as a photoanode for a photoelectrochemical cell (PEC). Nature 260, 312–313 (1976).
Hardee, K. L. & Bard, A. J. Semiconductor electrodes: v. The application of chemically vapor deposited iron oxide films photosensitized electrolysis. J. Electrochem. Soc. 123, 1024–1026 (1976).
Zhong, D. K., Sun, J., Inumaru, H. & Gamelin, D. R. Solar water oxidation by composite catalyst/α-Fe2O3 photoanodes. J. Am. Chem. Soc. 131, 6086–6087 (2009).
Zhong, D. K. & Gamelin, D. R. Photoelectrochemical water oxidation by cobalt catalyst (“Co–Pi”)/α-Fe2O3 Composite photoanodes: oxygen evolution and resolution of a kinetic bottleneck. J. Am. Chem. Soc. 132, 4202–4207 (2010).
Zhong, D. K., Cornuz, M., Sivula, K., Grätzel, M. & Gamelin, D. R. Photo-assisted electrodeposition of cobalt–phosphate (Co–Pi) catalyst on hematite photoanodes for solar water oxidation. Energy Environ. Sci. 4, 1759–1764 (2011).
Seabold, J. A. & Choi, K.-S. Effect of a cobalt-based oxygen evolution catalyst on the stability and the selectivity of photo-oxidation reactions of a WO3 photoanode. Chem. Mater. 23, 1105–1112 (2011).
Pijpers, J. J. H., Winkler, M. T., Surendranath, Y., Buonassisi, T. & Nocera, D. G. Light-induced water oxidation at silicon electrodes functionalized with a cobalt oxygen-evolving catalyst. Proc. Natl Acad. Sci. USA 108, 10056–10061 (2011).
Abdi, F. F. & van de Krol, R. Nature and light dependence of bulk recombination in Co-Pi-catalyzed BiVO4 photoanodes. J. Phys. Chem. C 116, 9398–9404 (2012).
Abdi, F. F., Firet, N. & van de Krol, R. Efficient BiVO4 thin film photoanodes modified with cobalt phosphate catalyst and W-doping. ChemCatChem 5, 490–496 (2013).
Pilli, S. K. et al. Light induced water oxidation on cobalt-phosphate (Co–Pi) catalyst modified semi-transparent, porous SiO2–BiVO4 electrodes. Phys. Chem. Chem. Phys. 14, 7032–7039 (2012).
Khnayzer, R. S. et al. Structure and activity of photochemically deposited “CoPi” oxygen evolving catalyst on titania. ACS Catal. 2, 2150–2160 (2012).
Li, Y. et al. Efficient and stable photoelectrochemical seawater splitting with TiO2@g-C3N4 nanorod arrays decorated by Co–Pi. J. Phys. Chem. C 119, 20283–20292 (2015).
Kim, J. H. et al. Wireless solar water splitting device with robust cobalt-catalyzed, dual-doped BiVO4 photoanode and perovskite solar cell in tandem: a dual absorber artificial leaf. ACS Nano 9, 11820–11829 (2015).
Choi, S. K., Choi, W. & Park, H. Solar water oxidation using nickel–borate coupled BiVO4 photoelectrodes. Phys. Chem. Chem. Phys. 15, 6499–6507 (2013).
Pilli, S. K., Summers, K. & Chidambaram, D. Ni–Ci oxygen evolution catalyst integrated BiVO4 photoanodes for solar induced water oxidation. RSC Adv. 5, 47080–47089 (2015).
Jin, T., Diao, P., Xu, D. & Wu, Q. High-aspect-ratio WO3 nanoneedles modified with nickel–borate for efficient photoelectrochemical water oxidation. Electrochim. Acta 114, 271–277 (2013).
Kleiman-Shwarsctein, A., Hu, Y.-S., Stucky, G. D. & McFarland, E. W. NiFe-oxide electrocatalysts for the oxygen evolution reaction on Ti doped hematite photoelectrodes. Electrochem. Commun. 11, 1150–1153 (2009).
Zeng, Q. et al. A novel in situ preparation method for nanostructured α-Fe2O3 films from electrodeposited Fe films for efficient photoelectrocatalytic water splitting and the degradation of organic pollutants. J. Mater. Chem. A 3, 4345–4353 (2015).
Tilley, S. D., Cornuz, M., Sivula, K. & Grätzel, M. Light-induced water splitting with hematite: improved nanostructure and iridium oxide catalysis. Angew. Chem. Int. Ed. 49, 6405–6408 (2010).
Kenney, M. J. et al. High-performance silicon photoanodes passivated with ultrathin nickel films for water oxidation. Science 342, 836–840 (2013).
Kim, T. W. & Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343, 990–994 (2014).
Rocheleau, R. E., Miller, E. L. & Misra, A. High-efficiency photoelectrochemical hydrogen production using multijunction amorphous silicon photoelectrodes. Energy Fuels 12, 3–10 (1998). Possibly the first monolithic photoelectrochemical solar-to-hydrogen device, one that still ranks as among the most efficient.
Verlage, E. et al. A monolithically integrated, intrinsically safe, 10% efficient, solar-driven water-splitting system based on active, stable earth-abundant electrocatalysts in conjunction with tandem iii–v light absorbers protected by amorphous TiO2 films. Energy Environ. Sci. 8, 3166–3172 (2015). A recent example of a highly efficient photoelectrochemical solar-to-hydrogen device, showing how effectively device design has been optimized since Rocheleau's report.
Reece, S. Y. et al. Wireless solar water splitting using silicon-based semiconductors and earth-abundant catalysts. Science 334, 645–648 (2011). The first effective photoelectrochemical solar-to-hydrogen device that operated at near-neutral pH.
Esiner, S. et al. Photoelectrochemical water splitting in an organic artificial leaf. J. Mater. Chem. A 3, 23936–23945 (2015).
Sathre, R. et al. Life-cycle net energy assessment of large-scale hydrogen production via photoelectrochemical water splitting. Energy Environ. Sci. 7, 3264–3278 (2014).
Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 78, 661–669 (2005).
Berger, A., Segalman, R. A. & Newman, J. Material requirements for membrane separators in a water-splitting photoelectrochemical cell. Energy Environ. Sci. 7, 1468–1476 (2014).
Symes, M. D. & Cronin, L. Decoupling hydrogen and oxygen evolution during water splitting using a proton-coupled-electron buffer. Nat. Chem. 5, 403–409 (2013).
Rausch, B., Symes, M. D. & Cronin, L. A. Bio-inspired, small molecule electron-coupled-proton buffer for decoupling the half-reactions of electrolytic water splitting. J. Am. Chem. Soc. 135, 13656–13659 (2013).
Rausch, B., Symes, M. D., Chisholm, G. & Cronin, L. Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting. Science 345, 1326–1330 (2014).
Bloor, L. G. et al. Solar-driven water oxidation and decoupled hydrogen production mediated by an electron-coupled-proton buffer. J. Am. Chem. Soc. 138, 6707–6710 (2016).
Acknowledgements
M.D.S. thanks the University of Glasgow for a Lord Kelvin Adam Smith Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Glossary
- Tafel slopes
-
Tafel slopes are the gradient of the linear portion of a graph of overpotential versus the logarithm of the current density for a given electrocatalyst performing a particular reaction. Hence, the value of each slope gives an idea as to how the electrocatalytic performance of a material changes over a given potential range. These slopes are often quoted in millivolts per decade, where a decade in this sense relates to a decade of current density on the log scale. Catalysts with lower slopes require smaller increments of overpotential to give increased current densities, making them more effective.
- Faradaic yield
-
In electrochemistry, this is the ratio between the amount of product actually detected and quantified, and the amount of that product that could theoretically have been formed based on the charge passed in the experiment.
- Exchange current densities
-
Exchange current densities reflect the intrinsic rate of electron transfer between an analyte in solution and the electrode, and can thus be viewed as a measure of the effectiveness of a catalyst for a particular electrochemical reaction under a particular set of conditions (the greater the magnitude of the exchange current density, the greater the activity of the catalyst).
- Photosystem II
-
Photosystem II is a key protein complex involved in photosynthesis. It uses sunlight to oxidize water, producing high energy electrons for subsequent chemical reactions.
- Reversible hydrogen electrode
-
(RHE). The RHE is based on the following electrochemical half-reaction: 2e− + 2H+ → H2. Therefore, it is a subtype of the standard hydrogen electrode. However, the measured potential of the RHE does not change with pH, which means it can be used to make a ready comparison between the theoretical position of onset of the HER (always 0 V versus RHE) and the position of a redox event of interest, regardless of the pH of the solution.
- Solar-to-hydrogen conversion efficiency
-
The energy that would be released upon complete oxidation of the hydrogen produced by, for example, an artificial photosynthesis system in a given time, divided by the energy required by the artificial photosynthesis system to produce that amount of hydrogen.
Rights and permissions
About this article
Cite this article
Roger, I., Shipman, M. & Symes, M. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat Rev Chem 1, 0003 (2017). https://doi.org/10.1038/s41570-016-0003
Published:
DOI: https://doi.org/10.1038/s41570-016-0003
This article is cited by
-
All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production
Nature Energy (2024)
-
Microenvironment reconstitution of highly active Ni single atoms on oxygen-incorporated Mo2C for water splitting
Nature Communications (2024)
-
Solar reforming as an emerging technology for circular chemical industries
Nature Reviews Chemistry (2024)
-
MXene supported PtCo bimetallic catalyst for hydrogen evolution in acidic conditions
Frontiers in Energy (2024)
-
Enhanced active edge sites on MoSe2 nanostructures for stable electrocatalytic and photocatalytic hydrogen evolution reaction
Ionics (2024)