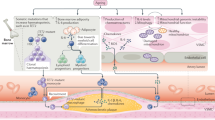
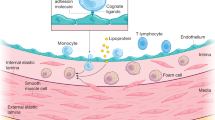
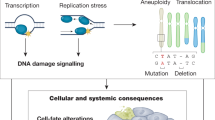
Abstract
Normal circulatory function is a key determinant of disease-free life expectancy (healthspan). Indeed, pathologies affecting the cardiovascular system, which are growing in prevalence, are the leading cause of global morbidity, disability and mortality, whereas the maintenance of cardiovascular health is necessary to promote both organismal healthspan and lifespan. Therefore, cardiovascular ageing might precede or even underlie body-wide, age-related health deterioration. In this Review, we posit that eight molecular hallmarks are common denominators in cardiovascular ageing, namely disabled macroautophagy, loss of proteostasis, genomic instability (in particular, clonal haematopoiesis of indeterminate potential), epigenetic alterations, mitochondrial dysfunction, cell senescence, dysregulated neurohormonal signalling and inflammation. We also propose a hierarchical order that distinguishes primary (upstream) from antagonistic and integrative (downstream) hallmarks of cardiovascular ageing. Finally, we discuss how targeting each of the eight hallmarks might be therapeutically exploited to attenuate residual cardiovascular risk in older individuals.
Key points
-
Age is one of the strongest determinants of cardiovascular health.
-
The cardiovascular system operates under constant mechanical and metabolic stress, which progressively increases the risk of dysfunction at the molecular, cellular and whole-organ levels with increasing age.
-
Disabled macroautophagy, loss of proteostasis, genomic instability, epigenetic alterations, mitochondrial dysfunction, cell senescence, dysregulated neurohormonal signalling and inflammation emerge as common molecular hallmarks of cardiovascular ageing.
-
The hallmarks of cardiovascular ageing are strongly intertwined, and accentuation or attenuation of individual hallmarks affects most, if not all, the others.
-
Targeting the hallmarks of cardiovascular ageing holds promise for the treatment of major cardiovascular disorders and to reduce residual cardiovascular risk beyond the management of conventional risk factors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Senyo, S. E. et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436 (2013).
Bergmann, O. et al. Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015).
Ji, H. et al. Sex differences in myocardial and vascular aging. Circ. Res. 130, 566–577 (2022).
Ungvari, Z. et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 15, 555–565 (2018).
Ungvari, Z., Tarantini, S., Sorond, F., Merkely, B. & Csiszar, A. Mechanisms of vascular aging, a geroscience perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 75, 931–941 (2020).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a ‘set up’ for vascular disease. Circulation 107, 139–146 (2003).
Lakatta, E. G. & Levy, D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation 107, 346–354 (2003).
North, B. J. & Sinclair, D. A. The intersection between aging and cardiovascular disease. Circ. Res. 110, 1097–1108 (2012).
Michos, E. D., McEvoy, J. W. & Blumenthal, R. S. Lipid management for the prevention of atherosclerotic cardiovascular disease. N. Engl. J. Med. 381, 1557–1567 (2019).
Fuchs, F. D. & Whelton, P. K. High blood pressure and cardiovascular disease. Hypertension 75, 285–292 (2020).
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Lieb, W., Enserro, D. M., Larson, M. G. & Vasan, R. S. Residual cardiovascular risk in individuals on lipid-lowering treatment: quantifying absolute and relative risk in the community. Open Heart 5, e000722 (2018).
Lieb, W., Enserro, D. M., Sullivan, L. M. & Vasan, R. S. Residual cardiovascular risk in individuals on blood pressure-lowering treatment. J. Am. Heart Assoc. 4, e002155 (2015).
Vanuzzo, D. The epidemiological concept of residual risk. Intern. Emerg. Med. 6, 45–51 (2011).
Moturi, S., Ghosh-Choudhary, S. K. & Finkel, T. Cardiovascular disease and the biology of aging. J. Mol. Cell Cardiol. 167, 109–117 (2022).
Zhernakova, D. V. et al. Age-dependent sex differences in cardiometabolic risk factors. Nat. Cardiovasc. Res. 1, 844–854 (2022).
Hägg, S. & Jylhävä, J. Sex differences in biological aging with a focus on human studies. eLife 10, e63425 (2021).
Abdellatif, M. et al. Fine-tuning cardiac insulin-like growth factor 1 receptor signaling to promote health and longevity. Circulation 145, 1853–1866 (2022).
Grunewald, M. et al. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science 373, eabc8479 (2021).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: an expanding universe. Cell 186, 243–278 (2023).
Levine, B. & Kroemer, G. Biological functions of autophagy genes: a disease perspective. Cell 176, 11–42 (2019).
Abdellatif, M. & Kroemer, G. Co-ordinated mitochondrial degradation by autophagy and heterophagy in cardiac homeostasis. Cardiovasc. Res. 117, e1–e3 (2021).
Nicolás-Ávila, J. A. et al. A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109.e23 (2020).
Abdellatif, M., Sedej, S., Carmona-Gutierrez, D., Madeo, F. & Kroemer, G. Autophagy in cardiovascular aging. Circ. Res. 123, 803–824 (2018).
Abdellatif, M., Ljubojevic-Holzer, S., Madeo, F. & Sedej, S. Autophagy in cardiovascular health and disease. Prog. Mol. Biol. Transl. Sci. 172, 87–106 (2020).
Picca, A. et al. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat. Rev. Cardiol. 15, 543–554 (2018).
Bravo-San Pedro, J. M., Kroemer, G. & Galluzzi, L. Autophagy and mitophagy in cardiovascular disease. Circ. Res. 120, 1812–1824 (2017).
Kanamori, H. et al. Impact of autophagy on prognosis of patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 79, 789–801 (2022).
Jefferies, J. L. & Saffitz, J. E. Autophagy and reverse remodeling: a new biomarker in heart failure? J. Am. Coll. Cardiol. 79, 802–804 (2022).
Pietrocola, F. et al. Aspirin recapitulates features of caloric restriction. Cell Rep. 22, 2395–2407 (2018).
Farah, B. L. et al. β-Adrenergic agonist and antagonist regulation of autophagy in HepG2 cells, primary mouse hepatocytes, and mouse liver. PLoS One 9, e98155 (2014).
Kania, E. et al. Verapamil treatment induces cytoprotective autophagy by modulating cellular metabolism. FEBS J. 284, 1370–1387 (2017).
Rubinsztein, D. C., Mariño, G. & Kroemer, G. Autophagy and aging. Cell 146, 682–695 (2011).
Kaushik, S. et al. Autophagy and the hallmarks of aging. Ageing Res. Rev. 72, 101468 (2021).
Klionsky, D. J. et al. Autophagy in major human diseases. EMBO J. 40, e108863 (2021).
LaRocca, T. J. et al. Translational evidence that impaired autophagy contributes to arterial ageing. J. Physiol. 590, 3305–3316 (2012).
Linton, P.-J., Gurney, M., Sengstock, D., Mentzer, R. M. & Gottlieb, R. A. This old heart: cardiac aging and autophagy. J. Mol. Cell Cardiol. 83, 44–54 (2015).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12, 1–222 (2016).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17, 1–382 (2021).
Cho, J. M. et al. Late-in-life treadmill training rejuvenates autophagy, protein aggregate clearance, and function in mouse hearts. Aging Cell 20, e13467 (2021).
Wu, B. et al. Aldehyde dehydrogenase 2 activation in aged heart improves the autophagy by reducing the carbonyl modification on SIRT1. Oncotarget 7, 2175–2188 (2016).
Chang, K. et al. TGFB-INHB/activin signaling regulates age-dependent autophagy and cardiac health through inhibition of MTORC2. Autophagy 16, 1807–1822 (2020).
Ma, L. et al. Restoring pharmacologic preconditioning in the aging heart: role of mitophagy/autophagy. J. Gerontol. A Biol. Sci. Med. Sci. 72, 489–498 (2017).
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F. & Kroemer, G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821 (2015).
Kane, A. E. & Sinclair, D. A. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ. Res. 123, 868–885 (2018).
Harraz, O. F. & Jensen, L. J. Vascular calcium signalling and ageing. J. Physiol. 599, 5361–5377 (2021).
Shaikh, S. et al. Regulation of cardiomyocyte autophagy by calcium. Am. J. Physiol. Endocrinol. Metab. 310, E587–E596 (2016).
Behringer, E. J. & Segal, S. S. Impact of aging on calcium signaling and membrane potential in endothelium of resistance arteries: a role for mitochondria. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1627–1637 (2017).
Wang, J. et al. Spermidine alleviates cardiac aging by improving mitochondrial biogenesis and function. Aging 12, 650–671 (2020).
Pucciarelli, S. et al. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 15, 590–595 (2012).
Madeo, F., Eisenberg, T., Pietrocola, F. & Kroemer, G. Spermidine in health and disease. Science 359, eaan2788 (2018).
LaRocca, T. J., Gioscia-Ryan, R. A., Hearon, C. M. & Seals, D. R. The autophagy enhancer spermidine reverses arterial aging. Mech. Ageing Dev. 134, 314–320 (2013).
Eisenberg, T. et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22, 1428–1438 (2016).
Taneike, M. et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy 6, 600–606 (2010).
Ljubojević-Holzer, S. et al. Loss of autophagy protein ATG5 impairs cardiac capacity in mice and humans through diminishing mitochondrial abundance and disrupting Ca2+ cycling. Cardiovasc. Res. 118, 1492–1505 (2022).
Michiels, C. F., Fransen, P., De Munck, D. G., De Meyer, G. R. Y. & Martinet, W. Defective autophagy in vascular smooth muscle cells alters contractility and Ca2+ homeostasis in mice. Am. J. Physiol. Heart Circ. Physiol. 308, H557–H567 (2015).
Grootaert, M. O. et al. Defective autophagy in vascular smooth muscle cells accelerates senescence and promotes neointima formation and atherogenesis. Autophagy 11, 2014–2032 (2015).
Torisu, T. et al. Autophagy regulates endothelial cell processing, maturation and secretion of von Willebrand factor. Nat. Med. 19, 1281–1287 (2013).
Hsieh, P. N. et al. A conserved KLF-autophagy pathway modulates nematode lifespan and mammalian age-associated vascular dysfunction. Nat. Commun. 8, 914 (2017).
Fernández, Á. F. et al. Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature 558, 136–140 (2018).
Alfaras, I. et al. Pharmacological strategies to retard cardiovascular aging. Circ. Res. 118, 1626–1642 (2016).
Voglhuber, J., Ljubojevic-Holzer, S., Abdellatif, M. & Sedej, S. Targeting cardiovascular risk factors through dietary adaptations and caloric restriction mimetics. Front. Nutr. 8, 758058 (2021).
Weiss, E. P. & Fontana, L. Caloric restriction: powerful protection for the aging heart and vasculature. Am. J. Physiol. Heart Circ. Physiol. 301, H1205–H1219 (2011).
Madeo, F., Carmona-Gutierrez, D., Hofer, S. J. & Kroemer, G. Caloric restriction mimetics against age-associated disease: targets, mechanisms, and therapeutic potential. Cell Metab. 29, 592–610 (2019).
Quarles, E. et al. Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell 19, e13086 (2020).
Lesniewski, L. A. et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell 16, 17–26 (2017).
Kaplon, R. E. et al. Oral trehalose supplementation improves resistance artery endothelial function in healthy middle-aged and older adults. Aging 8, 1167–1183 (2016).
Flynn, J. M. et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12, 851–862 (2013).
López-Otín, C. & Kroemer, G. Hallmarks of health. Cell 184, 1929–1939 (2021).
Hipp, M. S., Kasturi, P. & Hartl, F. U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 20, 421–435 (2019).
Kaushik, S. & Cuervo, A. M. The coming of age of chaperone-mediated autophagy. Nat. Rev. Mol. Cell Biol. 19, 365–381 (2018).
Alberti, S. & Hyman, A. A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 22, 196–213 (2021).
Gouveia, M., Xia, K., Colón, W., Vieira, S. I. & Ribeiro, F. Protein aggregation, cardiovascular diseases, and exercise training: where do we stand? Ageing Res. Rev. 40, 1–10 (2017).
Henning, R. H. & Brundel, B. J. J. M. Proteostasis in cardiac health and disease. Nat. Rev. Cardiol. 14, 637–653 (2017).
González-López, E. et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur. Heart J. 36, 2585–2594 (2015).
Rainer, P. P. et al. Desmin phosphorylation triggers preamyloid oligomers formation and myocyte dysfunction in acquired heart failure. Circ. Res. 122, e75–e83 (2018).
Hofmann, C., Katus, H. A. & Doroudgar, S. Protein misfolding in cardiac disease. Circulation 139, 2085–2088 (2019).
McLendon, P. M. & Robbins, J. Proteotoxicity and cardiac dysfunction. Circ. Res. 116, 1863–1882 (2015).
Martin, T. G. et al. Cardiomyocyte contractile impairment in heart failure results from reduced BAG3-mediated sarcomeric protein turnover. Nat. Commun. 12, 2942 (2021).
Bulteau, A.-L., Szweda, L. I. & Friguet, B. Age-dependent declines in proteasome activity in the heart. Arch. Biochem. Biophys. 397, 298–304 (2002).
Colotti, C. et al. Effects of aging and anti-aging caloric restrictions on carbonyl and heat shock protein levels and expression. Biogerontology 6, 397–406 (2005).
Dai, D. et al. Altered proteome turnover and remodeling by short‐term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539 (2014).
Ayyadevara, S. et al. Age- and hypertension-associated protein aggregates in mouse heart have similar proteomic profiles. Hypertension 67, 1006–1013 (2016).
Madrigal-Matute, J. et al. Protective role of chaperone-mediated autophagy against atherosclerosis. Proc. Natl Acad. Sci. USA 119, e2121133119 (2022).
Tabula Sapiens Consortium et al. The Tabula Sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022).
Marfella, R. et al. Effects of ubiquitin-proteasome system deregulation on the vascular senescence and atherosclerosis process in elderly patients. J. Gerontol. A Biol. Sci. Med. Sci. 63, 200–203 (2008).
Chin, J. H., Okazaki, M., Hu, Z. W., Miller, J. W. & Hoffman, B. B. Activation of heat shock protein (hsp)70 and proto-oncogene expression by alpha1 adrenergic agonist in rat aorta with age. J. Clin. Invest. 97, 2316–2323 (1996).
Degenhardt, K. et al. Medin aggregation causes cerebrovascular dysfunction in aging wild-type mice. Proc. Natl Acad. Sci. USA 117, 23925–23931 (2020).
Mucchiano, G., Cornwell, G. G. & Westermark, P. Senile aortic amyloid. Evidence for two distinct forms of localized deposits. Am. J. Pathol. 140, 871–877 (1992).
Wagner, J. et al. Medin co-aggregates with vascular amyloid-β in Alzheimer’s disease. Nature 612, 123–131 (2022).
Migrino, R. Q. et al. Cerebrovascular medin is associated with Alzheimer’s disease and vascular dementia. Alzheimers Dement. 12, e12072 (2020).
Rajasekaran, N. S. et al. Human αB-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell 130, 427–439 (2007).
Tannous, P. et al. Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc. Natl Acad. Sci. USA 105, 9745–9750 (2008).
Ma, X. et al. Transcription factor EB activation rescues advanced αB-crystallin mutation-induced cardiomyopathy by normalizing desmin localization. J. Am. Heart Assoc. 8, e010866 (2019).
Vicart, P. et al. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20, 92–95 (1998).
Nikolova, V. et al. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C-deficient mice. J. Clin. Invest. 113, 357–369 (2004).
Frock, R. L. et al. Cardiomyocyte-specific expression of lamin a improves cardiac function in Lmna−/− mice. PLoS One 7, e42918 (2012).
Galata, Z. et al. Amelioration of desmin network defects by αB-crystallin overexpression confers cardioprotection in a mouse model of dilated cardiomyopathy caused by LMNA gene mutation. J. Mol. Cell Cardiol. 125, 73–86 (2018).
Gupta, M. K. et al. Sumo E2 enzyme UBC9 is required for efficient protein quality control in cardiomyocytes. Circ. Res. 115, 721–729 (2014).
Pattison, J. S. et al. Cardiomyocyte expression of a polyglutamine preamyloid oligomer causes heart failure. Circulation 117, 2743–2751 (2008).
Blice-Baum, A. C. et al. Modest overexpression of FOXO maintains cardiac proteostasis and ameliorates age-associated functional decline. Aging Cell 16, 93–103 (2017).
Sanbe, A. et al. Protective effect of geranylgeranylacetone via enhancement of HSPB8 induction in desmin-related cardiomyopathy. PLoS One 4, e5351 (2009).
Ooie, T. et al. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation 104, 1837–1843 (2001).
Elliott, P. et al. Long-term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ. Heart Fail. 15, e008193 (2022).
Maurer, M. S. et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N. Engl. J. Med. 379, 1007–1016 (2018).
Fontana, L. Interventions to promote cardiometabolic health and slow cardiovascular ageing. Nat. Rev. Cardiol. 15, 566–577 (2018).
Yang, L. et al. Long-term calorie restriction enhances cellular quality-control processes in human skeletal muscle. Cell Rep. 14, 422–428 (2016).
Ono, T. et al. Age-associated increase of spontaneous mutant frequency and molecular nature of mutation in newborn and old lacZ-transgenic mouse. Mutat. Res. 447, 165–177 (2000).
De Majo, F. et al. Genomic instability in the naturally and prematurely aged myocardium. Proc. Natl Acad. Sci. USA 118, e2022974118 (2021).
Dollé, M. E., Snyder, W. K., Gossen, J. A., Lohman, P. H. & Vijg, J. Distinct spectra of somatic mutations accumulated with age in mouse heart and small intestine. Proc. Natl Acad. Sci. USA 97, 8403–8408 (2000).
Busuttil, R. A., Dollé, M., Campisi, J. & Vijga, J. Genomic instability, aging, and cellular senescence. Ann. N. Y. Acad. Sci. 1019, 245–255 (2004).
Oh, H. et al. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc. Natl Acad. Sci. USA 98, 10308–10313 (2001).
Terai, M. et al. Association of telomere shortening in myocardium with heart weight gain and cause of death. Sci. Rep. 3, 2401 (2013).
Takubo, K. et al. Telomere lengths are characteristic in each human individual. Exp. Gerontol. 37, 523–531 (2002).
Anderson, R. et al. Length-independent telomere damage drives post-mitotic cardiomyocyte senescence. EMBO J. 38, e100492 (2019).
Muñoz-Lorente, M. A., Cano-Martin, A. C. & Blasco, M. A. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 10, 4723 (2019).
Sharifi‐Sanjani, M. et al. Cardiomyocyte‐specific telomere shortening is a distinct signature of heart failure in humans. J. Am. Heart Assoc. 6, e005086 (2017).
Chang, A. C. Y. et al. Telomere shortening is a hallmark of genetic cardiomyopathies. Proc. Natl Acad. Sci. USA 115, 9276–9281 (2018).
Ungvari, Z., Tarantini, S., Donato, A. J., Galvan, V. & Csiszar, A. Mechanisms of vascular aging. Circ. Res. 123, 849–867 (2018).
Bloom, S. I. et al. Aging results in DNA damage and telomere dysfunction that is greater in endothelial versus vascular smooth muscle cells and is exacerbated in atheroprone regions. Geroscience 44, 2741–2755 (2022).
Martinet, W., Knaapen, M. W. M., De Meyer, G. R. Y., Herman, A. G. & Kockx, M. M. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation 106, 927–932 (2002).
Gray, K. et al. Effects of DNA damage in smooth muscle cells in atherosclerosis. Circ. Res. 116, 816–826 (2015).
Yabluchanskiy, A. et al. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience 42, 409–428 (2020).
Remes, T. M. et al. Radiation-induced accelerated aging of the brain vasculature in young adult survivors of childhood brain tumors. Neurooncol. Pract. 7, 415–427 (2020).
Andreassi, M. G. et al. Subclinical carotid atherosclerosis and early vascular aging from long-term low-dose ionizing radiation exposure: a genetic, telomere, and vascular ultrasound study in cardiac catheterization laboratory staff. JACC Cardiovasc. Interv. 8, 616–627 (2015).
Durik, M. et al. Nucleotide excision DNA repair is associated with age-related vascular dysfunction. Circulation 126, 468–478 (2012).
Ataei Ataabadi, E. et al. Vascular ageing features caused by selective DNA damage in smooth muscle cell. Oxid. Med. Cell Longev. 2021, 2308317 (2021).
Bautista-Niño, P. K. et al. Local endothelial DNA repair deficiency causes aging-resembling endothelial-specific dysfunction. Clin. Sci. 134, 727–746 (2020).
Matsumoto, T. et al. Aging-associated vascular phenotype in mutant mice with low levels of BubR1. Stroke 38, 1050–1056 (2007).
Jaiswal, S. & Libby, P. Clonal haematopoiesis: connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 17, 137–144 (2020).
Abelson, S. et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature 559, 400–404 (2018).
Jaiswal, S. et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 371, 2488–2498 (2014).
Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 371, 2477–2487 (2014).
Steensma, D. P. et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126, 9–16 (2015).
Xie, M. et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat. Med. 20, 1472–1478 (2014).
Jaiswal, S. et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl. J. Med. 377, 111–121 (2017).
Dorsheimer, L. et al. Association of mutations contributing to clonal hematopoiesis with prognosis in chronic ischemic heart failure. JAMA Cardiol. 4, 25–33 (2019).
Shi, C. et al. Clonal haematopoiesis of indeterminate potential: associations with heart failure incidence, clinical parameters and biomarkers. Eur. J. Heart Fail. 25, 4–13 (2023).
Devesa, A. et al. Bone marrow activation in response to metabolic syndrome and early atherosclerosis. Eur. Heart J. 43, 1809–1828 (2022).
Schloss, M. J., Swirski, F. K. & Nahrendorf, M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ. Res. 126, 1242–1259 (2020).
Fuster, J. J. et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355, 842–847 (2017).
Sano, S. et al. Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J. Am. Coll. Cardiol. 71, 875–886 (2018).
Sano, S. et al. CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ. Res. 123, 335–341 (2018).
Zekavat, S. M. et al. TP53-mediated clonal hematopoiesis confers increased risk for incident atherosclerotic disease. Nat. Cardiovasc. Res. 2, 144–158 (2023).
Abplanalp, W. T. et al. Clonal hematopoiesis-driver DNMT3A mutations alter immune cells in heart failure. Circ. Res. 128, 216–228 (2021).
Bick, A. G. et al. Genetic Interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation 141, 124–131 (2020).
Kabir, I. et al. The age of bone marrow dictates the clonality of smooth muscle-derived cells in atherosclerotic plaques. Nat. Aging 3, 64–81 (2023).
Potus, F. et al. Novel mutations and decreased expression of the epigenetic regulator TET2 in pulmonary arterial hypertension. Circulation 141, 1986–2000 (2020).
Shi, Y. et al. Epigenetic regulation in cardiovascular disease: mechanisms and advances in clinical trials. Signal. Transduct. Target. Ther. 7, 200 (2022).
Greco, C. M. & Condorelli, G. Epigenetic modifications and noncoding RNAs in cardiac hypertrophy and failure. Nat. Rev. Cardiol. 12, 488–497 (2015).
Schaum, N. et al. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602 (2020).
Stoeger, T. et al. Aging is associated with a systemic length-associated transcriptome imbalance. Nat. Aging 2, 1191–1206 (2022).
Bahar, R. et al. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature 441, 1011–1014 (2006).
Jusic, A. et al. Noncoding RNAs in age-related cardiovascular diseases. Ageing Res. Rev. 77, 101610 (2022).
Du, W. W. et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 38, 1402–1412 (2017).
Zhang, X., Azhar, G. & Wei, J. Y. The expression of microRNA and microRNA clusters in the aging heart. PLoS One 7, e34688 (2012).
Boon, R. A. et al. MicroRNA-34a regulates cardiac ageing and function. Nature 495, 107–110 (2013).
Ito, T., Yagi, S. & Yamakuchi, M. MicroRNA-34a regulation of endothelial senescence. Biochem. Biophys. Res. Commun. 398, 735–740 (2010).
Badi, I. et al. MicroRNA-34a induces vascular smooth muscle cells senescence by SIRT1 downregulation and promotes the expression of age-associated pro-inflammatory secretory factors. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1304–1311 (2015).
Badi, I. et al. miR-34a promotes vascular smooth muscle cell calcification by downregulating SIRT1 (Sirtuin 1) and Axl (AXL receptor tyrosine kinase). Arterioscler. Thromb. Vasc. Biol. 38, 2079–2090 (2018).
Huang, Z.-P. et al. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 112, 1234–1243 (2013).
Gupta, S. K. et al. Preclinical development of a microRNA-based therapy for elderly patients with myocardial infarction. J. Am. Coll. Cardiol. 68, 1557–1571 (2016).
Mattick, J. S. et al. Long non-coding RNAs: definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-022-00566-8 (2023).
Ponting, C. P. & Haerty, W. Genome-wide analysis of human long noncoding RNAs: a provocative review. Annu. Rev. Genomics Hum. Genet. 23, 153–172 (2022).
Trembinski, D. J. et al. Aging-regulated anti-apoptotic long non-coding RNA Sarrah augments recovery from acute myocardial infarction. Nat. Commun. 11, 2039 (2020).
Lu, T.-M. et al. Downregulation of Sirt1 as aging change in advanced heart failure. J. Biomed. Sci. 21, 57 (2014).
Donato, A. J. et al. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 589, 4545–4554 (2011).
Alcendor, R. R. et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 100, 1512–1521 (2007).
Guo, Y. et al. Endothelial SIRT1 prevents age-induced impairment of vasodilator responses by enhancing the expression and activity of soluble guanylyl cyclase in smooth muscle cells. Cardiovasc. Res. 115, 678–690 (2019).
Xu, S. et al. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. Aging 8, 1064–1082 (2016).
Li, W. et al. SIRT6 protects vascular smooth muscle cells from osteogenic transdifferentiation via Runx2 in chronic kidney disease. J. Clin. Invest. 132, e150051 (2022).
Sundaresan, N. R. et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 18, 1643–1650 (2012).
Zhang, W., Song, M., Qu, J. & Liu, G.-H. Epigenetic modifications in cardiovascular aging and diseases. Circ. Res. 123, 773–786 (2018).
Jeong, M. Y. et al. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci. Transl. Med. 10, eaao0144 (2018).
Travers, J. G. et al. HDAC inhibition reverses preexisting diastolic dysfunction and blocks covert extracellular matrix remodeling. Circulation 143, 1874–1890 (2021).
Wallner, M. et al. HDAC inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction. Sci. Transl. Med. 12, eaay7205 (2020).
Bagchi, R. A. & Weeks, K. L. Histone deacetylases in cardiovascular and metabolic diseases. J. Mol. Cell Cardiol. 130, 151–159 (2019).
Demos-Davies, K. M. et al. HDAC6 contributes to pathological responses of heart and skeletal muscle to chronic angiotensin-II signaling. Am. J. Physiol. Heart Circ. Physiol. 307, H252–H258 (2014).
Boucherat, O. et al. HDAC6: a novel histone deacetylase implicated in pulmonary arterial hypertension. Sci. Rep. 7, 4546 (2017).
Lin, Y.-H. et al. HDAC6 modulates myofibril stiffness and diastolic function of the heart. J. Clin. Invest. 132, e148333 (2022).
Malhotra, R. et al. HDAC9 is implicated in atherosclerotic aortic calcification and affects vascular smooth muscle cell phenotype. Nat. Genet. 51, 1580–1587 (2019).
Zhang, C. L. et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002).
Nguyen, A. T. et al. DOT1L regulates dystrophin expression and is critical for cardiac function. Genes Dev. 25, 263–274 (2011).
Stein, A. B. et al. Loss of H3K4 methylation destabilizes gene expression patterns and physiological functions in adult murine cardiomyocytes. J. Clin. Invest. 121, 2641–2650 (2011).
Delgado-Olguín, P. et al. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat. Genet. 44, 343–347 (2012).
Papait, R. et al. Histone methyltransferase G9a is required for cardiomyocyte homeostasis and hypertrophy. Circulation 136, 1233–1246 (2017).
Farina, F. M. et al. The epigenetic enzyme DOT1L orchestrates vascular smooth muscle cell-monocyte crosstalk and protects against atherosclerosis via the NF-κB pathway. Eur. Heart J. 43, 4562–4576 (2022).
Thienpont, B. et al. The H3K9 dimethyltransferases EHMT1/2 protect against pathological cardiac hypertrophy. J. Clin. Invest. 127, 335–348 (2017).
Lind, L., Ingelsson, E., Sundström, J., Siegbahn, A. & Lampa, E. Methylation-based estimated biological age and cardiovascular disease. Eur. J. Clin. Invest. 48, 12872 (2018).
Lo, Y.-H. & Lin, W.-Y. Cardiovascular health and four epigenetic clocks. Clin. Epigenetics 14, 73 (2022).
Zheng, Y. et al. Association of cardiovascular health through young adulthood with genome-wide DNA methylation patterns in midlife: the CARDIA study. Circulation 146, 94–109 (2022).
Movassagh, M. et al. Distinct epigenomic features in end-stage failing human hearts. Circulation 124, 2411–2422 (2011).
Haas, J. et al. Alterations in cardiac DNA methylation in human dilated cardiomyopathy. EMBO Mol. Med. 5, 413–429 (2013).
Pepin, M. E. et al. DNA methylation reprograms cardiac metabolic gene expression in end-stage human heart failure. Am. J. Physiol. Heart Circ. Physiol. 317, H674–H684 (2019).
Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115 (2013).
Pavanello, S. et al. The biological age of the heart is consistently younger than chronological age. Sci. Rep. 10, 10752 (2020).
Wu, T.-T. et al. Myocardial tissue-specific Dnmt1 knockout in rats protects against pathological injury induced by adriamycin. Lab. Invest. 100, 974–985 (2020).
Nührenberg, T. G. et al. Cardiac myocyte de novo DNA methyltransferases 3a/3b are dispensable for cardiac function and remodeling after chronic pressure overload in mice. PLoS One 10, e0131019 (2015).
Vujic, A. et al. Experimental heart failure modelled by the cardiomyocyte-specific loss of an epigenome modifier, DNMT3B. J. Mol. Cell Cardiol. 82, 174–183 (2015).
Okano, M., Bell, D. W., Haber, D. A. & Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99, 247–257 (1999).
Tyrrell, D. J. & Goldstein, D. R. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat. Rev. Cardiol. 18, 58–68 (2021).
Heyn, H. et al. Distinct DNA methylomes of newborns and centenarians. Proc. Natl Acad. Sci. USA 109, 10522–10527 (2012).
Zaina, S. et al. DNA methylation map of human atherosclerosis. Circ. Cardiovasc. Genet. 7, 692–700 (2014).
Dunn, J. et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J. Clin. Invest. 124, 3187–3199 (2014).
Amorim, J. A. et al. Mitochondrial and metabolic dysfunction in ageing and age-related diseases. Nat. Rev. Endocrinol. 18, 243–258 (2022).
Wallace, D. C. A mitochondrial bioenergetic etiology of disease. J. Clin. Invest. 123, 1405–1412 (2013).
Galluzzi, L., Kepp, O., Trojel-Hansen, C. & Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 111, 1198–1207 (2012).
Marchi, S., Guilbaud, E., Tait, S. W. G., Yamazaki, T. & Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 23, 159–173 (2023).
Lopez-Crisosto, C. et al. Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology. Nat. Rev. Cardiol. 14, 342–360 (2017).
Lesnefsky, E. J., Chen, Q. & Hoppel, C. L. Mitochondrial metabolism in aging heart. Circ. Res. 118, 1593–1611 (2016).
Dai, D.-F., Rabinovitch, P. S. & Ungvari, Z. Mitochondria and cardiovascular aging. Circ. Res. 110, 1109–1124 (2012).
Tian, R. et al. Unlocking the secrets of mitochondria in the cardiovascular system: path to a cure in heart failure — a report from the 2018 National Heart, Lung, and Blood Institute Workshop. Circulation 140, 1205–1216 (2019).
Bonora, M. et al. Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles. Nat. Rev. Cardiol. 16, 33–55 (2019).
Fannin, S. W., Lesnefsky, E. J., Slabe, T. J., Hassan, M. O. & Hoppel, C. L. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch. Biochem. Biophys. 372, 399–407 (1999).
Tatarková, Z. et al. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol. Res. 60, 281–289 (2011).
Lesnefsky, E. J. et al. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J. Mol. Cell Cardiol. 33, 37–47 (2001).
Messerer, J. et al. Spermidine supplementation influences mitochondrial number and morphology in the heart of aged mice. J. Anat. 242, 91–101 (2023).
Kates, A. M. et al. Impact of aging on substrate metabolism by the human heart. J. Am. Coll. Cardiol. 41, 293–299 (2003).
McMillin, J. B., Taffet, G. E., Taegtmeyer, H., Hudson, E. K. & Tate, C. A. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc. Res. 27, 2222–2228 (1993).
Ungvari, Z. et al. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 294, H2121–H2128 (2008).
Foote, K. et al. Restoring mitochondrial DNA copy number preserves mitochondrial function and delays vascular aging in mice. Aging Cell 17, e12773 (2018).
Park, S.-Y. et al. Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol. 222, e12893 (2018).
Gioscia-Ryan, R. A. et al. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 592, 2549–2561 (2014).
Ungvari, Z. et al. Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am. J. Physiol. Heart Circ. Physiol. 301, H363–H372 (2011).
Ungvari, Z. et al. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-κB activation in the nonhuman primate Macaca mulatta. J. Gerontol. A Biol. Sci. Med. Sci. 66, 866–875 (2011).
Tarantini, S. et al. Nrf2 deficiency exacerbates obesity-induced oxidative stress, neurovascular dysfunction, blood-brain barrier disruption, neuroinflammation, amyloidogenic gene expression, and cognitive decline in mice, mimicking the aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 73, 853–863 (2018).
Fulop, G. A. et al. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 40, 513–521 (2018).
Ungvari, Z. et al. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am. J. Physiol. Heart Circ. Physiol. 293, H37–H47 (2007).
Yu, H. et al. Role of the cGAS-STING pathway in aging-related endothelial dysfunction. Aging Dis. 13, 1901 (2022).
Rech, L. & Rainer, P. P. The innate immune cGAS-STING-pathway in cardiovascular diseases — a mini review. Front. Cardiovasc. Med. 8, 715903 (2021).
Pierce, G. L., Lesniewski, L. A., Lawson, B. R., Beske, S. D. & Seals, D. R. Nuclear factor-κB activation contributes to vascular endothelial dysfunction via oxidative stress in overweight/obese middle-aged and older humans. Circulation 119, 1284–1292 (2009).
Tyrrell, D. J. et al. Age-associated mitochondrial dysfunction accelerates atherogenesis. Circ. Res. 126, 298–314 (2020).
Abdellatif, M., Sedej, S. & Kroemer, G. NAD+ metabolism in cardiac health, aging, and disease. Circulation 144, 1795–1817 (2021).
Abdellatif, M., Bugger, H., Kroemer, G. & Sedej, S. NAD+ and vascular dysfunction: from mechanisms to therapeutic opportunities. J. Lipid Atheroscler. 11, 111–132 (2022).
Zimmermann, A., Madreiter-Sokolowski, C., Stryeck, S. & Abdellatif, M. Targeting the mitochondria-proteostasis axis to delay aging. Front. Cell Dev. Biol. 9, 656201 (2021).
Hoshino, A. et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 4, 2308 (2013).
Kubli, D. A., Quinsay, M. N. & Gustafsson, A. B. Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commun. Integr. Biol. 6, e24511 (2013).
Diao, R. Y. & Gustafsson, Å. B. Mitochondrial quality surveillance: mitophagy in cardiovascular health and disease. Am. J. Physiol. Cell Physiol. 322, C218–C230 (2022).
Billia, F. et al. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc. Natl Acad. Sci. USA 108, 9572–9577 (2011).
Zhu, X. et al. Fine-tuning of PGC1α expression regulates cardiac function and longevity. Circ. Res. 125, 707–719 (2019).
Song, M., Franco, A., Fleischer, J. A., Zhang, L. & Dorn, G. W. Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 26, 872–883.e5 (2017).
Wang, Q. et al. Deletion of PRKAA triggers mitochondrial fission by inhibiting the autophagy-dependent degradation of DNM1L. Autophagy 13, 404–422 (2017).
Peoples, J. N., Saraf, A., Ghazal, N., Pham, T. T. & Kwong, J. Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 51, 1–13 (2019).
Dai, D.-F. et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9, 536–544 (2010).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Yu, E. et al. Mitochondrial DNA damage can promote atherosclerosis independently of reactive oxygen species through effects on smooth muscle cells and monocytes and correlates with higher-risk plaques in humans. Circulation 128, 702–712 (2013).
Shabalina, I. G. et al. Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1. Aging 9, 315–339 (2017).
Fernandez-Caggiano, M. et al. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. Nat. Metab. 2, 1223–1231 (2020).
Zhang, Y. et al. Mitochondrial pyruvate carriers are required for myocardial stress adaptation. Nat. Metab. 2, 1248–1264 (2020).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Wilson, C., Lee, M. D., Buckley, C., Zhang, X. & McCarron, J. G. Mitochondrial ATP production is required for endothelial cell control of vascular tone. Function 4, zqac063 (2022).
Xiong, J. et al. A metabolic basis for endothelial-to-mesenchymal transition. Mol. Cell 69, 689–698.e7 (2018).
Tarantini, S. et al. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell 17, e12731 (2018).
Rossman, M. J. et al. Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71, 1056–1063 (2018).
Schriner, S. E. et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909–1911 (2005).
Yu, E. P. K. et al. Mitochondrial respiration is reduced in atherosclerosis, promoting necrotic core formation and reducing relative fibrous cap thickness. Arterioscler. Thromb. Vasc. Biol. 37, 2322–2332 (2017).
Kataura, T. et al. Autophagy promotes cell survival by maintaining NAD levels. Dev. Cell 57, 2584–2598.e11 (2022).
Abdellatif, M. et al. Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci. Transl. Med. 13, eabd7064 (2021).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 (2016).
Tarantini, S. et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 24, 101192 (2019).
Das, A. et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell 173, 74–89.e20 (2018).
Kiss, T. et al. Nicotinamide mononucleotide (NMN) supplementation promotes neurovascular rejuvenation in aged mice: transcriptional footprint of SIRT1 activation, mitochondrial protection, anti-inflammatory, and anti-apoptotic effects. Geroscience 42, 527–546 (2020).
Tarantini, S. et al. Treatment with the poly(ADP-ribose) polymerase inhibitor PJ-34 improves cerebromicrovascular endothelial function, neurovascular coupling responses and cognitive performance in aged mice, supporting the NAD+ depletion hypothesis of neurovascular aging. Geroscience 41, 533–542 (2019).
Mehdizadeh, M., Aguilar, M., Thorin, E., Ferbeyre, G. & Nattel, S. The role of cellular senescence in cardiac disease: basic biology and clinical relevance. Nat. Rev. Cardiol. 19, 250–264 (2022).
Gude, N. A., Broughton, K. M., Firouzi, F. & Sussman, M. A. Cardiac ageing: extrinsic and intrinsic factors in cellular renewal and senescence. Nat. Rev. Cardiol. 15, 523–542 (2018).
Gorgoulis, V. et al. Cellular senescence: defining a path forward. Cell 179, 813–827 (2019).
Di Micco, R., Krizhanovsky, V., Baker, D. & d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 22, 75–95 (2021).
Admasu, T. D., Rae, M. & Stolzing, A. Dissecting primary and secondary senescence to enable new senotherapeutic strategies. Ageing Res. Rev. 70, 101412 (2021).
González-Gualda, E., Baker, A. G., Fruk, L. & Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 288, 56–80 (2021).
Kohli, J. et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat. Protoc. 16, 2471–2498 (2021).
Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021).
Owens, W. A., Walaszczyk, A., Spyridopoulos, I., Dookun, E. & Richardson, G. D. Senescence and senolytics in cardiovascular disease: promise and potential pitfalls. Mech. Ageing Dev. 198, 111540 (2021).
Honda, S. et al. Cellular senescence promotes endothelial activation through epigenetic alteration, and consequently accelerates atherosclerosis. Sci. Rep. 11, 14608 (2021).
Wang, J. et al. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 132, 1909–1919 (2015).
Childs, B. G. et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 354, 472–477 (2016).
Dookun, E. et al. Clearance of senescent cells during cardiac ischemia-reperfusion injury improves recovery. Aging Cell 19, e13249 (2020).
Gevaert, A. B. et al. Endothelial senescence contributes to heart failure with preserved ejection fraction in an aging mouse model. Circ. Heart Fail. 10, e003806 (2017).
Moiseeva, V. et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature 613, 169–178 (2023).
Bloom, S. I., Islam, M. T., Lesniewski, L. A. & Donato, A. J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 20, 38–51 (2023).
Grosse, L. et al. Defined p16High senescent cell types are indispensable for mouse healthspan. Cell Metab. 32, 87–99.e6 (2020).
Kiss, T. et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 42, 429–444 (2020).
Chala, N. et al. Mechanical fingerprint of senescence in endothelial cells. Nano Lett. 21, 4911–4920 (2021).
Bhayadia, R., Schmidt, B. M. W., Melk, A. & Hömme, M. Senescence-induced oxidative stress causes endothelial dysfunction. J. Gerontol. A Biol. Sci. Med. Sci. 71, 161–169 (2016).
Yamazaki, Y. et al. Vascular cell senescence contributes to blood-brain barrier breakdown. Stroke 47, 1068–1077 (2016).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Meyer, K., Hodwin, B., Ramanujam, D., Engelhardt, S. & Sarikas, A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J. Am. Coll. Cardiol. 67, 2018–2028 (2016).
Garrido, A. M. et al. Efficacy and limitations of senolysis in atherosclerosis. Cardiovasc. Res. 118, 1713–1727 (2022).
Gasek, N. S., Kuchel, G. A., Kirkland, J. L. & Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 1, 870–879 (2021).
Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 24, 1246–1256 (2018).
Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15, 973–977 (2016).
Tarantini, S. et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience 43, 2427–2440 (2021).
Morgan, R. G. et al. Induced Trf2 deletion leads to aging vascular phenotype in mice associated with arterial telomere uncapping, senescence signaling, and oxidative stress. J. Mol. Cell Cardiol. 127, 74–82 (2019).
Leri, A. et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 22, 131–139 (2003).
Rossman, M. J. et al. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Heart Circ. Physiol. 313, H890–H895 (2017).
Ziff, O. J. & Kotecha, D. Digoxin: the good and the bad. Trends Cardiovasc. Med. 26, 585–595 (2016).
Triana-Martínez, F. et al. Identification and characterization of cardiac glycosides as senolytic compounds. Nat. Commun. 10, 4731 (2019).
Ma, T. K. W., Kam, K. K. H., Yan, B. P. & Lam, Y.-Y. Renin-angiotensin-aldosterone system blockade for cardiovascular diseases: current status. Br. J. Pharmacol. 160, 1273–1292 (2010).
Ruggenenti, P. et al. Renal and systemic effects of calorie restriction in patients with type 2 diabetes with abdominal obesity: a randomized controlled trial. Diabetes 66, 75–86 (2017).
Jia, G., Aroor, A. R., Hill, M. A. & Sowers, J. R. Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension 72, 537–548 (2018).
Conti, S., Cassis, P. & Benigni, A. Aging and the renin-angiotensin system. Hypertension 60, 878–883 (2012).
Yoon, H. E. et al. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid. Med. Cell Longev. 2016, 6731093 (2016).
Dai, D.-F. et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119, 2789–2797 (2009).
Dinh, Q. N. et al. Pressor response to angiotensin II is enhanced in aged mice and associated with inflammation, vasoconstriction and oxidative stress. Aging 9, 1595–1606 (2017).
Forrester, S. J. et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 98, 1627–1738 (2018).
Billet, S. et al. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J. Clin. Invest. 117, 1914–1925 (2007).
Habibi, J. et al. Mineralocorticoid receptor blockade improves diastolic function independent of blood pressure reduction in a transgenic model of RAAS overexpression. Am. J. Physiol. Heart Circ. Physiol. 300, H1484–H1491 (2011).
Xiao, H. D. et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am. J. Pathol. 165, 1019–1032 (2004).
Benigni, A. et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 119, 524–530 (2009).
Basso, N. et al. Protective effect of long-term angiotensin II inhibition. Am. J. Physiol. Heart Circ. Physiol. 293, H1351–H1358 (2007).
Inserra, F., Romano, L., Ercole, L., de Cavanagh, E. M. & Ferder, L. Cardiovascular changes by long-term inhibition of the renin-angiotensin system in aging. Hypertension 25, 437–442 (1995).
Keller, K., Kane, A., Heinze-Milne, S., Grandy, S. A. & Howlett, S. E. Chronic treatment with the ACE inhibitor enalapril attenuates the development of frailty and differentially modifies pro- and anti-inflammatory cytokines in aging male and female C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1149–1157 (2019).
McCurley, A. et al. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat. Med. 18, 1429–1433 (2012).
Paz Ocaranza, M. et al. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 17, 116–129 (2020).
Lymperopoulos, A., Rengo, G. & Koch, W. J. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ. Res. 113, 739–753 (2013).
Elia, A. et al. Aging is associated with cardiac autonomic nerve fiber depletion and reduced cardiac and circulating BDNF levels. J. Geriatr. Cardiol. 18, 549–559 (2021).
Ferrara, N. et al. β-Adrenergic receptor responsiveness in aging heart and clinical implications. Front. Physiol. 4, 396 (2014).
Stratton, J. R. et al. Differences in cardiovascular responses to isoproterenol in relation to age and exercise training in healthy men. Circulation 86, 504–512 (1992).
Xiao, R. P., Spurgeon, H. A., O’Connor, F. & Lakatta, E. G. Age-associated changes in β-adrenergic modulation on rat cardiac excitation-contraction coupling. J. Clin. Invest. 94, 2051–2059 (1994).
Engelhardt, S., Hein, L., Wiesmann, F. & Lohse, M. J. Progressive hypertrophy and heart failure in β1-adrenergic receptor transgenic mice. Proc. Natl Acad. Sci. USA 96, 7059–7064 (1999).
Milano, C. A. et al. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science 264, 582–586 (1994).
Du, X. Age-dependent cardiomyopathy and heart failure phenotype in mice overexpressing β2-adrenergic receptors in the heart. Cardiovasc. Res. 48, 448–454 (2000).
Bisognano, J. D. et al. Myocardial-directed overexpression of the human β1-adrenergic receptor in transgenic mice. J. Mol. Cell Cardiol. 32, 817–830 (2000).
Liggett, S. B. et al. Early and delayed consequences of β2-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation 101, 1707–1714 (2000).
No authors listed. Effect of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF). Lancet 353, 2001–2007 (1999).
No authors listed. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet 353, 9–13 (1999).
Ziff, O. J. et al. Beta-blocker efficacy across different cardiovascular indications: an umbrella review and meta-analytic assessment. BMC Med. 18, 103 (2020).
Sipahi, I. et al. β-Blockers and progression of coronary atherosclerosis: pooled analysis of 4 intravascular ultrasonography trials. Ann. Intern. Med. 147, 10–18 (2007).
Leosco, D. et al. Exercise training and β-blocker treatment ameliorate age-dependent impairment of β-adrenergic receptor signaling and enhance cardiac responsiveness to adrenergic stimulation. Am. J. Physiol. Heart Circ. Physiol. 293, H1596–H1603 (2007).
Spindler, S. R. et al. β1-Adrenergic receptor blockade extends the life span of Drosophila and long-lived mice. Age 35, 2099–2109 (2013).
Yan, L. et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell 130, 247–258 (2007).
del Villar, S. G., Westhoff, M., Voelker, T. L., Dickson, E. J. & Dixon, R. E. Restoration of β-adrenergic responsivity in the aging heart. Preprint at bioRxiv https://doi.org/10.1101/2022.08.23.504979 (2022).
Vögele, A. et al. Effectiveness and safety of beta blockers in the management of hypertension in older adults: a systematic review to help reduce inappropriate prescribing. BMC Geriatr. 17, 224 (2017).
Chan You, S. et al. Comprehensive comparative effectiveness and safety of first-line β-blocker monotherapy in hypertensive patients: a large-scale multicenter observational study. Hypertension 77, 1528–1538 (2021).
Junnila, R. K., List, E. O., Berryman, D. E., Murrey, J. W. & Kopchick, J. J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 9, 366–376 (2013).
Milman, S., Huffman, D. M. & Barzilai, N. The somatotropic axis in human aging: framework for the current state of knowledge and future research. Cell Metab. 23, 980–989 (2016).
McMullen, J. R. et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J. Biol. Chem. 279, 4782–4793 (2004).
Delaughter, M. C., Taffet, G. E., Fiorotto, M. L., Entman, M. L. & Schwartz, R. J. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 13, 1923–1929 (1999).
Ock, S. et al. Deletion of IGF-1 receptors in cardiomyocytes attenuates cardiac aging in male mice. Endocrinology 157, 336–345 (2016).
Mao, K. et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 9, 2394 (2018).
Inuzuka, Y. et al. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation 120, 1695–1703 (2009).
Hedges, C. P. et al. Dietary supplementation of clinically utilized PI3K p110α inhibitor extends the lifespan of male and female mice. Nat. Aging 3, 162–172 (2023).
D’Assante, R. et al. Myocardial expression of somatotropic axis, adrenergic signalling, and calcium handling genes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Esc. Heart Fail. 8, 1681–1686 (2021).
Vasan, R. S. et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann. Intern. Med. 139, 642–648 (2003).
Andreassen, M. et al. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur. J. Endocrinol. 160, 25–31 (2009).
Rahmani, J. et al. Association between IGF-1 levels ranges and all-cause mortality: a meta-analysis. Aging Cell 21, e13540 (2022).
Zhang, W. B., Ye, K., Barzilai, N. & Milman, S. The antagonistic pleiotropy of insulin-like growth factor 1. Aging Cell 20, e13443 (2021).
Fontana, L. et al. Identification of a metabolic signature for multidimensional impairment and mortality risk in hospitalized older patients. Aging Cell 12, 459–466 (2013).
Toth, L. et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience 44, 2771–2783 (2022).
Erlandsson, M. C. et al. Low serum IGF1 is associated with hypertension and predicts early cardiovascular events in women with rheumatoid arthritis. BMC Med. 17, 141 (2019).
Csiszar, A. et al. Endothelial function and vascular oxidative stress in long-lived GH/IGF-deficient Ames dwarf mice. Am. J. Physiol. Heart Circ. Physiol. 295, H1882–H1894 (2008).
Reddy, A. K. et al. Young little mice express a premature cardiovascular aging phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 69, 152–159 (2014).
Abbas, A. et al. The insulin-like growth factor-1 receptor is a negative regulator of nitric oxide bioavailability and insulin sensitivity in the endothelium. Diabetes 60, 2169–2178 (2011).
Higashi, Y. et al. Endothelial deficiency of insulin-like growth factor-1 receptor reduces endothelial barrier function and promotes atherosclerosis in Apoe-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 319, H730–H743 (2020).
Franceschi, C. et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254 (2000).
Ferrucci, L. & Fabbri, E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15, 505–522 (2018).
Engelen, S. E., Robinson, A. J. B., Zurke, Y.-X. & Monaco, C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat. Rev. Cardiol. 19, 522–542 (2022).
Libby, P., Ridker, P. M. & Maseri, A. Inflammation and atherosclerosis. Circulation 105, 1135–1143 (2002).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Joshi, N. V. et al. Systemic atherosclerotic inflammation following acute myocardial infarction: myocardial infarction begets myocardial infarction. J. Am. Heart Assoc. 4, e001956 (2015).
Esfahani, N. S. et al. Aging influences the cardiac macrophage phenotype and function during steady state and during inflammation. Aging Cell 20, e13438 (2021).
Koenig, A. L. et al. Single-cell transcriptomics reveals cell-type-specific diversification in human heart failure. Nat. Cardiovasc. Res. 1, 263–280 (2022).
Barcena de Arellano, M. L. et al. Sex differences in the aging human heart: decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging 11, 1918–1933 (2019).
Ramos, G. C. et al. Myocardial aging as a T-cell-mediated phenomenon. Proc. Natl Acad. Sci. USA 114, E2420–E2429 (2017).
Delgobo, M. et al. Terminally differentiated CD4+ T cells promote myocardial inflammaging. Front. Immunol. 12, 584538 (2021).
Dolejsi, T. et al. Adult T-cells impair neonatal cardiac regeneration. Eur. Heart J. 43, 2698–2709 (2022).
Desdín-Micó, G. et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 368, 1371–1376 (2020).
Maier, H. J. et al. Cardiomyocyte-specific IκB kinase (IKK)/NF-κB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc. Natl Acad. Sci. USA 109, 11794–11799 (2012).
Kubota, T. et al. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circ. Res. 81, 627–635 (1997).
Marín-Aguilar, F. et al. NLRP3 inflammasome suppression improves longevity and prevents cardiac aging in male mice. Aging Cell 19, e13050 (2020).
Hasegawa, Y. et al. Blockade of the nuclear factor-κB pathway in the endothelium prevents insulin resistance and prolongs life spans. Circulation 125, 1122–1133 (2012).
Tardif, J.-C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139, 1289–1299 (2019).
Ridker, P. M. et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 (2017).
Dunlay, S. M., Roger, V. L. & Redfield, M. M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 14, 591–602 (2017).
Paulus, W. J. & Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
Abdellatif, M. & Kroemer, G. Heart failure with preserved ejection fraction: an age-related condition. J. Mol. Cell Cardiol. 167, 83–84 (2022).
Withaar, C., Li, S., Meems, L. M. G., Silljé, H. H. W. & de Boer, R. A. Aging and HFpEF: are we running out of time? J. Mol. Cell Cardiol. 168, 33–34 (2022).
Kalogeropoulos, A. et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J. Am. Coll. Cardiol. 55, 2129–2137 (2010).
Solomon, S. D. et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 387, 1089–1098 (2022).
Anker, S. D. et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 385, 1451–1461 (2021).
Elrakaybi, A., Laubner, K., Zhou, Q., Hug, M. J. & Seufert, J. Cardiovascular protection by SGLT2 inhibitors – Do anti-inflammatory mechanisms play a role? Mol. Metab. 64, 101549 (2022).
Cowie, M. R. & Fisher, M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 17, 761–772 (2020).
Zannad, F. et al. Effect of empagliflozin on circulating proteomics in heart failure: mechanistic insights into the EMPEROR programme. Eur. Heart J. 43, 4991–5002 (2022).
Hoong, C. W. S. & Chua, M. W. J. SGLT2 Inhibitors as calorie restriction mimetics: insights on longevity pathways and age-related diseases. Endocrinology 162, bqab079 (2021).
Packer, M. SGLT2 inhibitors: role in protective reprogramming of cardiac nutrient transport and metabolism. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-022-00824-4 (2023).
Deng, Y. et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circ. Res. 128, 232–245 (2021).
Yang, H., Wang, H., Ren, J., Chen, Q. & Chen, Z. J. cGAS is essential for cellular senescence. Proc. Natl Acad. Sci. USA 114, E4612–E4620 (2017).
Sladitschek-Martens, H. L. et al. YAP/TAZ activity in stromal cells prevents ageing by controlling cGAS-STING. Nature 607, 790–798 (2022).
Kreienkamp, R. et al. A cell-intrinsic interferon-like response links replication stress to cellular aging caused by progerin. Cell Rep. 22, 2006–2015 (2018).
Olive, M. et al. Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 30, 2301–2309 (2010).
Pham, P. T. et al. STING, a cytosolic DNA sensor, plays a critical role in atherogenesis: a link between innate immunity and chronic inflammation caused by lifestyle-related diseases. Eur. Heart J. 42, 4336–4348 (2021).
King, K. R. et al. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat. Med. 23, 1481–1487 (2017).
Cao, D. J. et al. Cytosolic DNA sensing promotes macrophage transformation and governs myocardial ischemic injury. Circulation 137, 2613–2634 (2018).
Li, Q. et al. Inhibition of double-strand DNA-sensing cGAS ameliorates brain injury after ischemic stroke. EMBO Mol. Med. 12, e11002 (2020).
Rech, L. et al. Small molecule STING inhibition improves myocardial infarction remodeling. Life Sci. 291, 120263 (2022).
Mittelbrunn, M. & Kroemer, G. Hallmarks of T cell aging. Nat. Immunol. 22, 687–698 (2021).
Castoldi, F. et al. Autophagy-mediated metabolic effects of aspirin. Cell Death Discov. 6, 129 (2020).
McNeil, J. J. et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 379, 1509–1518 (2018).
Gaziano, J. M. et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 392, 1036–1046 (2018).
ASCEND Study Collaborative Group et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N. Engl. J. Med. 379, 1529–1539 (2018).
Castellano, J. M. et al. Polypill strategy in secondary cardiovascular prevention. N. Engl. J. Med. 387, 967–977 (2022).
Svensson, E. C. et al. TET2-Driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol. 7, 521–528 (2022).
Ridker, P. M. et al. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 391, 319–328 (2018).
Rajendran, P. et al. Autophagy and senescence: a new insight in selected human diseases. J. Cell Physiol. 234, 21485–21492 (2019).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science 366, eaan4673 (2019).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 143, e984–e1010 (2021).
Tong, M. et al. Mitophagy is essential for maintaining cardiac function during high fat diet-induced diabetic cardiomyopathy. Circ. Res. 124, 1360–1371 (2019).
Sánchez-Ceinos, J. et al. Impaired mRNA splicing and proteostasis in preadipocytes in obesity-related metabolic disease. eLife 10, e65996 (2021).
Park, Y. J., Han, S. M., Huh, J. Y. & Kim, J. B. Emerging roles of epigenetic regulation in obesity and metabolic disease. J. Biol. Chem. 297, 101296 (2021).
Abel, E. D. Obesity stresses cardiac mitochondria even when you are young. J. Am. Coll. Cardiol. 57, 586–589 (2011).
Chaib, S., Tchkonia, T. & Kirkland, J. L. Obesity, senescence, and senolytics. Handb. Exp. Pharmacol. 274, 165–180 (2022).
Montégut, L., Lopez-Otin, C., Magnan, C. & Kroemer, G. Old paradoxes and new opportunities for appetite control in obesity. Trends Endocrinol. Metab. 32, 264–294 (2021).
Sharma, A. M. & Engeli, S. Obesity and the renin–angiotensin–aldosterone system. Expert Rev. Endocrinol. Metab. 1, 255–264 (2006).
Khafagy, R. & Dash, S. Obesity and cardiovascular disease: the emerging role of inflammation. Front. Cardiovasc. Med. 8, 768119 (2021).
Ryder, J. R. et al. Accelerated early vascular aging among adolescents with obesity and/or type 2 diabetes mellitus. J. Am. Heart Assoc. 9, e014891 (2020).
Niemann, B. et al. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J. Am. Coll. Cardiol. 57, 577–585 (2011).
de Cabo, R. & Mattson, M. P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 381, 2541–2551 (2019).
Meydani, S. N. et al. Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: a randomized controlled trial in non-obese humans. Aging 8, 1416–1431 (2016).
Spadaro, O. et al. Caloric restriction in humans reveals immunometabolic regulators of health span. Science 375, 671–677 (2022).
Stekovic, S. et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 31, 878–881 (2020).
Sabatine, M. S. et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017).
Bangalore, S., Kumar, S., Wetterslev, J. & Messerli, F. H. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. Br. Med. J. 342, d2234 (2011).
Granger, C. B. et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 362, 772–776 (2003).
Cohn, J. N., Tognoni, G.; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N. Engl. J. Med. 345, 1667–1675 (2001).
Pfeffer, M. A. et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 362, 759–766 (2003).
McMurray, J. J. V. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
Flather, M. D. et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet 355, 1575–1581 (2000).
Dagenais, G. R., Pogue, J., Fox, K., Simoons, M. L. & Yusuf, S. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet 368, 581–588 (2006).
van Vark, L. C. et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur. Heart J. 33, 2088–2097 (2012).
SOLVD Investigators et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 325, 293–302 (1991).
CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N. Engl. J. Med. 316, 1429–1435 (1987).
Packer, M. et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 106, 2194–2199 (2002).
Flather, M. D. et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 26, 215–225 (2005).
Pitt, B. et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 341, 709–717 (1999).
Zannad, F. et al. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 364, 11–21 (2011).
Pitt, B. et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 348, 1309–1321 (2003).
Tabula Muris Consortium. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 583, 590–595 (2020).
Pinto, A. R. et al. Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 (2016).
Acknowledgements
The authors thank the reviewers for their valuable constructive feedback and apologize to the authors of several high-quality articles on cardiovascular ageing that could not be discussed and cited owing to space limitations. The authors are also grateful to Leen Assil Companioni (CBmed, Graz, Austria) for designing the figures before submission, Sylvère Durand (Institute Gustave Roussy, Villejuif, France) for spermidine measurements as well as Nathaly Anto Michel (Medical University of Graz, Graz, Austria) and Andrew L. Koenig (Washington University School of Medicine, St. Louis, MO, USA) for extracting and providing the single-cell data reported in Fig. 1. M.A. acknowledges support from the European Commission (H2020-MSCA-IF), the Austrian Society of Cardiology (Präsidentenstipendium-ÖKG), the Medical University of Graz (Start Fund), BioTechMed-Graz (Young Researcher Group) and the Austrian Science Fund (FWF; P34926). P.P.R. and S.S. acknowledge support from the Medical University of Graz (Flagship project VASC-HEALTH) and BioTechMed-Graz (Flagship project INTERACD+). G.K. is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; European Research Council Advanced Investigator Grand ‘ICD-Cancer’, Fondation pour la Recherche Médicale (FRM); a donation by Elior; Equipex Onco-Pheno-Screen; European Joint Programme on Rare Diseases (EJPRD); European Research Council (ICD-Cancer), European Union Horizon 2020 Projects Oncobiome and Crimson; Fondation Carrefour; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology (ANR-18-IDEX-0001); a Cancer Research ASPIRE Award from the Mark Foundation; the RHU Immunolife; Seerave Foundation; SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE); and SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris ANR-18-IDEX-0001.
Author information
Authors and Affiliations
Contributions
M.A. and G.K. conceptualized the article. The authors contributed substantially to all other aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
M.A. and S.S. are involved in patent applications related to the cardiometabolic effects of caloric restriction mimetics. G.K. holds research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, Osasuna Therapeutics, PharmaMar, Samsara Therapeutics, Sanofi, Tollys and Vascage. G.K. is a consultant for Reithera and is on the Board of Directors of the Bristol Myers Squibb Foundation, France. G.K. is also a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. G.K. is the inventor of patents covering therapeutic targeting of ageing, cancer, cystic fibrosis and metabolic disorders. G.K.’s wife, Laurence Zitvogel, has held research contracts with 9 Meters Biopharma, Daiichi Sankyo and Pilege, was on the Board of Directors of Transgene, is a co-founder of everImmune, and holds patents covering the treatment of cancer and therapeutic manipulation of the microbiota. G.K.’s brother, Romano Kroemer, was an employee of Sanofi and consulted for Boehringer-Ingelheim. P.P.R. declares no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Luigi Fontana and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Autophagy
-
An evolutionarily conserved homeostatic process through which old, dysfunctional or potentially hazardous cytoplasmic components undergo lysosomal degradation; three types of autophagy exist in mammalian cells: microautophagy, macroautophagy and chaperone-mediated autophagy.
- Clonal haematopoiesis of indeterminate potential
-
(CHIP). A process by which stem cells in the blood or bone marrow randomly accrues somatic mutations during ageing, leading to exaggerated representation of a single clone.
- Inflammageing
-
The phenomenon of low-grade, subclinical sterile inflammation commonly observed with ageing, leading to increased circulating and tissue cytokine levels.
- Inflammasome
-
A supramolecular complex, the formation of which is triggered by a range of exogenous and endogenous pro-inflammatory factors, culminating in the activation of caspase 1, which proteolytically activates the pro-inflammatory cytokines IL-1β and IL-18.
- Non-coding RNAs
-
(ncRNAs). RNA molecules that are not translated into proteins but regulate the expression levels of target genes; there are several subfamilies of ncRNAs, including microRNAs (<200 nucleotides), long non-coding RNAs (>200 nucleotides) and covalently closed circular RNAs.
- Proteostasis
-
A network of cellular processes that maintain the health and integrity of the proteome, including protein biogenesis, folding, trafficking and degradation.
- Senescence
-
Permanent arrest of the cell cycle with irreversible loss of replicative capacity, leading to the loss of specific cellular functions, and the acquisition of a secretory and metabolically active phenotype with pro-inflammatory features.
- Senolytic therapy
-
Elimination of senescent cells by molecules that specifically induce cell death in senescent cells to improve organismal health.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdellatif, M., Rainer, P.P., Sedej, S. et al. Hallmarks of cardiovascular ageing. Nat Rev Cardiol 20, 754–777 (2023). https://doi.org/10.1038/s41569-023-00881-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-023-00881-3
This article is cited by
-
Extracellular vesicle-derived TP53BP1, CD34, and PBX1 from human peripheral blood serve as potential biomarkers for the assessment and prediction of vascular aging
Hereditas (2024)
-
Mineralocorticoid receptor promotes cardiac macrophage inflammaging
Basic Research in Cardiology (2024)
-
3,4-dimethoxychalcone induces autophagy and reduces neointimal hyperplasia and aortic lesions in mouse models of atherosclerosis
Cell Death & Disease (2023)
-
Age-induced senescence impairs the neurovascular interface in the heart
Nature Reviews Cardiology (2023)
-
SGLT2 Inhibitors in Aging-Related Cardiovascular Disease: A Review of Potential Mechanisms
American Journal of Cardiovascular Drugs (2023)