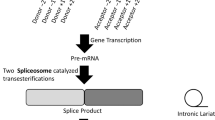
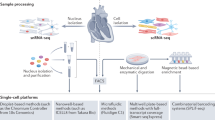
Abstract
Despite advances in therapeutics for heart failure and arrhythmias, a substantial proportion of patients with cardiomyopathy do not respond to interventions, indicating a need to identify novel modifiable myocardial pathobiology. Human genetic variation associated with severe forms of cardiomyopathy and arrhythmias has highlighted the crucial role of alternative splicing in myocardial health and disease, given that it determines which mature RNA transcripts drive the mechanical, structural, signalling and metabolic properties of the heart. In this Review, we discuss how the analysis of cardiac isoform expression has been facilitated by technical advances in multiomics and long-read and single-cell sequencing technologies. The resulting insights into the regulation of alternative splicing — including the identification of cardiac splice regulators as therapeutic targets and the development of a translational pipeline to evaluate splice modulators in human engineered heart tissue, animal models and clinical trials — provide a basis for improved diagnosis and therapy. Finally, we consider how the medical and scientific communities can benefit from facilitated acquisition and interpretation of splicing data towards improved clinical decision-making and patient care.
Key points
-
Human genetic variation associated with severe forms of cardiomyopathy and arrhythmia has highlighted the crucial role of alternative splicing in myocardial health and disease.
-
Alternative splicing governs major adaptations in cardiac physiology and pathology, including the re-expression of fetal and perinatal isoforms in heart failure.
-
Up to 10% of mutations in cardiac disease-related genes affect splice sites.
-
Splicing factor mutations alter the global protein composition of cardiomyocytes, resulting in complex disease phenotypes.
-
Technical advances have enabled the global analysis of cardiac isoform expression through multiomics and single-cell approaches, with implications for improved clinical decision-making and patient care.
-
Cardiac splice regulators can be targeted therapeutically through small-molecule and antisense oligonucleotide approaches, whereas splice site mutations are now accessible to gene editing and trans-splicing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pan, Q. et al. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40, 1413–1415 (2008).
Zhang, X. et al. Cell-type-specific alternative splicing governs cell fate in the developing cerebral cortex. Cell 166, 1147–1162.e15 (2016).
Olivieri, J. E. et al. RNA splicing programs define tissue compartments and cell types at single-cell resolution. eLife 10, e70692 (2021).
Wang, Z. & Burge, C. B. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA 14, 802–813 (2008).
Graveley, B. R. Sorting out the complexity of SR protein functions. RNA 6, 1197–1211 (2000).
Geuens, T., Bouhy, D. & Timmerman, V. The hnRNP family: insights into their role in health and disease. Hum. Genet. 135, 851–867 (2016).
Martí-Gómez, C. et al. Functional impact and regulation of alternative splicing in mouse heart development and disease. J. Cardiovasc. Transl. Res. 15, 1239–1255 (2022).
Travers, J. G., Tharp, C. A., Rubino, M. & McKinsey, T. A. Therapeutic targets for cardiac fibrosis: from old school to next-gen. J. Clin. Invest. 132, e148554 (2022).
Guo, W. et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat. Med. 18, 766–773 (2012).
Gittenberger-de Groot, A. C., Bartelings, M. M., Deruiter, M. C. & Poelmann, R. E. Basics of cardiac development for the understanding of congenital heart malformations. Pediatr. Res. 57, 169–176 (2005).
Olson, E. N. Gene regulatory networks in the evolution and development of the heart. Science 313, 1922–1927 (2006).
Opitz, C. A., Leake, M. C., Makarenko, I., Benes, V. & Linke, W. A. Developmentally regulated switching of titin size alters myofibrillar stiffness in the perinatal heart. Circ. Res. 94, 967–975 (2004).
Lahmers, S., Wu, Y., Call, D. R., Labeit, S. & Granzier, H. Developmental control of titin isoform expression and passive stiffness in fetal and neonatal myocardium. Circ. Res. 94, 505–513 (2004).
Freiburg, A. et al. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ. Res. 86, 1114–1121 (2000).
Agarkova, I., Auerbach, D., Ehler, E. & Perriard, J. C. A novel marker for vertebrate embryonic heart, the EH-myomesin isoform. J. Biol. Chem. 275, 10256–10264 (2000).
Gomes, A. V., Guzman, G., Zhao, J. & Potter, J. D. Cardiac troponin T isoforms affect the Ca2+ sensitivity and inhibition of force development. Insights into the role of troponin T isoforms in the heart. J. Biol. Chem. 277, 35341–35349 (2002).
Maytum, R., Bathe, F., Konrad, M. & Geeves, M. A. Tropomyosin exon 6b is troponin-specific and required for correct acto-myosin regulation. J. Biol. Chem. 279, 18203–18209 (2004).
Hoshijima, M. Mechanical stress-strain sensors embedded in cardiac cytoskeleton: Z disk, titin, and associated structures. Am. J. Physiol. Heart Circ. Physiol. 290, H1313–H1325 (2006).
Yamazaki, T. et al. Splice variants of enigma homolog, differentially expressed during heart development, promote or prevent hypertrophy. Cardiovasc. Res. 86, 374–382 (2010).
Huang, C. et al. Characterization and in vivo functional analysis of splice variants of cypher. J. Biol. Chem. 278, 7360–7365 (2003).
Weeland, C. J., van den Hoogenhof, M. M., Beqqali, A. & Creemers, E. E. Insights into alternative splicing of sarcomeric genes in the heart. J. Mol. Cell Cardiol. 81, 107–113 (2015).
Guo, Y. & Pu, W. T. Cardiomyocyte maturation: new phase in development. Circ. Res. 126, 1086–1106 (2020).
Link, S. et al. Diversity and developmental expression of L-type calcium channel β2 proteins and their influence on calcium current in murine heart. J. Biol. Chem. 284, 30129–30137 (2009).
Bayer, K. U. & Schulman, H. Regulation of signal transduction by protein targeting: the case for CaMKII. Biochem. Biophys. Res. Commun. 289, 917–923 (2001).
Xu, X. et al. ASF/SF2-regulated CaMKIIδ alternative splicing temporally reprograms excitation-contraction coupling in cardiac muscle. Cell 120, 59–72 (2005).
van den Hoogenhof, M. M. G. et al. RBM20 mutations induce an arrhythmogenic dilated cardiomyopathy related to disturbed calcium handling. Circulation 138, 1330–1342 (2018).
Giudice, J. et al. Alternative splicing regulates vesicular trafficking genes in cardiomyocytes during postnatal heart development. Nat. Commun. 5, 3603 (2014).
Wang, H. et al. Genome-wide analysis of alternative splicing during human heart development. Sci. Rep. 6, 35520 (2016).
Liu, C.-X. & Chen, L.-L. Circular RNAs: characterization, cellular roles, and applications. Cell 185, 2016–2034 (2022).
Mazin, P. V., Khaitovich, P., Cardoso-Moreira, M. & Kaessmann, H. Alternative splicing during mammalian organ development. Nat. Genet. 53, 925–934 (2021).
Kalsotra, A. et al. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc. Natl Acad. Sci. USA 105, 20333–20338 (2008).
Riquelme, C. A. et al. Fatty acids identified in the burmese python promote beneficial cardiac growth. Science 334, 528–531 (2011).
Secor, S. M. & Diamond, J. A vertebrate model of extreme physiological regulation. Nature 395, 659–662 (1998).
Maillet, M., van Berlo, J. H. & Molkentin, J. D. Molecular basis of physiological heart growth: fundamental concepts and new players. Nat. Rev. Mol. Cell Biol. 14, 38–48 (2012).
Song, H. K., Hong, S.-E., Kim, T. & Kim, D. H. Deep RNA sequencing reveals novel cardiac transcriptomic signatures for physiological and pathological hypertrophy. PLoS ONE 7, e35552 (2012).
Beqqali, A. Alternative splicing in cardiomyopathy. Biophys. Rev. 10, 1061–1071 (2018).
Neagoe, C. et al. Titin isoform switch in ischemic human heart disease. Circulation 106, 1333–1341 (2002).
Nagueh, S. F. et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation 110, 155–162 (2004).
Brauch, K. M. et al. Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy. J. Am. Coll. Cardiol. 54, 930–941 (2009).
Parikh, V. N. et al. Regional variation in RBM20 causes a highly penetrant arrhythmogenic cardiomyopathy. Circ. Heart Fail. 12, e005371 (2019).
Sedaghat-Hamedani, F. et al. Clinical genetics and outcome of left ventricular non-compaction cardiomyopathy. Eur. Heart J. 38, 3449–3460 (2017).
Fenix, A. M. et al. Gain-of-function cardiomyopathic mutations in RBM20 rewire splicing regulation and re-distribute ribonucleoprotein granules within processing bodies. Nat. Commun. 12, 6324 (2021).
Schneider, J. W. et al. Dysregulated ribonucleoprotein granules promote cardiomyopathy in RBM20 gene-edited pigs. Nat. Med. 26, 1788–1800 (2020).
Methawasin, M. et al. Experimentally increasing titin compliance in a novel mouse model attenuates the Frank-Starling mechanism but has a beneficial effect on diastole. Circulation 129, 1924–1936 (2014).
Hinze, F., Dieterich, C., Radke, M. H., Granzier, H. & Gotthardt, M. Reducing RBM20 activity improves diastolic dysfunction and cardiac atrophy. J. Mol. Med. 94, 1349–1358 (2016).
Methawasin, M. et al. Experimentally increasing the compliance of titin through RNA binding motif-20 (RBM20) inhibition improves diastolic function in a mouse model of heart failure with preserved ejection fraction. Circulation 134, 1085–1099 (2016).
Mazzarotto, F. et al. Reevaluating the genetic contribution of monogenic dilated cardiomyopathy. Circulation 141, 387–398 (2020).
Sturm, A. C. & Hershberger, R. E. Genetic testing in cardiovascular medicine: current landscape and future horizons. Curr. Opin. Cardiol. 28, 317–325 (2013).
Seeger, T. et al. A premature termination codon mutation in MYBPC3 causes hypertrophic cardiomyopathy via chronic activation of nonsense-mediated decay. Circulation 139, 799–811 (2019).
Glazier, A. A. et al. HSC70 is a chaperone for wild-type and mutant cardiac myosin binding protein C. JCI Insight 3, 99319 (2018).
Kumar, S. et al. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J. Am. Coll. Cardiol. 68, 2299–2307 (2016).
Sen-Chowdhry, S. et al. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J. Am. Coll. Cardiol. 52, 2175–2187 (2008).
Smith, E. D. et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 141, 1872–1884 (2020).
Patel, P. N. et al. Contribution of noncanonical splice variants to TTN truncating variant cardiomyopathy. Circ. Genom. Precis. Med. 14, e003389 (2021).
Haggerty, C. M. et al. Genomics-first evaluation of heart disease associated with titin-truncating variants. Circulation 140, 42–54 (2019).
Schafer, S. et al. Titin-truncating variants affect heart function in disease cohorts and the general population. Nat. Genet. 49, 46–53 (2017).
Li, D. et al. Identification of novel mutations in RBM20 in patients with dilated cardiomyopathy. Clin. Transl. Sci. 3, 90–97 (2010).
Lee, Y. & Rio, D. C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem. 84, 291–323 (2015).
Koelemen, J., Gotthardt, M., Steinmetz, L. M. & Meder, B. RBM20-Related cardiomyopathy: current understanding and future options. J. Clin. Med. 10, 4101 (2021).
Youn, J.-Y. et al. High-density proximity mapping reveals the subcellular organization of mRNA-associated granules and bodies. Mol. Cell 69, 517–532.e11 (2018).
Maatz, H. et al. RNA-binding protein RBM20 represses splicing to orchestrate cardiac pre-mRNA processing. J. Clin. Invest. 124, 3419–3430 (2014).
Rudolph, F. et al. Deconstructing sarcomeric structure-function relations in titin-BioID knock-in mice. Nat. Commun. 11, 3133 (2020).
Vieira-Vieira, C. H., Dauksaite, V., Sporbert, A., Gotthardt, M. & Selbach, M. Proteome-wide quantitative RNA-interactome capture identifies phosphorylation sites with regulatory potential in RBM20. Mol. Cell 82, 2069–2083.e8 (2022).
Ortiz-Sánchez, P. et al. Loss of SRSF3 in cardiomyocytes leads to decapping of contraction-related mRNAs and severe systolic dysfunction. Circ. Res. 125, 170–183 (2019).
Larrasa-Alonso, J. et al. The SRSF4-GAS5-glucocorticoid receptor axis regulates ventricular hypertrophy. Circ. Res. 129, 669–683 (2021).
Lee, J.-H. et al. Analysis of transcriptome complexity through RNA sequencing in normal and failing murine hearts. Circ. Res. 109, 1332–1341 (2011).
Amarasinghe, S. L. et al. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. 21, 30 (2020).
Zhu, C. et al. Single-molecule, full-length transcript isoform sequencing reveals disease-associated RNA isoforms in cardiomyocytes. Nat. Commun. 12, 4203 (2021).
Tabula Sapiens Consortium. The Tabula Sapiens: a multiple-organ, single-cell transcriptomic atlas of humans. Science 376, eabl4896 (2022).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Yekelchyk, M., Guenther, S., Preussner, J. & Braun, T. Mono- and multi-nucleated ventricular cardiomyocytes constitute a transcriptionally homogenous cell population. Basic Res. Cardiol. 114, 36 (2019).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Rebboah, E. et al. Mapping and modeling the genomic basis of differential RNA isoform expression at single-cell resolution with LR-Split-seq. Genome Biol. 22, 286 (2021).
Reichart, D. et al. Pathogenic variants damage cell composition and single cell transcription in cardiomyopathies. Science 377, eabo1984 (2022).
Rudolph, F. et al. Resolving titin’s lifecycle and the spatial organization of protein turnover in mouse cardiomyocytes. Proc. Natl Acad. Sci. USA 116, 25126–25136 (2019).
Liss, M. et al. Drug discovery with an RBM20 dependent titin splice reporter identifies cardenolides as lead structures to improve cardiac filling. PLoS ONE 13, e0198492 (2018).
König, J. et al. iCLIP reveals the function of hnRNP particles in splicing at individual nucleotide resolution. Nat. Struct. Mol. Biol. 17, 909–915 (2010).
Cooper, T. A. & Mattox, W. The regulation of splice-site selection, and its role in human disease. Am. J. Hum. Genet. 61, 259–266 (1997).
Goodall, G. J. & Wickramasinghe, V. O. RNA in cancer. Nat. Rev. Cancer 21, 22–36 (2021).
Parikh, V. N. & Ashley, E. A. Next-generation sequencing in cardiovascular disease: present clinical applications and the horizon of precision medicine. Circulation 135, 406–409 (2017).
Hershberger, R. E. et al. Genetic evaluation of cardiomyopathy: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 20, 899–909 (2018).
Baralle, D. & Baralle, M. Splicing in action: assessing disease causing sequence changes. J. Med. Genet. 42, 737–748 (2005).
Manning, K. S. & Cooper, T. A. The roles of RNA processing in translating genotype to phenotype. Nat. Rev. Mol. Cell Biol. 18, 102–114 (2016).
Chang, Y.-F., Imam, J. S. & Wilkinson, M. F. The nonsense-mediated decay RNA surveillance pathway. Annu. Rev. Biochem. 76, 51–74 (2007).
Ito, K. et al. Identification of pathogenic gene mutations in LMNA and MYBPC3 that alter RNA splicing. Proc. Natl Acad. Sci. USA 114, 7689–7694 (2017).
Holliday, M. et al. Transcriptome sequencing of patients with hypertrophic cardiomyopathy reveals novel splice-altering variants in MYBPC3. Circ. Genom. Precis. Med. 14, e003202 (2021).
Moolman, J. A. et al. A newly created splice donor site in exon 25 of the MyBP-C gene is responsible for inherited hypertrophic cardiomyopathy with incomplete disease penetrance. Circulation 101, 1396–1402 (2000).
Brodehl, A. et al. The desmin mutation DES-c.735G>C causes severe restrictive cardiomyopathy by inducing in-frame skipping of exon-3. Biomedicines 9, 1400 (2021).
Singer, E. S., Ingles, J., Semsarian, C. & Bagnall, R. D. Key value of RNA analysis of MYBPC3 splice-site variants in hypertrophic cardiomyopathy. Circ. Genom. Precis. Med. 12, e002368 (2019).
Abramowicz, A. & Gos, M. Splicing mutations in human genetic disorders: examples, detection, and confirmation. J. Appl. Genet. 59, 253–268 (2018).
Helms, A. S. et al. Sarcomere mutation-specific expression patterns in human hypertrophic cardiomyopathy. Circ. Cardiovasc. Genet. 7, 434–443 (2014).
Karakikes, I., Ameen, M., Termglinchan, V. & Wu, J. C. Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes. Circ. Res. 117, 80–88 (2015).
Tilgner, H. et al. Comprehensive transcriptome analysis using synthetic long-read sequencing reveals molecular co-association of distant splicing events. Nat. Biotechnol. 33, 736–742 (2015).
Jaganathan, K. et al. Predicting splicing from primary sequence with deep learning. Cell 176, 535–548.e24 (2019).
Lopes, L. R. et al. Cryptic splice-altering variants in MYBPC3 are a prevalent cause of hypertrophic cardiomyopathy. Circ. Genom. Precis. Med. 13, e002905 (2020).
Dauksaite, V. & Gotthardt, M. Molecular basis of titin exon exclusion by RBM20 and the novel titin splice regulator PTB4. Nucleic Acids Res. 46, 5227–5238 (2018).
Gao, G. et al. Enhanced risk profiling of implanted defibrillator shocks with circulating SCN5A mRNA splicing variants: a pilot trial. J. Am. Coll. Cardiol. 63, 2261–2269 (2014).
Liu, N. & Olson, E. N. CRISPR modeling and correction of cardiovascular disease. Circ. Res. 130, 1827–1850 (2022).
Briganti, F. et al. iPSC modeling of RBM20-deficient DCM identifies upregulation of RBM20 as a therapeutic strategy. Cell Rep. 32, 108117 (2020).
Meyer, S. M. et al. Small molecule recognition of disease-relevant RNA structures. Chem. Soc. Rev. 49, 7167–7199 (2020).
Salton, M. & Misteli, T. Small molecule modulators of pre-mRNA splicing in cancer therapy. Trends Mol. Med. 22, 28–37 (2016).
Singh, R. N., Ottesen, E. W. & Singh, N. N. The first orally deliverable small molecule for the treatment of spinal muscular atrophy. Neurosci. Insights 15, 2633105520973985 (2020).
Palacino, J. et al. SMN2 splice modulators enhance U1-pre-mRNA association and rescue SMA mice. Nat. Chem. Biol. 11, 511–517 (2015).
Keller, C. G. et al. An orally available, brain penetrant, small molecule lowers huntingtin levels by enhancing pseudoexon inclusion. Nat. Commun. 13, 1150 (2022).
Bull, M. et al. Alternative splicing of titin restores diastolic function in an HFpEF-like genetic murine model (TtnΔIAjxn). Circ. Res. 119, 764–772 (2016).
Radke, M. H. et al. Therapeutic inhibition of RBM20 improves diastolic function in a murine heart failure model and human engineered heart tissue. Sci. Transl. Med. 13, eabe8952 (2021).
Musunuru, K. et al. Genetic testing for inherited cardiovascular diseases: a scientific statement from the American Heart Association. Circ. Genom. Precis. Med. 13, e000067 (2020).
Minoche, A. E. et al. Genome sequencing as a first-line genetic test in familial dilated cardiomyopathy. Genet. Med. 21, 650–662 (2019).
Riepe, T. V., Khan, M., Roosing, S., Cremers, F. P. M. & ’t Hoen, P. A. C. Benchmarking deep learning splice prediction tools using functional splice assays. Hum. Mutat. 42, 799–810 (2021).
Marasco, L. E. & Kornblihtt, A. R. The physiology of alternative splicing. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-022-00545-z (2022).
Kornblihtt, A. R. et al. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat. Rev. Mol. Cell Biol. 14, 153–165 (2013).
Filippello, A., Lorenzi, P., Bergamo, E. & Romanelli, M. G. Identification of nuclear retention domains in the RBM20 protein. FEBS Lett. 587, 2989–2995 (2013).
Acknowledgements
This work was funded by Leducq TAN CASTT grant 21CVD02. Jacobo Lopez Carballo (Max Delbrück Center for Molecular Medicine in the Helmholtz Association, Germany) supported the data analysis and generation of the supplementary tables.
Author information
Authors and Affiliations
Contributions
M. Gotthardt, V.B.-L., V.N.P., M.F., S.S., M. Grosch, C.C. and L.L. researched data for the article. M. Gotthardt, V.B.-L., V.N.P., E.A., M.C.-F., B.M., M. Grosch, L.S. and L.L. discussed the content of the article. All the authors wrote the manuscript. M. Gotthardt and L.L. reviewed/edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
M. Gotthardt has a consultancy agreement with River BioMedics and has received speaker honoraria from Bayer. V.N.P. reports a sponsored research agreement with BioMarin and consulting relationships with Constantiam and viz.ai. E.A. reports sponsored research from Bristol Myers Squibb, has ownership interest in DeepCell, Nuevocor and Personalis, and is a board member of AstraZeneca. M.C.-F. is a cofounder and scientific adviser of GenoMed, a molecular diagnosis company. B.M. holds stocks in biotech and pharma, has received speaker honoraria from Bayer, Bristol Myers Squibb, Daiichi Sankyo, Novartis and Pfizer, and is on the Scientific Advisory Boards of Bristol Myers Squibb/Myokardia and Fleischhacker. L.S. is a co-founder of SOPHiA Genetics, as well as co-founder and board member of LevitasBio and Recombia Biosciences, and receives research support from GlaxoSmithKline. L.L. has sponsored research agreements from Bristol Myers Squibb and Edgewise Therapeutics, and is on the scientific advisory boards of Bristol Meyers Squibb/MyoKardia and Edgewise Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Samuel Dudley and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Alamut software: https://www.sophiagenetics.com/platform/alamut-visual-plus/
AltAnalyze: http://www.altanalyze.org/
ClinVar database: https://www.ncbi.nlm.nih.gov/clinvar/
Cytoscape: https://cytoscape.org/
Gene Ontology: https://www.ebi.ac.uk/QuickGO/
GTEx: https://gtexportal.org/home/
Human Protein Atlas: https://www.proteinatlas.org/
Oxford Nanopore Technology: https://nanoporetech.com
Pacific Biosciences: https://www.pacb.com
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gotthardt, M., Badillo-Lisakowski, V., Parikh, V.N. et al. Cardiac splicing as a diagnostic and therapeutic target. Nat Rev Cardiol 20, 517–530 (2023). https://doi.org/10.1038/s41569-022-00828-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00828-0
This article is cited by
-
RNA-binding proteins in cardiovascular biology and disease: the beat goes on
Nature Reviews Cardiology (2024)