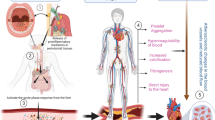
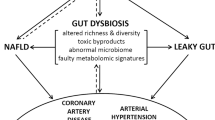
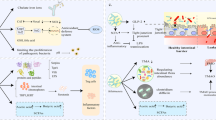
Abstract
Despite advances in our understanding of the pathophysiology of many cardiovascular diseases (CVDs) and expansion of available therapies, the global burden of CVD-associated morbidity and mortality remains unacceptably high. Important gaps remain in our understanding of the mechanisms of CVD and determinants of disease progression. In the past decade, much research has been conducted on the human microbiome and its potential role in modulating CVD. With the advent of high-throughput technologies and multiomics analyses, the complex and dynamic relationship between the microbiota, their ‘theatre of activity’ and the host is gradually being elucidated. The relationship between the gut microbiome and CVD is well established. Much less is known about the role of disruption (dysbiosis) of the oral microbiome; however, interest in the field is growing, as is the body of literature from basic science and animal and human investigations. In this Review, we examine the link between the oral microbiome and CVD, specifically coronary artery disease, stroke, peripheral artery disease, heart failure, infective endocarditis and rheumatic heart disease. We discuss the various mechanisms by which oral dysbiosis contributes to CVD pathogenesis and potential strategies for prevention and treatment.
Key points
-
The incidence and prevalence of cardiovascular diseases (CVDs) are increasing despite advances in our understanding of their pathophysiology and an expanded arsenal of treatment options.
-
An association between the oral microbiome (or oralome) and cardiovascular inflammation and CVD is supported by a growing body of epidemiological studies, systematic reviews and basic science investigations.
-
Validated links exist between oral dysbiosis and CVDs, including atherosclerotic diseases, heart failure, infective endocarditis and rheumatic heart disease.
-
The mechanisms by which oral dysbiosis contributes to CVD include immunomodulation; endothelial dysfunction; molecular mimicry and antibody cross-reactivity; protein citrullination; platelet activation, aggregation and thrombogenesis; arterial invasion; and systemic inflammation.
-
Targeting oral dysbiosis in a clinical setting could be an important component of CVD management.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J. Am. Coll. Cardiol. 76, 2982–3021 (2020).
Bowry, A. D., Lewey, J., Dugani, S. B. & Choudhry, N. K. The burden of cardiovascular disease in low- and middle-income countries: epidemiology and management. Can. J. Cardiol. 31, 1151–1159 (2015).
Tang, W. H. W., Kitai, T. & Hazen, S. L. Gut microbiota in cardiovascular health and disease. Circ. Res. 120, 1183–1196 (2017).
Fan, Y. & Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71 (2021).
Degruttola, A. K., Low, D., Mizoguchi, A. & Mizoguchi, E. Current understanding of dysbiosis in disease in human and animal models. Inflamm. Bowel Dis. 22, 1137–1150 (2016).
Shreiner, A. B., Kao, J. Y. & Young, V. B. The gut microbiome in health and in disease. Curr. Opin. Gastroenterol. 31, 69–75 (2015).
Gilbert, J. et al. Current understanding of the human microbiome. Nat. Med. 24, 392–400 (2018).
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533 (2016).
Hasan, N. & Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 7, e7502 (2019).
The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Thaiss, C. A., Zmora, N., Levy, M. & Elinav, E. The microbiome and innate immunity. Nature 535, 65–74 (2016).
Honda, K. & Littman, D. R. The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84 (2016).
Nikitakis, N. G., Papaioannou, W., Sakkas, L. I. & Kousvelari, E. The autoimmunity-oral microbiome connection. Oral. Dis. 23, 828–839 (2017).
Dhadse, P., Gattani, D. & Mishra, R. The link between periodontal disease and cardiovascular disease: how far we have come in last two decades? J. Indian Soc. Periodontol. 14, 148–154 (2010).
Leishman, S. J., Do, H. L. & Ford, P. J. Cardiovascular disease and the role of oral bacteria. J. Oral. Microbiol. 2, 5781 (2010).
Kholy, K.El, Genco, R. J. & Dyke, T. E.Van Oral infections and cardiovascular disease. Trends Endocrinol. Metab. 26, 315–321 (2015).
Albandar, J. M. & Kingman, A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988-1994. J. Periodontol. 70, 30–43 (1999).
Dotre, S. V., Davane, M. S. & Nagoba, B. S. Peridontitis, bacteremia and infective endocarditis: a review study. Arch. Pediatr. Infect. Dis. 5, e41067 (2017).
Lockhart, P. B. et al. Bacteremia associated with toothbrushing and dental extraction. Circulation 117, 3118–3125 (2008).
Lockhart, P. B. et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J. Am. Dent. Assoc. 140, 1238–1244 (2009).
Bartova, J. et al. Periodontitis as a risk factor of atherosclerosis. J. Immunol. Res. 2014, 636893 (2014).
Gorelick, P. B. Stroke prevention therapy beyond antithrombotics: unifying mechanisms in ischemic stroke pathogenesis and implications for therapy: an invited review. Stroke 33, 862–875 (2002).
Raber-Durlacher, J. E. et al. Periodontal status and bacteremia with oral viridans streptococci and coagulase negative staphylococci in allogeneic hematopoietic stem cell transplantation recipients: a prospective observational study. Support. Care Cancer 21, 1621–1627 (2013).
Forner, L., Larsen, T., Kilian, M. & Holmstrup, P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 33, 401–407 (2006).
Sreedevi, M., Ramesh, A. & Dwarakanath, C. Periodontal status in smokers and nonsmokers: a clinical, microbiological, and histopathological study. Int. J. Dent. 2012, 571590 (2012).
Libby, P. et al. Atherosclerosis. Nat. Rev. Dis. Prim. 5, 56 (2019).
Cosselman, K. E., Navas-Acien, A. & Kaufman, J. D. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 12, 627–642 (2015).
Zühlke, L. et al. Cardiovascular medicine and research in sub-Saharan Africa: challenges and opportunities. Nat. Rev. Cardiol. 16, 642–644 (2019).
Weber, C. & Noels, H. Atherosclerosis: current pathogenesis and therapeutic options. Nat. Med. 17, 1410–1422 (2011).
Sanz, M. et al. Periodontitis and cardiovascular diseases: consensus report. J. Clin. Periodontol. 47, 268–288 (2020).
DeStefano, F., Anda, R. F., Kahn, H. S., Williamson, D. F. & Russell, C. M. Dental disease and risk of coronary heart disease and mortality. BMJ 306, 688–691 (1993).
Dietrich, T., Sharma, P. & Walter, C. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Clin. Periodontol. 40 (Suppl. 14), S70–S84 (2013).
Sen, S., Giamberardino, L. D. & Moss, K. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke 49, 355–362 (2018).
Cowan, L. T., Lakshminarayan, K. & Lutsey, P. L. Periodontal disease and incident venous thromboembolism: the Atherosclerosis Risk in Communities study. J. Clin. Periodontol. 46, 12–19 (2019).
Humphrey, L. L., Fu, R. & Buckley, D. I. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J. Gen. Intern. Med. 23, 2079–2086 (2008).
Mattila, K. J., Nieminen, M. S. & Valtonen, V. V. Association between dental health and acute myocardial infarction. BMJ 298, 779–781 (1989).
Chen, Y. W. et al. Periodontitis may increase the risk of peripheral arterial disease. Eur. J. Vasc. Endovasc. Surg. 35, 153–158 (2008).
Cauley, J. A., Kassem, A. M., Lane, N. E. & Thorson, S. Prevalent peripheral arterial disease and inflammatory burden. BMC Geriatr. 16, 213 (2016).
Soto‐Barreras, U., Olvera‐Rubio, J. O. & Loyola‐Rodriguez, J. P. Peripheral arterial disease associated with caries and periodontal disease. J. Periodontol. 84, 486–494 (2013).
Chandy, S., Joseph, K. & Sankaranarayanan, A. Evaluation of C-reactive protein and fibrinogen in patients with chronic and aggressive periodontitis: a clinico-biochemical study. J. Clin. Diagn. Res. 11, ZC41–ZC45 (2017).
Fentoglu, O. & Bozkurt, F. Y. The bi-directional relationship between periodontal disease and hyperlipidemia. Eur. J. Dent. 2, 142–146 (2008).
Golpasand, H. L., Zakavi, F., Hajizadeh, F. & Saleki, M. The association between hyperlipidemia and periodontal infection. Iran. Red. Crescent Med. J. 16, e6577 (2014).
Herzberg, M. C. Platelet-streptococcal interactions in endocarditis. Crit. Rev. Oral. Biol. Med. 7, 222–236 (1996).
Nakano, K., Nomura, R., Matsumoto, M. & Ooshima, T. Roles of oral bacteria in cardiovascular diseases – from molecular mechanisms to clinical cases: cell-surface structures of novel serotype k streptococcus mutans strains and their correlation to virulence. J. Pharmacol. Sci. 113, 120–125 (2010).
Schenkein, H. A. & Loos, B. G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Clin. Periodontol. 40 (Suppl. 14), S51–S69 (2013).
Hashizume-Takizawa, T., Yamaguchi, Y. & Kobayashi, R. Oral challenge with Streptococcus sanguinis induces aortic inflammation and accelerates atherosclerosis in spontaneously hyperlipidemic mice. Biochem. Biophys. Res. Commun. 520, 507–513 (2019).
Chhibber-Goel, J., Singhal, V. & Bhowmik, D. Linkages between oral commensal bacteria and atherosclerotic plaques in coronary artery disease patients. NPJ Biofilms Microbiomes 2, 7 (2016).
Radaic, A. & Kapila, Y. L. The oralome and its dysbiosis: new insights into oral microbiome–host interactions. Comp. Struct. Biotechnol. J. 19, 1335–1360 (2021).
Schenkein, H. A., Papapanou, P. N., Genco, R. & Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 83, 90–106 (2000).
Pussinen, P. J. et al. Endotoxemia, immune response to periodontal pathogens, and systemic inflammation associate with incident cardiovascular disease events. Arterioscler. Thromb. Vasc. Biol. 27, 1433–1439 (2007).
Aarabi, G., Heydecke, G. & Seedorf, U. Roles of oral infections in the pathomechanism of atherosclerosis. Int. J. Mol. Sci. 19, 1978 (2018).
Saini, R., Marawar, P. P., Shete, S. & Periodontitis, S. S. A true infection. J. Glob. Infect. Dis. 1, 149–150 (2009).
Borén, J. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 41, 2313–2330 (2020).
Virani, S. S. et al. 2021 ACC expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia. J. Am. Coll. Card. 78, 960–993 (2021).
Rosenson, R. S. et al. HDL and atherosclerotic cardiovascular disease: genetic insights into complex biology. Nat. Rev. Cardiol. 15, 9–19 (2018).
Sammalkorpi, K., Valtonen, V., Kerttula, Y., Nikkila, E. & Taskinen, M. R. Changes in serum lipoprotein pattern induced by acute infections. Metabolism 37, 859–865 (1988).
Popa, C., Netea, M. G., van Riel, P. L., van der Meer, J. W. & Stalenhoef, A. F. The role of TNF-α in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J. Lipid Res. 48, 751–762 (2007).
Berbee, J. F., Havekes, L. M. & Rensen, P. C. Apolipoproteins modulate the inflammatory response to lipopolysaccharide. J. Endotoxin Res. 11, 97–103 (2005).
Auerbach, B. J. & Parks, J. S. Lipoprotein abnormalities associated with lipopolysaccharide-induced lecithin: cholesterol acyltransferase and lipase deficiency. J. Biol. Chem. 264, 10264–10270 (1989).
Feingold, K. R., Memon, R. A., Moser, A. H., Shigenaga, J. K. & Grunfeld, C. Endotoxin and interleukin-1 decrease hepatic lipase mRNA levels. Atherosclerosis 142, 379–387 (1999).
Khovidhunkit, W. et al. Apolipoproteins A-IV and A-V are acute-phase proteins in mouse HDL. Atherosclerosis 176, 37–44 (2004).
Hossain, E. et al. Lipopolysaccharide augments the uptake of oxidized LDL by up-regulating lectin-like oxidized LDL receptor-1 in macrophages. Mol. Cell Biochem. 400, 29–40 (2015).
Levine, B., Kalman, J. & Mayer, L. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 323, 236–241 (1990).
Mann, D. L. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ. Res. 116, 1254–1268 (2015).
Liu, L., Wang, Y. & Cao, Z. Y. Up-regulated TLR4 in cardiomyocytes exacerbates heart failure after long-term myocardial infarction. J. Cell Mol. Med. 19, 2728–2740 (2015).
Swirski, F. K. & Nahrendorf, M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 18, 733–744 (2018).
Kobayashi, R., Ogawa, Y., Hashizume-Takizawa, T. & Kurita-Ochiai, T. Oral bacteria affect the gut microbiome and intestinal immunity. Pathog. Dis. 78, ftaa024 (2020).
Kitai, T. & Tang, W. H. W. Gut microbiota in cardiovascular disease and heart failure. Clin. Sci. 132, 85–91 (2018).
Francisqueti-Ferron, F. V. et al. The role of gut dysbiosis-associated inflammation in heart failure. Rev. Assoc. Med. Bras. 68, 1120–1124 (2022).
Piya, M. K., Harte, A. L. & McTernan, P. G. Metabolic endotoxaemia: is it more than just a gut feeling? Curr. Opin. Lipidol. 24, 78–85 (2013).
Kallio, K. A. E., Hätönen, K. A. & Lehto, M. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol. 52, 395–404 (2015).
Rogler, G. & Rosano, G. The heart and the gut. Eur. Heart J. 35, 426–430 (2014).
Pasini, E., Aquilani, R. & Testa, C. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 4, 220–227 (2016).
Niebauer, J., Volk, H. D. & Kemp, M. Endotoxin and immune activation in chronic heart failure: a prospective cohort study. Lancet 353, 1838–1842 (1999).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Genth-Zotz, S., Haehling, S. & Bolger, A. P. Pathophysiologic quantities of endotoxin-induced tumor necrosis factor-alpha release in whole blood from patients with chronic heart failure. Am. J. Cardiol. 90, 1226–1230 (2002).
Jamart, C., Gomes, A. V. & Dewey, S. Regulation of ubiquitin-proteasome and autophagy pathways after acute LPS and epoxomicin administration in mice. BMC Musculoskelet. Disord. 15, 166 (2014).
Yao, Z., Mates, J. M. & Cheplowitz, A. M. Blood-borne lipopolysaccharide is rapidly eliminated by liver sinusoidal endothelial cells via high-density lipoprotein. J. Immunol. 197, 2390–2399 (2016).
Tsukamoto, H., Takeuchi, S. & Kubota, K. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1–IKKε–IRF3 axis activation. J. Biol. Chem. 293, 10186–10201 (2018).
Sandek, A., Bjarnason, I. & Volk, H. D. Studies on bacterial endotoxin and intestinal absorption function in patients with chronic heart failure. Int. J. Cardiol. 157, 80–85 (2012).
Habib, G. et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology. Eur. Heart J. 36, 3075–3128 (2015).
Kanafani, Z. A., Mahfouz, T. H. & Kanj, S. S. Infective endocarditis at a tertiary care centre in Lebanon: predominance of streptococcal infection. J. Infect. 45, 152–159 (2002).
Barrau, K., Boulamery, A. & Imbert, G. Causative organisms of infective endocarditis according to host status. Clin. Microbiol. Infect. 10, 302–308 (2004).
Tariq, M., Alam, M. & Munir, G. Infective endocarditis: a five-year experience at a tertiary care hospital in Pakistan. Int. J. Infect. Dis. 8, 163–170 (2004).
Nakatani, S., Mitsutake, K. & Ohara, T. Recent picture of infective endocarditis in Japan–lessons from Cardiac Disease Registration (CADRE-IE). Circ. J. 77, 1558–1564 (2013).
Horliana, A. C. et al. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: a systematic review. PLoS ONE 9, e98271 (2014).
Vikram, H. R. The long and short of vegetations in infective endocarditis. Expert. Rev. Anti Infect. Ther. 5, 529–533 (2007).
Drangsholt, M. T. A new causal model of dental diseases associated with endocarditis. Ann. Periodontol. 3, 184–196 (1998).
Moreillon, P., Que, Y. A. & Bayer, A. S. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. North. Am. 16, 297–318 (2002).
Brown, M. & Griffin, G. E. Immune responses in endocarditis. Heart 79, 1–2 (1998).
Gould, K., Ramirez-Ronda, C. H., Holmes, R. K. & Sanford, J. P. Adherence of bacteria to heart valves in vitro. J. Clin. Invest. 56, 1364–1370 (1975).
Kuusela, P., Vartio, T., Vuento, M. & Myhre, E. B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect. Immun. 50, 77–81 (1985).
Mandell, G.L. & Korzeniowski, O.M. Atlas of Infectious Diseases Vol. 10 (Current Medicine, 1997).
Durack, D. T. & Beeson, P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br. J. Exp. Pathol. 53, 44–49 (1972).
Carapetis, J. R. et al. Acute rheumatic fever and rheumatic heart disease. Nat. Rev. Dis. Prim. 2, 15084 (2016).
Mayosi, B., Robertson, K. & Volmink, J. The Drakensberg declaration on the control of rheumatic fever and rheumatic heart disease in Africa. S Afr. Med. J. 96 (3 Pt 2), 246 (2006).
Watkins, D. A., Beaton, A. Z. & Carapetis, J. R. Rheumatic heart disease worldwide. JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 72, 1397–1416 (2018).
McDonald, M., Currie, B. J. & Carapetis, J. R. Acute rheumatic fever: a chink in the chain that links the heart to the throat? Lancet Infect. Dis. 4, 240–245 (2004).
Dinkla, K. et al. Crucial role of the CB3-region of collagen IV in PARF-induced acute rheumatic fever. PLoS ONE 4, e4666 (2009).
Tandon, R., Sharma, M. & Chandrashekhar, Y. Revisiting the pathogenesis of rheumatic fever and carditis. Nat. Rev. Cardiol. 10, 171–177 (2013).
Martins, T. B., Hoffman, J. L. & Augustine, N. H. Comprehensive analysis of antibody responses to streptococcal and tissue antigens in patients with acute rheumatic fever. Int. Immunol. 20, 445–452 (2008).
Entine, M. A survey of dental diseases as a diagnostic aid in rheumatic fever. J. Am. Dent. Assoc. 38, 303–308 (1949).
Peres, M. A., Macpherson, L. M. D. & Weyant, R. J. Oral diseases: a global public health challenge. Lancet 394, 249–260 (2019).
Petersen, P. E., Bourgeois, D. & Ogawa, H. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 83, 661–669 (2005).
Easley, M. W. Celebrating 50 years of fluoridation: a public health success story. Br. Dent. J. 178, 72–75 (1995).
Grembowski, D., Fiset, L., Spadafora, A. & Milgrom, P. Fluoridation effects on periodontal disease among adults. J. Periodontal Res. 28, 166–172 (1993).
Anuradha, K. P., Chadrashekar, J. & Ramesh, N. Prevalence of periodontal disease in endemically flourosed areas of Davangere Taluk, India. Indian J. Dent. Res. 13, 15–19 (2002).
Thongboonkerd, V., Luengpailin, J. & Cao, J. Fluoride exposure attenuates expression of Streptococcus pyogenes virulence factors. J. Biol. Chem. 277, 16599–16605 (2002).
Surve, N. Z. et al. A longitudinal study of antibody responses to selected host antigens in rheumatic fever and rheumatic heart disease. J. Med. Microbiol. https://doi.org/10.1099/jmm.0.001355 (2021).
Pilapitiya, D. H. et al. Antibody responses to collagen peptides and streptococcal collagen-like 1 proteins in acute rheumatic fever patients. Pathog. Dis. 79, ftab033 (2021).
Maresz, K. J. et al. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 9, e1003627 (2013).
Yamada, R., Suzuki, A., Chang, X. & Yamamoto, K. Citrullinated proteins in rheumatoid arthritis. Front. Biosci. 10, 54–64 (2005).
Koziel, J., Mydel, P. & Potempa, J. The link between periodontal disease and rheumatoid arthritis: an updated review. Curr. Rheumatol. Rep. 16, 408 (2014).
Into, T. et al. Arginine-specific gingipains from Porphyromonas gingivalis deprive protective functions of secretory leucocyte protease inhibitor in periodontal tissue. Clin. Exp. Immunol. 145, 545–554 (2006).
Pathirana, R. D., O’Brien-Simpson, N. M., Veith, P. D., Riley, P. F. & Reynolds, E. C. Characterization of proteinase-adhesin complexes of Porphyromonas gingivalis. Microbiology 152, 2381–2394 (2006).
Ruggiero, S. et al. Cleavage of extracellular matrix in periodontitis: gingipains differentially affect cell adhesion activities of fibronectin and tenascin-C. Biochim. Biophys. Acta 1832, 517–526 (2013).
Liu, B. et al. Deep sequencing of the oral microbiome reveals signatures of periodontal disease. PLoS ONE 7, e37919 (2012).
Sintim, H. O. & Gürsoy, U. K. Biofilms as “connectors” for oral and systems medicine: a new opportunity for biomarkers, molecular targets, and bacterial eradication. OMICS 20, 3–11 (2016).
Sudhakara, P., Gupta, A., Bhardwaj, A. & Wilson, A. Oral dysbiotic communities and their implications in systemic diseases. Dent. J. 6, 10 (2018).
Olsen, I. & Hajishengallis, G. Major neutrophil functions subverted by Porphyromonas gingivalis. J. Oral. Microbiol. 8, 30936 (2016).
Darveau, R. P. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 8, 481–490 (2010).
Hajishengallis, E. & Hajishengallis, G. Neutrophil homeostasis and periodontal health in children and adults. J. Dent. Res. 93, 231–237 (2014).
Hajishengallis, G. & Harokopakis, E. Porphyromonas gingivalis interactions with complement receptor 3 (CR3): innate immunity or immune evasion? Front. Biosci. 12, 4547–4557 (2007).
Hajishengallis, G., Shakhatreh, M. A., Wang, M. & Liang, S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J. Immunol. 179, 2359–2367 (2007).
Zargar, A. et al. Bacterial secretions of nonpathogenic Escherichia coli elicit inflammatory pathways: a closer investigation of interkingdom signaling. mBio 6, e00025-15 (2015).
Yee, M., Kim, S. & Sethi, P. Porphyromonas gingivalis stimulates IL-6 and IL-8 secretion in GMSM-K, HSC-3 and H413 oral epithelial cells. Anaerobe 28, 62–67 (2014).
Imamura, T., Potempa, J., Tanase, S. & Travis, J. Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from Porphyromonas gingivalis. J. Biol. Chem. 272, 16062–16067 (1997).
Roth, G. A., Moser, B. & Huang, S. J. Infection with a periodontal pathogen induces procoagulant effects in human aortic endothelial cells. J. Thromb. Haemost. 4, 2256–2261 (2006).
Roth, G. A., Moser, B. & Roth-Walter, F. Infection with periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis 190, 271–281 (2007).
Moran, A. P., Prendergast, M. M. & Appelmelk, B. J. Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol. Med. Microbiol. 16, 105–115 (1996).
Neves, A. L., Coelho, J. & Couto, L. Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 51, R51–R64 (2013).
Liljestrand, J. M. et al. Immunologic burden links periodontitis to acute coronary syndrome. Atherosclerosis 268, 177–184 (2018).
Jia, R., Kurita-Ochiai, T., Oguchi, S. & Yamamoto, M. Periodontal pathogen accelerates lipid peroxidation and atherosclerosis. J. Dent. Res. 92, 247–252 (2013).
Tuomainen, A. M., Jauhiainen, M. & Kovanen, P. T. Aggregatibacter actinomycetemcomitans induces MMP-9 expression and proatherogenic lipoprotein profile in apoE-deficient mice. Microb. Pathog. 44, 111–117 (2007).
Lundberg, J. O., Weitzberg, E. & Gladwin, M. T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug. Discov. 7, 156–167 (2008).
Goh, C. E. et al. Association between nitrate‐reducing oral bacteria and cardiometabolic outcomes: results from ORIGINS. J. Am. Heart Assoc. 8, e013324 (2019).
Aurer, A., Aleksic, J., Ivic-Kardum, M., Aurer, J. & Culo, F. Nitric oxide synthesis is decreased in periodontitis. J. Clin. Periodontol. 28, 565–568 (2001).
Kleinbongard, P. et al. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic. Biol. Med. 40, 295–302 (2006).
Desvarieux, M. et al. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J. Hypertens. 28, 1413–1421 (2010).
Tonetti, M. S. et al. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 356, 911–920 (2007).
Mollenhauer, J. & Schulmeister, A. The humoral immune response to heat shock proteins. Experientia 48, 644–649 (1992).
Suarez, L. J., Garzon, H., Arboleda, S. & Rodriguez, A. Oral dysbiosis and autoimmunity: from local periodontal responses to an imbalanced systemic immunity. A review. Front. Immunol. 11, 591255 (2020).
Wick, G., Perschinka, H. & Xu, Q. Autoimmunity and atherosclerosis. Am. Heart J. 138, 444–449 (1999).
Wick, G., Jakic, B. & Buszko, M. The role of heat shock proteins in atherosclerosis. Nat. Rev. Cardiol. 11, 516–529 (2014).
Perschinka, H. et al. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 1060–1065 (2003).
Tutturen, A. E. V., Fleckenstein, B. & Souza, G. A. Assessing the citrullinome in rheumatoid arthritis synovial fluid with and without enrichment of citrullinated peptides. J. Proteome Res. 13, 2867–2873 (2014).
Steendam, K., Tilleman, K. & Ceuleneer, M. Citrullinated vimentin as an important antigen in immune complexes from synovial fluid of rheumatoid arthritis patients with antibodies against citrullinated proteins. Arthritis Res. Ther. 12, R132 (2010).
Fert-Bober, J. & Sokolove, J. Proteomics of citrullination in cardiovascular disease. Proteom. Clin. Appl. 8, 522–533 (2014).
Weyrich, A. S. & Zimmerman, G. A. Platelets: signaling cells in the immune continuum. Trends Immunol. 25, 489–495 (2004).
Kerrigan, S. W. et al. Role of Streptococcus gordonii surface proteins SspA/SspB and Hsa in platelet function. Infect. Immun. 75, 5740–5747 (2007).
Fitzgerald, F. Jr, Foster, T. J. & Cox, D. The interaction of bacterial pathogens with platelets. Nat. Rev. Microbiol. 4, 445–457 (2006).
Kerrigan, S. W. & Cox, D. Platelet-bacterial interactions. Cell Mol. Life Sci. 67, 513–523 (2010).
Erickson, P. R. & Herzberg, M. C. The Streptococcus sanguis platelet aggregation-associated protein. J. Biol. Chem. 268, 1646–1649 (1993).
Plummer, C., Wu, H. & Kerrigan, S. W. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br. J. Haematol. 129, 101–109 (2005).
Takahashi, Y., Ruhl, S. & Yoon, J. W. Adhesion of viridans group streptococci to sialic acid-, galactose-, and N-acetylgalactosamine-containing receptors. Oral. Microbiol. Immunol. 17, 257–262 (2002).
Taniguchi, N. et al. Defect of glucosyltransferases reduces platelet aggregation activity of Streptococcus mutans: analysis of clinical strains isolated from oral cavities. Arch. Oral. Biol. 55, 410–416 (2010).
Nakano, K. et al. Detection of oral bacteria in cardiovascular specimens. Oral. Microbiol. Immunol. 24, 64–68 (2009).
Kozarov, E. V., Dorn, B. R., Shelburne, C. E., Dunn, W. A. Jr & Progulske-Fox, A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 25, e17–e18 (2005).
Torrungruang, K., Jitpakdeebordin, S., Charatkulangkun, O. & Gleebbua, Y. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia co-infection are associated with severe Periodontitis in a Thai population. PLoS ONE 10, e0136646 (2015).
Pucar, A., Milasin, J. & Lekovic, V. Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary arteries. J. Periodontol. 78, 677–682 (2007).
Ford, P. J., Gemmell, E. & Hamlet, S. M. Cross-reactivity of GroEL antibodies with human heat shock protein 60 and quantification of pathogens in atherosclerosis. Oral. Microbiol. Immunol. 20, 296–302 (2005).
Kozarov, E., Sweier, D., Shelburne, C., Progulske-Fox, A. & Lopatin, D. Detection of bacterial DNA in atheromatous plaques by quantitative PCR. Microbes Infect. 8, 687–693 (2006).
Rafferty, B. et al. Impact of monocytic cells on recovery of uncultivable bacteria from atherosclerotic lesions. J. Intern. Med. 270, 273–280 (2011).
Amar, S., Wu, S.-C. & Madan, M. Is Porphyromonas gingivalis cell invasion required for atherogenesis? Pharmacotherapeutic implications. J. Immunol. 182, 1584–1592 (2009).
Amin, M. N. et al. Inflammatory cytokines in the pathogenesis of cardiovascular disease and cancer. SAGE Open Med. 8, 2050312120965752 (2020).
Kaptoge, S. et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur. Heart J. 35, 578–589 (2014).
Lagrand, W. K. et al. C-reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation 100, 96–102 (1999).
Danesh, J. et al. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur. Heart J. 20, 954–959 (1999).
Cardoso, E. M., Reis, C. & Manzanares-Cespedes, M. C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 130, 98–104 (2018).
Polepalle, T., Moogala, S., Boggarapu, S., Pesala, D. S. & Palagi, F. B. Acute phase proteins and their role in periodontitis: a review. J. Clin. Diagn. Res. 9, ZE01–ZE05 (2015).
Amar, S. et al. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler. Thromb. Vasc. Biol. 23, 1245–1249 (2003).
Fukuchi, Y. et al. Immunohistochemical detection of oxidative stress biomarkers, dityrosine and Nε-(hexanoyl)lysine, and C-reactive protein in rabbit atherosclerotic lesions. J. Atheroscler. Thromb. 15, 185–192 (2008).
Tsutsui, H., Kinugawa, S. & Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 301, H2181–H2190 (2011).
Fagundes, N. C. F. et al. Periodontitis as a risk factor for stroke: a systematic review and meta-analysis. Vasc. Health Risk Manag. 15, 519–532 (2019).
Kurita-Ochiai, T., Jia, R., Cai, Y., Yamaguchi, Y. & Yamamoto, M. Periodontal disease-induced atherosclerosis and oxidative stress. Antioxidants 4, 577–590 (2015).
Dahiya, P. et al. Reactive oxygen species in periodontitis. J. Indian Soc. Periodontol. 17, 411–416 (2013).
Takimoto, E. & Kass, D. A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49, 241–248 (2007).
Madan, M. & Amar, S. Toll-like receptor-2 mediates diet and/or pathogen associated atherosclerosis: proteomic findings. PLoS ONE 3, e3204 (2008).
Chi, H., Messas, E., Levine, R. A., Graves, D. T. & Amar, S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model. Circulation 110, 1678–1685 (2004).
Madan, M., Bishayi, B., Hoge, M. & Amar, S. Atheroprotective role of interleukin-6 in diet- and/or pathogen-associated atherosclerosis using an ApoE heterozygote murine model. Atherosclerosis 197, 504–514 (2008).
Reiss, A. B., Siegart, N. M. & De Leon, J. Interleukin-6 in atherosclerosis: atherogenic or atheroprotective? Clin. Lipidol. 12, 14–23 (2017).
Ser, H. L., Letchumanan, V., Goh, B. H., Wong, S. H. & Lee, L. H. The use of fecal microbiome transplant in treating human diseases: too early for poop? Front. Microbiol. 12, 519836 (2021).
Gupta, S., Allen-Vercoe, E. & Petrof, E. O. Fecal microbiota transplantation: in perspective. Ther. Adv. Gastroenterol. 9, 229–239 (2016).
Aron-Wisnewsky, J. et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut 68, 70–82 (2019).
Debédat, J., Clément, K. & Aron-Wisnewsky, J. Gut microbiota dysbiosis in human obesity: impact of bariatric surgery. Curr. Obes. Rep. 8, 229–242 (2019).
Džunková, M. et al. Salivary microbiome composition changes after bariatric surgery. Sci. Rep. 10, 20086 (2020).
Stefura, T. et al. Changes in the composition of oral and intestinal microbiota after sleeve gastrectomy and Roux-en-Y gastric bypass and their impact on outcomes of bariatric surgery. Obes. Surg. 32, 1439–1450 (2022).
Durand, R. et al. Dental caries are positively associated with periodontal disease severity. Clin. Oral. Investig. 23, 3811–3819 (2019).
Kilian, M. The oral microbiome – friend or foe? Eur. J. Oral. Sci. 126, 5–12 (2018).
Sälzer, S., Graetz, C., Dörfer, C. E., Slot, D. E. & Weijden, F. A. Contemporary practices for mechanical oral hygiene to prevent periodontal disease. Periodontology 84, 35–44 (2000).
Chapple, I. L. C. et al. Primary prevention of periodontitis: managing gingivitis. J. Clin. Periodontol. 42 (Suppl. 16), S71–S76 (2015).
Innes, N. & Fee, P. A. Is personal oral hygiene advice effective in preventing coronal dental caries? Evid. Based Dent. 20, 52–53 (2019).
Ten Cate, J. M. & Featherstone, J. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit. Rev. Oral. Biol. Med. 2, 283–296 (1991).
Joshipura, K., Muñoz-Torres, F., Fernández-Santiago, J., Patel, R. P. & Lopez-Candales, A. Over-the-counter mouthwash use, nitric oxide and hypertension risk. Blood Press. 29, 103–112 (2020).
Kapil, V. et al. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free. Radic. Biol. Med. 55, 93–100 (2013).
Dagher, A. & Hannan, N. Mouthwash: more harm than good? Br. Dent. J. 226, 240 (2019).
D’Aiuto, F. et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 6, 954–965 (2018).
Cavero-Redondo, I., Peleteiro, B., Alvarez-Bueno, C., Rodriguez-Artalejo, F. & Martinez-Vizcaino, V. Glycated haemoglobin A1c as a risk factor of cardiovascular outcomes and all-cause mortality in diabetic and non-diabetic populations: a systematic review and meta-analysis. BMJ Open 7, e015949 (2017).
Mulhall, H., Huck, O. & Amar, S. Porphyromonas gingivalis, a long-range pathogen: systemic impact and therapeutic implications. Microorganisms 8, 869 (2020).
Wong, J. M. W. et al. Gut microbiota, diet, and heart disease. J. AOAC Int. 95, 24–30 (2012).
Hara, H., Haga, S., Aoyama, Y. & Kiriyama, S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 129, 942–948 (1999).
Granado-Serrano, A. B. et al. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 9, 1772 (2019).
Parada Venegas, D. et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10, 277 (2019).
Satija, A. & Hu, F. B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 28, 437–441 (2018).
Tian, Y. et al. The microbiome modulating activity of bile acids. Gut Microbes 11, 979–996 (2020).
Tang, W. H. W., Li, D. Y. & Hazen, S. L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 16, 137–154 (2019).
Li, Y. T., Swales, K. E., Thomas, G. J., Warner, T. D. & Bishop-Bailey, D. Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler. Thromb. Vasc. Biol. 27, 2606–2611 (2007).
Wang, Y. D. et al. Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology 48, 1632–1643 (2008).
Xu, Y. et al. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 64, 1072–1085 (2016).
Cipriani, S., Mencarelli, A., Palladino, G. & Fiorucci, S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J. Lipid Res. 51, 771–784 (2010).
von Haehling, S. et al. Ursodeoxycholic acid in patients with chronic heart failure: a double-blind, randomized, placebo-controlled, crossover trial. J. Am. Coll. Cardiol. 59, 585–592 (2012).
Nascimento, M. M. Approaches to modulate biofilm ecology. Dent. Clin. North. Am. 63, 581–594 (2019).
Shin, J. M. et al. Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front. Microbiol. 6, 617 (2015).
Altman, H. et al. In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J. Antimicrob. Chemother. 58, 198–201 (2006).
Sol, A. et al. LL-37 opsonizes and inhibits biofilm formation of Aggregatibacter actinomycetemcomitans at subbactericidal concentrations. Infect. Immun. 81, 3577–3585 (2013).
Libby, P. et al. Inflammation, immunity, and infection in atherothrombosis. J. Am. Coll. Cardiol. 72, 2071–2081 (2018).
Libby, P. Interleukin-1 beta as a target for atherosclerosis therapy: biological basis of CANTOS and beyond. J. Am. Coll. Cardiol. 70, 2278–2289 (2017).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Umu, O. C. O., Rudi, K. & Diep, D. B. Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microb. Ecol. Health Dis. 28, 1348886 (2017).
Maitre, Y. et al. Pre and probiotics involved in the modulation of oral bacterial species: new therapeutic leads in mental disorders? Microorganisms 9, 1450 (2021).
Jimenez-Hernandez, N. et al. Modulation of saliva microbiota through prebiotic intervention in HIV-infected individuals. Nutrients 11, 1346 (2019).
Nascimento, M. M. Potential uses of arginine in dentistry. Adv. Dent. Res. 29, 98–103 (2018).
Agnello, M. et al. Arginine improves pH homeostasis via metabolism and microbiome modulation. J. Dent. Res. 96, 924–930 (2017).
Rosier, B. T., Buetas, E., Moya-Gonzalvez, E. M., Artacho, A. & Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 10, 12895 (2020).
Slomka, V. et al. Oral prebiotics and the influence of environmental conditions in vitro. J. Periodontol. 89, 708–717 (2018).
Nguyen, T. et al. Probiotics, including nisin-based probiotics, improve clinical and microbial outcomes relevant to oral and systemic diseases. Periodontol 82, 173–185 (2020).
Nguyen, T., Brody, H., Radaic, A. & Kapila, Y. L. Probiotics for periodontal health–current molecular findings. Periodontol 87, 254–267 (2021).
Khalaf, H. et al. Antibacterial effects of Lactobacillus and bacteriocin PLNC8 ab on the periodontal pathogen Porphyromonas gingivalis. BMC Microbiol. 16, 188 (2016).
Terai, T. et al. Screening of probiotic candidates in human oral bacteria for the prevention of dental disease. PLoS ONE 10, e0128657 (2015).
Preshaw, P. M. et al. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis: a review. J. Clin. Periodontol. 31, 697–707 (2004).
Ramamurthy, N. S. et al. Inhibition of matrix metalloproteinase-mediated periodontal bone loss in rats: a comparison of 6 chemically modified tetracyclines. J. Periodontol. 73, 726–734 (2002).
Jiao, Y., Tay, F. R., Niu, L. N. & Chen, J. H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral. Sci. 11, 28 (2019).
Bernegossi, J. et al. Peptide KSL-W-loaded mucoadhesive liquid crystalline vehicle as an alternative treatment for multispecies oral biofilm. Molecules 21, E37 (2015).
Liu, Y. L., Nascimento, M. & Burne, R. A. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int. J. Oral. Sci. 4, 135–140 (2012).
He, J. et al. l-Arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J. Bacteriol. 198, 2651–2661 (2016).
Fabbri, S. et al. High-velocity microsprays enhance antimicrobial activity in Streptococcus mutans biofilms. J. Dent. Res. 95, 1494–1500 (2016).
Gao, L. et al. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 101, 272–284 (2016).
Kovacic, S. & Bakran, M. Genetic susceptibility to atherosclerosis. Stroke Res. Treat. 2012, 362941 (2012).
Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 47, C7–C12 (2006).
Malekmohammad, K., Bezsonov, E. E. & Rafieian-Kopaei, M. Role of lipid accumulation and inflammation in atherosclerosis: focus on molecular and cellular mechanisms. Front. Cardiovasc. Med. 8, 707529 (2021).
Lehti, S. et al. Extracellular lipids accumulate in human carotid arteries as distinct three-dimensional structures and have proinflammatory properties. Am. J. Pathol. 188, 525–538 (2018).
Geng, Y. J. & Libby, P. Progression of atheroma: a struggle between death and procreation. Arterioscler. Thromb. Vasc. Biol. 22, 1370–1380 (2002).
Bentzon, J. F., Otsuka, F., Virmani, R. & Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 114, 1852–1866 (2014).
Sakakura, K. et al. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 22, 399–411 (2013).
Berg, G. et al. Microbiome definition re-visited: old concepts and new challenges. Microbiome 8, 103 (2020).
Verma, D., Garg, P. K. & Dubey, A. K. Insights into the human oral microbiome. Arch. Microbiol. 200, 525–540 (2018).
Aas, J. A., Paster, B. J. & Stokes, L. N. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43, 5721–5732 (2005).
Dewhirst, F. E., Chen, T. & Izard, J. The human oral microbiome. J. Bacteriol. 192, 5002–5017 (2010).
Palmer, R. J. Composition and development of oral bacterial communities. Periodontology 64, 20–39 (2014).
Sutter, V. L. Anaerobes as normal oral flora. Rev. Infect. Dis. 6 (Suppl. 1), S62–S66 (1984).
Deo, P. N. & Deshmukh, R. Oral microbiome: unveiling the fundamentals. J. Oral. Maxillofac. Pathol. 23, 122–128 (2019).
Thomas, T., Gilbert, J. & Meyer, F. Metagenomics – a guide from sampling to data analysis. Microb. Inf. Exp. 2, 3 (2012).
Ranjan, R., Rani, A., Metwally, A., McGee, H. S. & Perkins, D. L. Analysis of the microbiome: advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem. Biophys. Res. Commun. 469, 967–977 (2016).
Lowe, R., Shirley, N. & Bleackley, M. Transcriptomics technologies. PLoS Comput. Biol. 13, e1005457 (2017).
Rifai, N., Gillette, M. A. & Carr, S. A. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotechnol. 24, 971–983 (2006).
Gilbert, J. A., Field, D. & Huang, Y. Detection of large numbers of novel sequences in the metatranscriptomes of complex marine microbial communities. PLoS ONE 3, e3042 (2008).
Jones, N. R. V., Tong, T. Y. N. & Monsivais, P. Meeting UK dietary recommendations is associated with higher estimated consumer food costs: an analysis using the National Diet and Nutrition Survey and consumer expenditure data, 2008–2012. Public Health Nutr. 21, 948–956 (2018).
Bhupathiraju, S. N., Wedick, N. M. & Pan, A. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am. J. Clin. Nutr. 98, 1514–1523 (2013).
Bahadoran, Z., Mirmiran, P. & Kabir, A. The nitrate-independent blood pressure-lowering effect of beetroot juice: a systematic review and meta-analysis. Adv. Nutr. 8, 830–838 (2017).
Larsen, F. J., Ekblom, B. & Sahlin, K. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 355, 2792–2793 (2006).
Broxterman, R. M., Salle, D. T. & Zhao, J. Influence of dietary inorganic nitrate on blood pressure and vascular function in hypertension: prospective implications for adjunctive treatment. J. Appl. Physiol. 127, 1085–1094 (2019).
D’El-Rei, J., Cunha, A. R. & Trindade, M. Beneficial effects of dietary nitrate on endothelial function and blood pressure levels. Int. J. Hypertens. 2016, 6791519 (2016).
Velmurugan, S., Gan, J. M. & Rathod, K. S. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am. J. Clin. Nutr. 103, 25–38 (2016).
Lidder, S. & Webb, A. J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate–nitrite–nitric oxide pathway. Br. J. Clin. Pharmacol. 75, 677–696 (2013).
Jackson, J. K., Patterson, A. J. & MacDonald-Wick, L. K. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: a systematic review and meta-analysis of human evidence. Nutr. Rev. 76, 348–371 (2018).
Petersson, J., Carlström, M. & Schreiber, O. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic. Biol. Med. 46, 1068–1075 (2006).
Mitsui, T. & Harasawa, R. The effects of essential oil, povidone-iodine, and chlorhexidine mouthwash on salivary nitrate/nitrite and nitrate-reducing bacteria. J. Oral. Sci. 59, 597–601 (2017).
Bondonno, C. P., Liu, A. H. & Croft, K. D. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am. J. Hypertens. 28, 572–575 (2015).
Deehan, E. C., Yang, C. & Perez-Muñoz, M. E. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 27, 389–404 (2020).
Perry, R. J., Peng, L. & Barry, N. A. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534, 213–217 (2016).
Whelton, S. P., Hyre, A. D. & Pedersen, B. Effect of dietary fiber intake on blood pressure: a meta-analysis of randomized, controlled clinical trials. J. Hypertens. 23, 475–481 (2005).
Pluznick, J. L., Protzko, R. J. & Gevorgyan, H. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl Acad. Sci. USA 110, 4410–4415 (2013).
Natarajan, N., Hori, D. & Flavahan, S. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol. Genomics 48, 826–834 (2016).
Li, J., Zhao, F. & Wang, Y. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5, 14 (2017).
Espina, M., Gabarrini, G. & Harmsen, H. Talk to your gut: the oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol. Rev. 43, 1–18 (2019).
Brusca, S. B., Abramson, S. B. & Scher, J. U. Microbiome and mucosal inflammation as extra-articular triggers for rheumatoid arthritis and autoimmunity. Curr. Opin. Rheumatol. 26, 101–107 (2014).
Chen, B., Sun, L. & Zhang, X. Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J. Autoimmun. 83, 31–42 (2017).
Saini, R., Saini, S. & Sharma, S. Biofilm: a dental microbial infection. J. Nat. Sci. Biol. Med. 2, 71–75 (2011).
Pischon, N., Pischon, T. & Kröger, J. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J. Periodontol. 79, 979–986 (2008).
Meulen, T., Harmsen, H. & Bootsma, H. Dysbiosis of the buccal mucosa microbiome in primary Sjögren’s syndrome patients. Rheumatol 57, 2225–2234 (2018).
Gent, A. E., Hellier, M. D. & Grace, R. H. Inflammatory bowel disease and domestic hygiene in infancy. Lancet 343, 766–767 (1994).
Li, B. Z., Zhou, H. Y. & Guo, B. Dysbiosis of oral microbiota is associated with systemic lupus erythematosus. Arch. Oral. Biol. 113, 104708 (2020).
Kitamoto, S., Nagao-Kitamoto, H. & Jiao, Y. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 182, 447–462 (2020).
Acknowledgements
A.T. acknowledges funding from the Skye Foundation, the Life Healthcare Group and The Mandela Rhodes Foundation. E.N.L. acknowledges funding from the National Research Foundation of South Africa and the Carnegie Corporation of New York. N.A.B.N. acknowledges funding from the National Research Foundation, South African Medical Research Council, Medical Research Council United Kingdom, and the Lily and Ernst Hausmann Trust. The funders had no role in the design and conduct of the research.
Author information
Authors and Affiliations
Contributions
E.N.L. and N.A.B.N. conceived the microbiome project. A.T., E.N.L. and N.A.B.N. researched data for the article, and A.T. compiled the figures. All the authors contributed to discussion of content, writing of the manuscript and reviewing/editing the article before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Bruno Bohn, Ryan Demmer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Dysbiosis
-
Any change to the composition or function, in terms of abundance and diversity, of resident commensal microbes relative to the composition or role in healthy individuals. Owing to the close, bidirectional orchestration of these communities and human physiology, perturbations in abundance and diversity can lead to inflammatory and metabolic abnormalities that contribute to a plethora of diseases.
- Endotoxaemia
-
Translocation of lipopolysaccharide in the circulation, which can arise from infections or commensal bacteria. The oral cavity is an important source of endotoxaemia. The mouth harbours approximately 100–200 commensal bacterial species and has higher phylogenetic diversity than any other location in the body.
- Eubiosis
-
A healthy or optimal microbiome, one with diversity and uniformity of respective microbiota, at abundances characteristically found in healthy individuals. A eubiotic microbiome confers a protective and beneficial physiological effect, helping to maintain the optimal homeostatic balance while ensuring appropriate training and maintenance of the immunological system.
- Microbiome
-
A characteristic community of microbiota, including bacteria, archaea, fungi, viruses, protists and algae, occupying a specific habitat, including their ‘theatre of activity’, consisting of various microbial structural elements (proteins, lipids and polysaccharides and nucleic acids), internal and external structural elements (including microbial metabolites and mobile genetic elements) and the immediate environmental conditions. Together, these elements form unique ecological niches that are dynamic and adaptable through time, integrating with host physiology to influence function and health.
- Multiomics
-
A comprehensive, or global, assessment of a set of molecules. The term is commonly applied to high-throughput technologies in the fields of genomics, proteomics, transcriptomics, metabolomics and lipidomics. When these fields are combined, specifically looking at data from a ‘multiomics’ perspective, the flow of information that underlies disease, from gene to phenotype, can be elucidated.
- Oralome
-
The overarching term using to summarize the dynamic interactions between the ecological community of oral microorganisms, including bacteria, fungi, viruses, archaea and protozoa that live within the oral cavity of the host.
- Periodontitis
-
A chronic inflammatory disease associated with destruction of connective tissue of gingiva, periodontal ligament and alveolar bone following untreated or improperly treated gingivitis. Bacterial biofilms (dental plaque), predominantly composed of the viridans group streptococci, are the primary aetiological factors for the inflammatory process of gingivitis, leading to subsequent destruction of periodontal tissues.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tonelli, A., Lumngwena, E.N. & Ntusi, N.A.B. The oral microbiome in the pathophysiology of cardiovascular disease. Nat Rev Cardiol 20, 386–403 (2023). https://doi.org/10.1038/s41569-022-00825-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00825-3
This article is cited by
-
Bridging the gap between omics research and dental practice
BDJ Open (2024)
-
Association between oral microbial dysbiosis and poor functional outcomes in stroke-associated pneumonia patients
BMC Microbiology (2023)
-
Circular RNA ZBTB46 depletion alleviates the progression of Atherosclerosis by regulating the ubiquitination and degradation of hnRNPA2B1 via the AKT/mTOR pathway
Immunity & Ageing (2023)
-
Bugs as features (part 1): concepts and foundations for the compositional data analysis of the microbiome–gut–brain axis
Nature Mental Health (2023)
-
Occlusive membranes for guided regeneration of inflamed tissue defects
Nature Communications (2023)