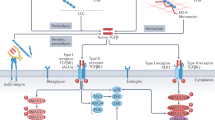
Abstract
Left ventricular hypertrophy is a leading risk factor for cardiovascular morbidity and mortality. Although reverse ventricular remodelling was long thought to be irreversible, evidence from the past three decades indicates that this process is possible with many existing heart disease therapies. The regression of pathological hypertrophy is associated with improved cardiac function, quality of life and long-term health outcomes. However, less than 50% of patients respond favourably to most therapies, and the reversibility of remodelling is influenced by many factors, including age, sex, BMI and disease aetiology. Cardiac hypertrophy also occurs in physiological settings, including pregnancy and exercise, although in these cases, hypertrophy is associated with normal or improved ventricular function and is completely reversible postpartum or with cessation of training. Studies over the past decade have identified the molecular features of hypertrophy regression in health and disease settings, which include modulation of protein synthesis, microRNAs, metabolism and protein degradation pathways. In this Review, we summarize the evidence for hypertrophy regression in patients with current first-line pharmacological and surgical interventions. We further discuss the molecular features of reverse remodelling identified in cell and animal models, highlighting remaining knowledge gaps and the essential questions for future investigation towards the goal of designing specific therapies to promote regression of pathological hypertrophy.
Key points
-
Pathological cardiac hypertrophy is a leading risk factor for cardiovascular morbidity and mortality and is associated with increased fibrosis and apoptosis that lead to ventricular stiffness, risk of arrhythmia and impaired cardiac function.
-
Partial reversal of cardiac hypertrophy occurs with many existing heart failure therapies, including renin–angiotensin–aldosterone system, β-adrenergic receptor, calcium-channel and SGLT2 antagonists, but is achieved in only a subset of patients.
-
The potential for reverse remodelling in heart disease is influenced by many factors, including biological sex, genetics, duration of hypertension, BMI, age and disease aetiology.
-
Exercise-induced and pregnancy-induced physiological cardiac hypertrophy is driven by cellular mechanisms distinct from those in pathological hypertrophy, and is completely reversible, whereas moderate exercise in heart failure antagonizes pathological cellular pathways and promotes hypertrophy regression.
-
At the molecular level, hypertrophy regression is associated with metabolic shifts, reduced protein synthesis, extracellular matrix remodelling and altered activity of proteolytic pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ahmad, F. B. & Anderson, R. N. The leading causes of death in the US for 2020. JAMA 325, 1829–1830 (2021).
Bluemke, D. A. et al. The relationship of left ventricular mass and geometry to incident cardiovascular events. The MESA (Multi-Ethnic Study of Atherosclerosis) study. J. Am. Coll. Cardiol. 52, 2148–2155 (2008).
Levy, D., Garrison, R. J., Savage, D. D., Kannel, W. B. & Castelli, W. P. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N. Engl. J. Med. 322, 1561–1566 (1990).
Izumi, C. et al. Effect of left ventricular reverse remodeling on long-term outcomes after aortic valve replacement. Am. J. Cardiol. 124, 105–112 (2019).
Daubert, M. A. et al. NT-proBNP goal achievement is associated with significant reverse remodeling and improved clinical outcomes in HFrEF. JACC Heart Fail. 7, 158–168 (2019).
Kim, G. H., Uriel, N. & Burkhoff, D. Reverse remodelling and myocardial recovery in heart failure. Nat. Rev. Cardiol. 15, 83–96 (2018).
Rawlins, J., Bhan, A. & Sharma, S. Left ventricular hypertrophy in athletes. Eur. J. Echocardiogr. 10, 350–356 (2009).
Swoboda, P. P. et al. Regression of left ventricular mass in athletes undergoing complete detraining is mediated by decrease in intracellular but not extracellular compartments. Circ. Cardiovasc. Imaging 12, e009417 (2019).
Chung, E. & Leinwand, L. A. Pregnancy as a cardiac stress model. Cardiovascular Res. 101, 561–570 (2014).
Nauta, J. F. et al. Concentric vs. eccentric remodelling in heart failure with reduced ejection fraction: clinical characteristics, pathophysiology and response to treatment. Eur. J. Heart Fail. 22, 1147–1155 (2020).
Melenovsky, V. Cardiac adaptation to volume overload. Card. Adaptations: Mol. Mechanisms 4, 167–199 (2013).
Litviňuková, M. et al. Cells of the adult human heart. Nature 588, 466–472 (2020).
Carreño, J. E., Apablaza, F., Ocaranza, M. P. & Jalil, J. E. Cardiac hypertrophy: molecular and cellular events. Rev. Esp. Cardiol. 59, 473–486 (2006).
Bergmann, O. et al. Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015).
Nakamura, M. & Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15, 387–407 (2018).
Weeks, K. L. & McMullen, J. R. The athlete’s heart vs. the failing heart: Can signaling explain the two distinct outcomes? Physiology 26, 97–105 (2011).
Troncoso, R., Ibarra, C., Vicencio, J. M., Jaimovich, E. & Lavandero, S. New insights into IGF-1 signaling in the heart. Trends Endocrinol. Metab. 25, 128–137 (2014).
Dale Abel, E. Insulin signaling in the heart. Am. J. Physiol. Endocrinol. Metab. 321, 130–145 (2021).
Nagao, H. et al. Distinct signaling by insulin and IGF-1 receptors and their extra- and intracellular domains. Proc. Natl Acad. Sci. USA 118, e2019474118 (2021).
Riehle, C. et al. Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol. Cell. Biol. 34, 3450–3460 (2014).
McMullen, J. R. et al. The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3-kinase(p110α) pathway. J. Biol. Chem. 279, 4782–4793 (2004).
McMullen, J. R. et al. Phosphoinositide 3-kinase(p110α) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc. Natl Acad. Sci. USA 100, 12355–12360 (2003).
Skurk, C. et al. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J. Biol. Chem. 280, 20814–20823 (2005).
Boström, P. et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 143, 1072–1083 (2010).
Haq, S. et al. Glycogen synthase kinase-3β is a negative regulator of cardiomyocyte hypertrophy. J. Cell Biol. 151, 117–129 (2000).
Sciarretta, S., Forte, M., Frati, G. & Sadoshima, J. New insights into the role of mtor signaling in the cardiovascular system. Circ. Res. 122, 489–505 (2018).
Bueno, O. F. et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 19, 6341–6350 (2000).
Ojamaa, K. Signaling mechanisms in thyroid hormone-induced cardiac hypertrophy. Vasc. Pharmacol. 52, 113–119 (2010).
Simoncini, T. et al. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature 407, 538–541 (2000).
Chang, K. C. et al. Thyroid hormone improves function and Ca2+ handling in pressure overload hypertrophy. Association with increased sarcoplasmic reticulum Ca2+-ATPase and α-myosin heavy chain in rat hearts. J. Clin. Invest. 100, 1742–1749 (1997).
Trivieri, M. G. et al. Cardiac-specific elevations in thyroid hormone enhance contractility and prevent pressure overload-induced cardiac dysfunction. Proc. Natl Acad. Sci. USA 103, 6043–6048 (2006).
Iliopoulou, I. et al. Time-dependent and independent effects of thyroid hormone administration following myocardial infarction in rats. Mol. Med. Rep. 18, 864–876 (2018).
Pantos, C. et al. Thyroid hormone at supra-physiological dose optimizes cardiac geometry and improves cardiac function in rats with old myocardial infarction. J. Physiol. Pharmacol. 60, 49–56 (2009).
Ojamaa, K., Kenessey, A., Shenoy, R. & Klein, I. Thyroid hormone metabolism and cardiac gene expression after acute myocardial infarction in the rat. Am. J. Physiol. Endocrinol. Metab. 279, E1319–E1324 (2000).
Riquelme, C. A. et al. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science 334, 528–531 (2011).
Liu, X. et al. MiR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 21, 584–595 (2015).
Shi, J. et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics 7, 664–676 (2017).
Gao, R. et al. Long noncoding RNA cardiac physiological hypertrophy-associated regulator induces cardiac physiological hypertrophy and promotes functional recovery after myocardial ischemia-reperfusion injury. Circulation 144, 303–317 (2021).
Li, H. et al. lncExACT1 and DCHS2 regulate physiological and pathological cardiac growth. Circulation 145, 1218–1233 (2022).
Gogiraju, R., Bochenek, M. L. & Schäfer, K. Angiogenic endothelial cell signaling in cardiac hypertrophy and heart failure. Front. Cardiovasc. Med. 6, 20 (2019).
Oka, T., Akazawa, H., Naito, A. T. & Komuro, I. Angiogenesis and cardiac hypertrophy: Maintenance of cardiac function and causative roles in heart failure. Circ. Res. 114, 565–571 (2014).
Shiojima, I. et al. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J. Clin. Invest. 115, 2108–2118 (2005).
Oldfield, C. J., Duhamel, T. A. & Dhalla, N. S. Mechanisms for the transition from physiological to pathological cardiac hypertrophy. Can. J. Physiol. Pharmacol. 98, 74–84 (2020).
Lymperopoulos, A., Rengo, G. & Koch, W. J. Adrenergic nervous system in heart failure: pathophysiology and therapy. Circ. Res. 113, 739–753 (2013).
Morel, E. et al. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ. Res. 97, 1296–1304 (2005).
Métrich, M. et al. Epac activation induces histone deacetylase nuclear export via a Ras-dependent signalling pathway. Cell Signal. 22, 1459–1468 (2010).
Métrich, M. et al. Epac mediates β-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ. Res. 102, 959–965 (2008).
Osadchii, O. E. Cardiac hypertrophy induced by sustained β-adrenoreceptor activation: pathophysiological aspects. Heart Fail. Rev. 12, 66–86 (2007).
Sato, P. Y., Chuprun, J. K., Schwartz, M. & Koch, W. J. The evolving impact of G protein-coupled receptor kinases in cardiac health and disease. Physiol. Rev. 95, 377–404 (2015).
Hullmann, J. E. et al. GRK5-mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circ. Res. 115, 976–985 (2014).
Martini, J. S. et al. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc. Natl Acad. Sci. USA 105, 12457–12462 (2008).
Gold, J. I., Gao, E., Shang, X., Premont, R. T. & Koch, W. J. Determining the absolute requirement of G protein-coupled receptor kinase 5 for pathological cardiac hypertrophy: short communication. Circ. Res. 111, 1048–1053 (2012).
Rapacciuolo, A. et al. Important role of endogenous norepinephrine and epinephrine in the development of in vivo pressure-overload cardiac hypertrophy. J. Am. Coll. Cardiol. 38, 876–882 (2001).
Dash, R. et al. Differential regulation of p38 mitogen-activated protein kinase mediates gender-dependent catecholamine-induced hypertrophy. Cardiovasc. Res. 57, 704–714 (2003).
Calvieri, C., Rubattu, S. & Volpe, M. Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides. J. Mol. Med. 90, 5–13 (2012).
Hall, E. J. et al. Cardiac natriuretic peptide deficiency sensitizes the heart to stress-induced ventricular arrhythmias via impaired CREB signalling. Cardiovasc. Res. 118, 2124–2138 (2022).
Holtwick, R. et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J. Clin. Invest. 111, 1399–1407 (2003).
Potter, L. R., Yoder, A. R., Flora, D. R., Antos, L. K. & Dickey, D. M. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 191, 341–366 (2009).
Rainer, P. P. & Kass, D. A. Old dog, new tricks: novel cardiac targets and stress regulation by protein kinase G. Cardiovasc. Res. 111, 154–162 (2016).
Klaiber, M. et al. A cardiac pathway of cyclic GMP-independent signaling of guanylyl cyclase A, the receptor for atrial natriuretic peptide. Proc. Natl Acad. Sci. USA 108, 18500–18505 (2011).
Vinnakota, S. & Chen, H. H. The importance of natriuretic peptides in cardiometabolic diseases. J. Endocr. Soc. 4, bvaa052 (2020).
Tsai, E. J. & Kass, D. A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 122, 216–238 (2009).
Takimoto, E. et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 11, 214–222 (2005).
Orsborne, C., Chaggar, P. S., Shaw, S. M. & Williams, S. G. The renin-angiotensin-aldosterone system in heart failure for the non-specialist: the past, the present and the future. Postgrad. Med. J. 93, 29–37 (2017).
Zhang, C. L. et al. Plasma endothelin-1-related peptides as the prognostic biomarkers for heart failure: a PRISMA-compliant meta-analysis. Medicine 96, e9342 (2017).
Yamazaki, T. et al. Angiotensin II partly mediates mechanical stress-induced cardiac hypertrophy. Circ. Res. 77, 258–265 (1995).
Zablocki, D. & Sadoshima, J. Solving the cardiac hypertrophy riddle: the angiotensin II–mechanical stress connection. Circ. Res. 113, 1192–1195 (2013).
Lyon, R. C., Zanella, F., Omens, J. H. & Sheikh, F. Mechanotransduction in cardiac hypertrophy and failure. Circ. Res. 116, 1462–1476 (2015).
Molkentin, J. D. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc. Res. 63, 467–475 (2004).
Wilkins, B. J. et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ. Res. 94, 110–118 (2004).
Mehta, P. K. & Griendling, K. K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 292, C82–C97 (2007).
Zhang, C. L. et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002).
Wang, Z., Zhao, Y. T. & Zhao, T. C. Histone deacetylases in modulating cardiac disease and their clinical translational and therapeutic implications. Exp. Biol. Med. 246, 213–225 (2021).
Singh, M. V. et al. Angiotensin II-induced hypertension and cardiac hypertrophy are differentially mediated by TLR3- and TLR4-dependent pathways. Am. J. Physiol. Heart Circ. Physiol. 316, H1027–H1038 (2019).
Pierdomenico, S. D., Lapenna, D. & Cuccurullo, F. Regression of echocardiographic left ventricular hypertrophy after 2 years of therapy reduces cardiovascular risk in patients with essential hypertension. Am. J. Hypertens. 21, 464–470 (2008).
Moon, M. G. et al. Reverse remodelling by sacubitril/valsartan predicts the prognosis in heart failure with reduced ejection fraction. ESC Heart Fail. 8, 2058–2069 (2021).
Sayer, G. & Bhat, G. The renin-angiotensin-aldosterone system and heart failure. Cardiol. Clin. 32, 21–32 (2014).
Yasunari, K. et al. Comparative effects of valsartan versus amlodipine on left ventricular mass and reactive oxygen species formation by monocytes in hypertensive patients with left ventricular hypertrophy. J. Am. Coll. Cardiol. 43, 2116–2123 (2004).
Misra, K. H., Das, M. C. & Ramani, Y. J. Effect of telmisartan on the regression of the left ventricular hypertrophy in the patients of essential hypertension. J. Clin. Diagn. Res. 7, 1352–1355 (2013).
Nalbantgil, S. et al. Effects of valsartan and enalapril on regression of left ventricular hypertrophy in patients with mild to moderate hypertension: a randomized, double-blind study. Curr. Ther. Res. Clin. Exp. 61, 331–338 (2000).
Dyadyk, A. I. et al. ACE inhibitors captopril and enalapril induce regression of left ventricular hypertrophy in hypertensive patients with chronic renal failure. Nephrol. Dial. Transplant. 12, 945–951 (1997).
Hernandez, D. et al. Regression of left ventricular hypertrophy by lisinopril after renal transplantation: role of ACE gene polymorphism. Kidney Int. 58, 889–897 (2000).
Nakashima, Y., Fouad, F. M. & Tarazi, R. C. Regression of left ventricular hypertrophy from systemic hypertension by enalapril. Am. J. Cardiol. 53, 1044–1049 (1984).
Konstam, M. A. et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dysfunction in patients with heart failure. Circulation 86, 431–438 (1992).
Wong, M. et al. Valsartan benefits left ventricular structure and function in heart failure: Val-HeFT echocardiographic study. J. Am. Coll. Cardiol. 40, 970–975 (2002).
Wong, M. et al. Severity of left ventricular remodeling defines outcomes and response to therapy in heart failure: valsartan heart failure trial (Val-HeFT) echocardiographic data. J. Am. Coll. Cardiol. 43, 2022–2027 (2004).
Cuspidi, C. et al. Effects of angiotensin II receptor blockade-based therapy with losartan on left ventricular hypertrophy and geometry in previously treated hypertensive patients. Blood Press. 15, 107–115 (2006).
Edwards, N. C., Steeds, R. P., Stewart, P. M., Ferro, C. J. & Townend, J. N. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease. a randomized controlled trial. J. Am. Coll. Cardiol. 54, 505–512 (2009).
Feniman Stefano, G. M. M. et al. Spironolactone is secure and reduces left ventricular hypertrophy in hemodialysis patients. Ther. Adv. Cardiovasc. Dis. 9, 158–167 (2015).
Edelmann, F. et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA 309, 781–791 (2013).
Packer, M. et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 131, 54–61 (2015).
Martens, P., Beliën, H., Dupont, M., Vandervoort, P. & Mullens, W. The reverse remodeling response to sacubitril/valsartan therapy in heart failure with reduced ejection fraction. Cardiovasc. Ther. 36, e12435 (2018).
Piña, I. L. et al. Improvement of health status following initiation of sacubitril/valsartan in heart failure and reduced ejection fraction. JACC Heart Fail. 9, 42–51 (2021).
Januzzi, J. L. et al. Association of change in N-terminal pro-B-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA 322, 1085–1095 (2019).
Ibrahim, N. E. et al. Racial and ethnic differences in biomarkers, health status, and cardiac remodeling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Circ. Heart Fail. 13, E007829 (2020).
Ibrahim, N. E. et al. Sex-based differences in biomarkers, health status, and reverse cardiac remodelling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. Eur. J. Heart Fail. 22, 2018–2025 (2020).
Wang, Y. et al. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J. Am. Heart Assoc. 8, e012272 (2019).
Safdar, O. et al. Impact of sacubitril/valsartan in cardiac reverse remodeling in ischemic vs. nonischemic cardiomyopathy. J. Heart Lung Transplant. 39, S239–S240 (2020).
Lønnebakken, M. T. et al. Left ventricular hypertrophy regression during antihypertensive treatment in an outpatient clinic (the Campania salute network). J. Am. Heart Assoc. 6, e004152 (2017).
Gerdts, E. et al. Impact of age on left ventricular hypertrophy regression during antihypertensive treatment with losartan or atenolol (the LIFE study). J. Hum. Hypertens. 18, 417–422 (2004).
Xie, X. et al. Early prediction of left ventricular reverse remodeling in first-diagnosed idiopathic dilated cardiomyopathy: a comparison of linear model, random forest, and extreme gradient boosting. Front. Cardiovasc. Med. 8, 684004 (2021).
Cannella, G. et al. Prolonged therapy with ACE inhibitors induces a regression of left ventricular hypertrophy of dialyzed uremic patients independently from hypotensive effects. Am. J. Kidney Dis. 30, 659–664 (1997).
Bristow, M. R. et al. β1- and β2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor down-regulation in heart failure. Circ. Res. 59, 297–309 (1986).
de Lucia, C., Eguchi, A. & Koch, W. J. New insights in cardiac β-adrenergic signaling during heart failure and aging. Front. Pharmacol. 9, 904 (2018).
Bristow, M. R. et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. Circulation 94, 2807–2816 (1996).
Colucci, W. S. et al. Metoprolol reverses left ventricular remodeling in patients with asymptomatic systolic dysfunction: the REversal of VEntricular Remodeling with Toprol-XL (REVERT) trial. Circulation 116, 49–56 (2007).
Waagstein, F. et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Lancet 342, 1441–1446 (1993).
Packer, M. et al. Double-blind, placebo-controlled study of the effects of carvedilol in patients with moderate to severe heart failure: the PRECISE trial. Circulation 94, 2793–2799 (1996).
Godfraind, T. Discovery and development of calcium channel blockers. Front. Pharmacol. 8, 286 (2017).
Singh, N. K., Gupta, S. K. & Agrawal, B. V. Left ventricular mass regression with amlodipine in elderly hypertensives. Clin. Exp. Hypertens. 21, 113–119 (1999).
Adalet, K. et al. The effect of amlodipine on the mass and functions of the left ventricle in patients with primary hypertension and left ventricular hypertrophy. Curr. Ther. Res. 56, 607–616 (1995).
Fak, A. S., Okucu, M., Tezcan, H., Bodur, G. & Oktay, A. The effects of amlodipine on left ventricular mass and diastolic function in concentric and eccentric left ventricular hypertrophy. J. Cardiovasc. Pharmacol. Ther. 1, 95–100 (1996).
Szlachcic, J., Tubau, J. F., Vollmer, C. & Massie, B. M. Effect of diltiazem on left ventricular mass and diastolic filling in mild to moderate hypertension. Am. J. Cardiol. 63, 198–201 (1989).
Natale, E. et al. The effect of verapamil on left ventricular remodelling and diastolic function after acute myocardial infarction (the Verapamil Infarction Study on Remodelling and Relaxation – VISOR). Cardiovasc. Drugs Ther. 13, 315–324 (1999).
Ahmed, S. N., Jhaj, R., Sadasivam, B. & Joshi, R. Regression of the left ventricular hypertrophy in patients with essential hypertension on standard drug therapy. Discoveries 8, e115 (2020).
Sun, S., Lu, F., Zhao, Y., Liu, Z. & Wang, S. Effects and reversal of left ventricular hypertrophy of amlodipine plus amiloride/hydrochlorothiazide versus amlodipine plus telmisartan in patients with mild to moderate hypertension. Int. J. Cardiol. 152, S24 (2011).
Polonia, J. et al. Lisinopril and diltiazem reduce left ventricular mass without changing blood pressure in normotensive subjects with exaggerated blood pressure response to exercise. Rev. Port. Cardiol. 15, 185–193 (1996).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019).
Anker, S. D. et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 385, 1451–1461 (2021).
Verma, S. et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation 140, 1693–1702 (2019).
Brown, A. J. M. et al. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. Eur. Heart J. 41, 3421–3432 (2020).
Mohan, M. et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur. Heart J. 40, 3409–3417 (2019).
Guazzi, M., Vicenzi, M., Arena, R. & Guazzi, M. D. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: result of a 1-year, prospective, randomized, placebo-controlled study. Circ. Heart Fail. 4, 8–17 (2011).
Paoletti, E. et al. Everolimus for regression of left ventricular hypertrophy of renal transplant recipients: a randomized controlled trial [abstract 16]. Am. J. Transplant. 12 (Suppl. 3), 31 (2012).
Paoletti, E. et al. Effect of sirolimus on left ventricular hypertrophy in kidney transplant recipients: a 1-year nonrandomized controlled trial. Am. J. Kidney Dis. 52, 324–330 (2008).
Saberi, S. et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM cardiac magnetic resonance substudy analysis. Circulation 143, 606–608 (2021).
Koga-Ikuta, A. et al. Reverse remodelling after aortic valve replacement for chronic aortic regurgitation. Interact. Cardiovasc. Thorac. Surg. 33, 10–18 (2021).
Lindman, B. R. et al. Early regression of severe left ventricular hypertrophy after transcatheter aortic valve replacement is associated with decreased hospitalizations. JACC Cardiovasc. Interv. 7, 662–673 (2014).
Monrad, E. S. et al. Time course of regression of left ventricular hypertrophy after aortic valve replacement. Circulation 77, 1345–1355 (1988).
Treibel, T. A. et al. Reverse myocardial remodeling following valve replacement in patients with aortic stenosis. J. Am. Coll. Cardiol. 71, 860–871 (2018).
Flett, A. S. et al. Diffuse myocardial fibrosis in severe aortic stenosis: an equilibrium contrast cardiovascular magnetic resonance study. Eur. Heart J. Cardiovasc. Imaging 13, 819–826 (2012).
Lund, O. & Erlandsen, M. Changes in left ventricular function and mass during serial investigations after valve replacement for aortic stenosis. J. Heart Valve Dis. 9, 583–593 (2000).
Lund, O., Emmertsen, K., Dørup, I., Jensen, F. T. & Flø, C. Regression of left ventricular hypertrophy during 10 years after valve replacement for aortic stenosis is related to the preoperative risk profile. Eur. Heart J. 24, 1437–1446 (2003).
Pibarot, P., Dumesnil, J. G., Leblanc, M. H., Cartier, P. & Metras, J. Changes in left ventricular mass and function after aortic valve replacement: a comparison between stentless and stented bioprosthetic valves. J. Am. Soc. Echocardiogr. 12, 981–987 (1999).
Walther, T. et al. Prospectively randomized evaluation of stentless versus conventional biological aortic valves: impact on early regression of left ventricular hypertrophy. Circulation 100(Suppl. 2), Ii-6–Ii-10 (1999).
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease a scientific statement from the American Heart Association. Circulation 143, E984–E1010 (2021).
Ippisch, H. M. et al. Reversibility of cardiac abnormalities in morbidly obese adolescents. J. Am. Coll. Cardiol. 51, 1342–1348 (2008).
Cunha, L. et al. Evolutive echocardiographic study of the structural and functional heart alterations in obese individuals after bariatric surgery. Arq. Bras. Cardiol. 87, 615–622 (2006).
Hsuan, C. F. et al. The effect of surgical weight reduction on left ventricular structure and function in severe obesity. Obesity 18, 1188–1193 (2010).
Kanoupakis, E. et al. Left ventricular function and cardiopulmonary performance following surgical treatment of morbid obesity. Obes. Surg. 11, 552–558 (2001).
Syed, M., Torosoff, M., Rosati, C., Alger, S. & Fein, S. Effect of comorbidities and medications on left ventricular mass regression after bariatric surgery. J. Clin. Hypertens. 12, 223–227 (2010).
Jhaveri, R. R. et al. Cardiac remodeling after substantial weight loss: a prospective cardiac magnetic resonance study after bariatric surgery. Surg. Obes. Relat. Dis. 5, 648–652 (2009).
Bristow, M. R. et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 350, 2140–2150 (2004).
Linde, C. et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J. Am. Coll. Cardiol. 52, 1834–1843 (2008).
Moss, A. J. et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 361, 1329–1338 (2009).
St John Sutton, M. et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation 107, 1985–1990 (2003).
Linde, C. et al. Long-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the resynchronization reverses remodeling in systolic left ventricular dysfunction (REVERSE) study. Eur. Heart J. 34, 2592–2599 (2013).
Naqvi, S. Y. et al. Left ventricular reverse remodeling in cardiac resynchronization therapy and long-term outcomes. JACC Clin. Electrophysiol. 5, 1001–1010 (2019).
Matsumoto, K. et al. Reverse remodelling induces progressive ventricular resynchronization after cardiac resynchronization therapy ‘from vicious to virtuous cycle’. Eur. J. Echocardiogr. 12, 782–789 (2011).
Alvarez-Alvarez, B. et al. Long-term cardiac reverse remodeling after cardiac resynchronization therapy. J. Arrhythmia 37, 653–659 (2021).
Jefferson, H. L. et al. Left ventricular assist devices: a comprehensive review of major clinical trials, devices, and future directions. J. Card. Surg. 36, 1480–1491 (2021).
Burkhoff, D., Topkara, V. K., Sayer, G. & Uriel, N. Reverse remodeling with left ventricular assist devices. Circ. Res. 128, 1594–1612 (2021).
Bruckner, B. A. et al. Regression of fibrosis and hypertrophy in failing myocardium following mechanical circulatory support. J. Heart Lung Transplant. 20, 457–464 (2001).
Madigan, J. D. et al. Time course of reverse remodeling of the left ventricle during support with a left ventricular assist device. J. Thorac. Cardiovasc. Surg. 121, 902–908 (2001).
Zafeiridis, A., Jeevanandam, V., Houser, S. R. & Margulies, K. B. Regression of cellular hypertrophy after left ventricular assist device support. Circulation 98, 656–662 (1998).
Barbone, A. et al. Comparison of right and left ventricular responses to left ventricular assist device support in patients with severe heart failure: a primary role of mechanical unloading underlying reverse remodeling. Circulation 104, 670–675 (2001).
Johnson, E. J., Dieter, B. P. & Marsh, S. A. Evidence for distinct effects of exercise in different cardiac hypertrophic disorders. Life Sci. 123, 100–106 (2015).
Chen, Y. M., Li, Z. B., Zhu, M. & Cao, Y. M. Effects of exercise training on left ventricular remodelling in heart failure patients: an updated meta-analysis of randomised controlled trials. Int. J. Clin. Pract. 66, 782–791 (2012).
Kokkinos, P. F. et al. Effects of regular exercise on blood pressure and left ventricular hypertrophy in African-American men with severe hypertension. N. Engl. J. Med. 333, 1462–1467 (1995).
Haykowsky, M. et al. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials 12, 92 (2011).
Delagardelle, C. et al. Reverse remodelling through exercise training is more pronounced in non-ischemic heart failure. Clin. Res. Cardiol. 97, 865–871 (2008).
Takatsu, M. et al. Calorie restriction attenuates cardiac remodeling and diastolic dysfunction in a rat model of metabolic syndrome. Hypertension 62, 957–965 (2013).
Okoshi, K. et al. Influence of intermittent fasting on myocardial infarction-induced cardiac remodeling. BMC Cardiovasc. Disord. 19, 126 (2019).
An, H. S. et al. Caloric restriction reverses left ventricular hypertrophy through the regulation of cardiac iron homeostasis in impaired leptin signaling mice. Sci. Rep. 10, 7176 (2020).
De Lucia, C. et al. Long-term caloric restriction improves cardiac function, remodeling, adrenergic responsiveness, and sympathetic innervation in a model of postischemic heart failure. Circ. Heart Fail. 11, e004153 (2018).
Sciarretta, S. et al. Caloric restriction mimetics for the treatment of cardiovascular diseases. Cardiovasc. Res. 117, 1434–1449 (2021).
Hall, C., Gehmlich, K., Denning, C. & Pavlovic, D. Complex relationship between cardiac fibroblasts and cardiomyocytes in health and disease. J. Am. Heart Assoc. 10, e019338 (2021).
Dobson, L. E. et al. Acute reverse remodelling after transcatheter aortic valve implantation: a link between myocardial fibrosis and left ventricular mass regression. Can. J. Cardiol. 32, 1411–1418 (2016).
Petrov, G. et al. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation 122, S23–S28 (2010).
Puls, M. et al. Impact of myocardial fibrosis on left ventricular remodelling, recovery, and outcome after transcatheter aortic valve implantation in different haemodynamic subtypes of severe aortic stenosis. Eur. Heart J. 41, 1903–1914 (2020).
Lewis, G. A. et al. Pirfenidone in heart failure with preserved ejection fraction: a randomized phase 2 trial. Nat. Med. 27, 1477–1482 (2021).
Frieler, R. A. & Mortensen, R. M. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 131, 1019–1030 (2015).
Ma, X. L. et al. Rituximab prevents and reverses cardiac remodeling by depressing B cell function in mice. Biomed. Pharmacother. 114, 108804 (2019).
Szardien, S. et al. Regression of cardiac hypertrophy by granulocyte colony-stimulating factor-stimulated interleukin-1β synthesis. Eur. Heart J. 33, 595–605 (2012).
Chung, E., Yeung, F. & Leinwand, L. A. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J. Appl. Physiol. 112, 1564–1575 (2012).
Chung, E., Heimiller, J. & Leinwand, L. A. Distinct cardiac transcriptional profiles defining pregnancy and exercise. PLoS One 7, e42297 (2012).
Mone, S. M., Sanders, S. P. & Colan, S. D. Control mechanisms for physiological hypertrophy of pregnancy. Circulation 94, 667–672 (1996).
Robson, S. C., Dunlop, W., Moore, M. & Hunter, S. Haemodynamic changes during the puerperium: a Doppler and M‐mode echocardiographic study. BJOG . Int. J. Obstet. Gynaecol. 94, 1028–1039 (1987).
Clapp, J. F. & Capeless, E. Cardiovascular function before, during, and after the first and subsequent pregnancies. Am. J. Cardiol. 80, 1469–1473 (1997).
Umar, S. et al. Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J. Appl. Physiol. 113, 1253–1259 (2012).
Gonzalez, A. M. D. et al. Hypertrophy signaling during peripartum cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 293, H3008–H3013 (2007).
Iorga, A., Dewey, S., Partow-Navid, R., Gomes, A. V. & Eghbali, M. Pregnancy is associated with decreased cardiac proteasome activity and oxidative stress in mice. PLoS One 7, e48601 (2012).
Limon-Miranda, S. et al. Pregnancy differentially regulates the collagens types I and III in left ventricle from rat heart. Biomed. Res. Int. 2014, 984785 (2014).
Parrott, M. E. et al. Feature article: Maternal cardiac messenger RNA expression of extracellular matrix proteins in mice during pregnancy and the postpartum period. Exp. Biol. Med. 243, 1220–1232 (2018).
Bond, C. F. Blood volume changes in the lactating rat. Endocrinology 63, 285–289 (1958).
Lunsford, T. Cardiac Hypertrophy and Regresion during Postpartum in C57B1/6 Mice. Thesis, Texas Tech Univ. Honors College (2014).
Frenzel, H. et al. Regression of cardiac hypertrophy: morphometric and biochemical studies in rat heart after swimming training. J. Mol. Cell. Cardiol. 20, 737–751 (1988).
Rubin, D. A. et al. Endocrine response to acute resistance exercise in obese versus lean physically active men. Eur. J. Appl. Physiol. 115, 1359–1366 (2015).
Lin, H. et al. Antihypertrophic memory after regression of exercise-induced physiological myocardial hypertrophy is mediated by the long noncoding RNA Mhrt779. Circulation 143, 2277–2292 (2021).
Wei, X. et al. Myocardial hypertrophic preconditioning attenuates cardiomyocyte hypertrophy and slows progression to heart failure through upregulation of S100A8/A9. Circulation 131, 1506–1517 (2015).
Kinney LaPier, T. L. & Rodnick, K. J. Effects of aerobic exercise on energy metabolism in the hypertensive rat heart. Phys. Ther. 81, 1006–1017 (2001).
Chen, L., Song, J. & Hu, S. Metabolic remodeling of substrate utilization during heart failure progression. Heart Fail. Rev. 24, 143–154 (2019).
Gupte, A. A. et al. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ. Cardiovasc. Genet. 7, 266–276 (2014).
Blaxall, B. C., Tschannen-Moran, B. M., Milano, C. A. & Koch, W. J. Differential gene expression and genomic patient stratification following left ventricular assist device support. J. Am. Coll. Cardiol. 41, 1096–1106 (2003).
Schaefer, A. et al. Analysis of fibrosis in control or pressure overloaded rat hearts after mechanical unloading by heterotopic heart transplantation. Sci. Rep. 9, 5710 (2019).
Gao, X. M. et al. Regression of pressure overload-induced left ventricular hypertrophy in mice. Am. J. Physiol. Heart Circ. Physiol. 288, H2702–H2707 (2005).
Diakos, N. A. et al. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: implications for cardiac reloading and conditioning. JACC Basic. Transl. Sci. 1, 432–444 (2016).
Badolia, R. et al. The role of nonglycolytic glucose metabolism in myocardial recovery upon mechanical unloading and circulatory support in chronic heart failure. Circulation 142, 259–274 (2020).
Heerdt, P. M. et al. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation 102, 2713–2719 (2000).
Birks, E. J. Molecular changes after left ventricular assist device support for heart failure. Circ. Res. 113, 777–791 (2013).
Ogletree-Hughes, M. L. et al. Mechanical unloading restores β-adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 104, 881–886 (2001).
Li, Y. Y. et al. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation 104, 1147–1152 (2001).
Felkin, L. E., Lara-Pezzi, E. A., Hall, J. L., Birks, E. J. & Barton, P. J. R. Reverse remodelling and recovery from heart failure are associated with complex patterns of gene expression. J. Cardiovasc. Transl. Res. 4, 321–331 (2011).
Wohlschlaeger, J. et al. Ventricular unloading is associated with increased 20S proteasome protein expression in the myocardium. J. Heart Lung Transplant. 29, 125–132 (2010).
Kassiotis, C. et al. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation 120, S191–S197 (2009).
Willis, M. S. et al. Muscle ring finger 1 mediates cardiac atrophy in vivo. Am. J. Physiol. Heart Circ. Physiol. 296, H997–H1006 (2009).
Baskin, K. K. et al. MAFbx/Atrogin-1 is required for atrophic remodeling of the unloaded heart. J. Mol. Cell. Cardiol. 72, 168–176 (2014).
Razeghi, P. et al. Mechanical unloading of the heart activates the calpain system. J. Mol. Cell. Cardiol. 42, 449–452 (2007).
Hariharan, N. et al. Autophagy plays an essential role in mediating regression of hypertrophy during unloading of the heart. PLoS One 8, e51632 (2013).
Hall, J. L. et al. Genomic profiling of the human heart before and after mechanical support with a ventricular assist device reveals alterations in vascular signaling networks. Physiol. Genomics 17, 283–291 (2004).
Yang, D. K. et al. Gene profiling during regression of pressure overload-induced cardiac hypertrophy. Physiol. Genomics 30, 1–7 (2007).
Melman, Y. F. et al. Circulating microRNA-30d is associated with response to cardiac resynchronization therapy in heart failure and regulates cardiomyocyte apoptosis: a translational pilot study. Circulation 131, 2202–2216 (2015).
Wang, S. B., Murray, C. I., Chung, H. S. & Van Eyk, J. E. Redox regulation of mitochondrial ATP synthase. Trends Cardiovasc. Med. 23, 14–18 (2013).
Agnetti, G. et al. Modulation of mitochondrial proteome and improved mitochondrial function by biventricular pacing of dyssynchronous failing hearts. Circ. Cardiovasc. Genet. 3, 78–87 (2010).
Cho, H., Barth, A. S. & Tomaselli, G. F. Basic science of cardiac resynchronization therapy: molecular and electrophysiological mechanisms. Circ. Arrhythm. Electrophysiol. 5, 594–603 (2012).
Vanderheyden, M. et al. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy. Responders versus nonresponders. J. Am. Coll. Cardiol. 51, 129–136 (2008).
Hosen, M. R. et al. Circulating microRNA-122-5p is associated with a lack of improvement in left ventricular function after transcatheter aortic valve replacement and regulates viability of cardiomyocytes through extracellular vesicles. Circulation https://doi.org/10.1161/CIRCULATIONAHA.122.060258 (2022).
Khamis, T., Alsemeh, A. E. & Abdullah, D. M. Sacubitril/valsartan (LCZ696) ameliorates hyperthyroid-induced cardiac hypertrophy in male rats through modulation of miR-377, let-7b, autophagy, and fibrotic signaling pathways. Sci. Rep. 12, 14654 (2022).
Muehleman, D. L., Crocini, C., Swearingen, A. R., Ozeroff, C. D. & Leinwand, L. A. Regression from pathological hypertrophy in mice is sexually dimorphic and stimulus specific. Am. J. Physiol. Heart Circ. Physiol. 322, H785–H797 (2022).
Mondaca-Ruff, D. et al. Hydrochlorothiazide reduces cardiac hypertrophy, fibrosis and rho-kinase activation in DOCA-salt induced hypertension. J. Cardiovasc. Pharmacol. Ther. 26, 724–735 (2021).
Garfinkel, A. C., Seidman, J. G. & Seidman, C. E. Genetic pathogenesis of hypertrophic and dilated cardiomyopathy. Heart Fail. Clin. 14, 139–146 (2018).
Dal Ferro, M. et al. Association between mutation status and left ventricular reverse remodelling in dilated cardiomyopathy. Heart 103, 1704–1710 (2017).
Escobar-Lopez, L. et al. Association of genetic variants with outcomes in patients with nonischemic dilated cardiomyopathy. J. Am. Coll. Cardiol. 78, 1682–1699 (2021).
Verdonschot, J. A. J. et al. Clinical phenotype and genotype associations with improvement in left ventricular function in dilated cardiomyopathy. Circ. Heart Fail. 11, e005220 (2018).
Tobita, T. et al. Genetic basis of cardiomyopathy and the genotypes involved in prognosis and left ventricular reverse remodeling. Sci. Rep. 8, 1998 (2018).
Petto, J. et al. Reverse myocardial remodeling in hypertrophic cardiomyopathy: little explored benefit of exercise. Int. J. Exerc. Sci. 14, 1018–1026 (2021).
Snir, A. W., Connelly, K. A., Goodman, J. M., Dorian, D. & Dorian, P. Exercise in hypertrophic cardiomyopathy: restrict or rethink. Am. J. Physiol. Heart Circ. Physiol. 320, H2101–H2111 (2021).
Konhilas, J. P. et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ. Res. 98, 540–548 (2006).
Marin, T. M. et al. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J. Clin. Invest. 121, 1026–1043 (2011).
Rau, C. D. et al. Mapping genetic contributions to cardiac pathology induced by beta-adrenergic stimulation in mice. Circ. Cardiovasc. Genet. 8, 40–49 (2015).
Wang, J. J. C. et al. Genetic dissection of cardiac remodeling in an isoproterenol-induced heart failure mouse model. PLoS Genet. 12, e1006038 (2016).
Regitz-Zagrosek, V., Oertelt-Prigione, S., Seeland, U. & Hetzer, R. Sex and gender differences in myocardial hypertrophy and heart failure. Circ. J. 74, 1265–1273 (2010).
Beale, A. L., Meyer, P., Marwick, T. H., Lam, C. S. P. & Kaye, D. M. Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 138, 198–205 (2018).
Lilli, A. et al. Cardiac resynchronization therapy: gender related differences in left ventricular reverse remodeling. Pacing Clin. Electrophysiol. 30, 1349–1355 (2007).
Kenigsberg, B. B. et al. Sex-associated differences in cardiac reverse remodeling in patients supported by contemporary left ventricular assist devices. J. Card. Fail. 26, 494–504 (2020).
Aimo, A. et al. Effect of sex on reverse remodeling in chronic systolic heart failure. JACC Heart Fail. 5, 735–742 (2017).
Stangl, V. et al. Impact of gender on three-month outcome and left ventricular remodeling after transfemoral transcatheter aortic valve implantation. Am. J. Cardiol. 110, 884–890 (2012).
García, R. et al. Sex-specific regulation of miR-29b in the myocardium under pressure overload is associated with differential molecular, structural and functional remodeling patterns in mice and patients with aortic stenosis. Cells 9, 833 (2020).
Savage, P. S., Campbell, P. C. & Adams, S. A. Gender differences in reverse cardiac remodeling following initiation of sacubitril/valsartan in heart failure with reduced ejection fraction: real world experience [abstract]. Eur. Heart J. 42, (Suppl. 1) ehab724.0900 (2021).
Ibrahim, N. E. et al. Sex-based differences in biomarkers, quality of life, and reverse cardiac remodeling in patients with heart failure with reduced ejection fraction treated with sacubitril/valsartan. J. Card. Fail. 26, S9 (2020).
Paolini, C. et al. Effects and clinical implications of sacubitril/valsartan on left ventricular reverse remodeling in patients affected by chronic heart failure: a 24-month follow-up. IJC Heart Vasc. 35, 100821 (2021).
Bella, J. N. et al. Sex-related difference in regression of left ventricular hypertrophy with antihypertensive treatment: the LIFE study. J. Hum. Hypertens. 18, 411–416 (2004).
Ruppert, M. et al. Sex similarities and differences in the reverse and anti-remodeling effect of pressure unloading therapy in a rat model of aortic banding and debanding. Am. J. Physiol. Heart Circ. Physiol. 323, H204–H222 (2022).
Barkhudaryan, A., Scherbakov, N., Springer, J. & Doehner, W. Cardiac muscle wasting in individuals with cancer cachexia. Esc. Heart Fail. 4, 458–467 (2017).
Vernice, N. A., Meydan, C., Afshinnekoo, E. & Mason, C. E. Long-term spaceflight and the cardiovascular system. Precis. Clin. Med. 3, 284–291 (2020).
Samarel, A. M., Parmacek, M. S., Magid, N. M., Decker, R. S. & Lesch, M. Protein synthesis and degradation during starvation-induced cardiac atrophy in rabbits. Circ. Res. 60, 933–941 (1987).
Belloum, Y., Rannou-Bekono, F. & Favier, F. B. Cancer-induced cardiac cachexia: pathogenesis and impact of physical activity (review). Oncol. Rep. 37, 2543–2552 (2017).
Inui, A. Cancer anorexia-cachexia syndrome: current issues in research and management. CA Cancer J. Clin. 52, 72–91 (2002).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Cosper, P. F. & Leinwand, L. A. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 71, 1710–1720 (2011).
Tian, M., Asp, M. L., Nishijima, Y. & Belury, M. A. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. Int. J. Oncol. 39, 1321–1326 (2011).
Springer, J. et al. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur. Heart J. 35, 932–941 (2014).
Manne, N. D. P. K. et al. Altered cardiac muscle mTOR regulation during the progression of cancer cachexia in the ApcMin/+ mouse. Int. J. Oncol. 42, 2134–2140 (2013).
Murphy, K. T. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. Am. J. Physiol. Heart Circ. Physiol. 310, H466–H477 (2016).
Willis, M. S. et al. Doxorubicin exposure causes subacute cardiac atrophy dependent on the striated muscle-specific ubiquitin ligase MuRF1. Circ. Heart Fail. 12, e005234 (2019).
MacNamara, J. P. et al. Cardiac effects of repeated weightlessness during extreme duration swimming compared with spaceflight. Circulation 143, 1533–1535 (2021).
Perhonen, M. A. et al. Cardiac atrophy after bed rest and spaceflight. J. Appl. Physiol. 91, 645–653 (2001).
Feger, B. J. et al. Microgravity induces proteomics changes involved in endoplasmic reticulum stress and mitochondrial protection. Sci. Rep. 6, 34091 (2016).
Liang, L. et al. Calpain activation mediates microgravity-induced myocardial abnormalities in mice via p38 and ERK1/2 MAPK pathways. J. Biol. Chem. 295, 16840–16851 (2020).
Walls, S. et al. Prolonged exposure to microgravity reduces cardiac contractility and initiates remodeling in Drosophila. Cell Rep. 33, 108445 (2020).
Kumar, A., Tahimic, C. G. T., Almeida, E. A. C. & Globus, R. K. Spaceflight modulates the expression of key oxidative stress and cell cycle related genes in heart. Int. J. Mol. Sci. 22, 9088 (2021).
Hu, C., Zhang, X., Teng, T., Ma, Z. G. & Tang, Q. Z. Cellular senescence in cardiovascular diseases: a systematic review. Aging Dis. 13, 103–128 (2022).
Baba, H. A. et al. Dynamic regulation of MEK/Erks and Akt/GSK-3β in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc. Res. 59, 390–399 (2003).
Gopinathannair, R. et al. Device therapy and arrhythmia management in left ventricular assist device recipients: a scientific statement from the American Heart Association. Circulation 139, E967–E989 (2019).
Jakovljevic, D. G. et al. Left ventricular assist device as a bridge to recovery for patients with advanced heart failure. J. Am. Coll. Cardiol. 69, 1924–1933 (2017).
Rodgers, B. D. & Ward, C. W. Myostatin/activin receptor ligands in muscle and the development status of attenuating drugs. Endocr. Rev. 43, 329–365 (2022).
Smith, R. C. et al. Inhibition of myostatin prevents microgravity-induced loss of skeletal muscle mass and strength. PLoS One 15, e0230818 (2020).
Loumaye, A. et al. Role of activin A and myostatin in human cancer cachexia. J. Clin. Endocrinol. Metab. 100, 2030–2038 (2015).
Allen, D. L., Cleary, A. S., Lindsay, S. F., Loh, A. S. & Reed, J. M. Myostatin expression is increased by food deprivation in a muscle-specific manner and contributes to muscle atrophy during prolonged food deprivation in mice. J. Appl. Physiol. 109, 692–701 (2010).
Han, H. Q., Zhou, X., Mitch, W. E. & Goldberg, A. L. Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 45, 2333–2347 (2013).
Reis Filho, J. R. A. R., Cardoso, J. N., Cardoso, C. M. R. & Pereira-Barretto, A. C. Reverse cardiac remodeling: a marker of better prognosis in heart failure. Arq. Bras. Cardiol. 104, 502–506 (2015).
Boulet, J. & Mehra, M. R. Left ventricular reverse remodeling in heart failure: remission to recovery. Struct. Heart 5, 466–481 (2021).
Picca, M., Bisceglia, J., Zocca, A. & Pelosi, G. Effects of enalapril and amlodipine on left ventricular hypertrophy and function in essential hypertension. Clin. Drug. Investig. 13, 29–35 (1997).
Greenberg, B. et al. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction results of the SOLVD echocardiography substudy. Circulation 91, 2573–2581 (1995).
Desai, A. S. et al. Effect of sacubitril-valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 322, 1077–1084 (2019).
Seeman, T. et al. Regression of left-ventricular hypertrophy in children and adolescents with hypertension during ramipril monotherapy. Am. J. Hypertens. 20, 990–996 (2007).
Park, K. et al. The impact of a dose of the angiotensin receptor blocker valsartan on post-myocardial infarction ventricular remodelling. Esc. Heart Fail. 5, 354–363 (2018).
Degirmenci, H. et al. Comparison of effects of nebivolol, carvedilol and irbesartan on left ventricular hypertrophy associated with hypertension. Eur. Rev. Med. Pharmacol. Sci. 18, 630–637 (2014).
Barrios, V. et al. Regression of left ventricular hypertrophy by a candesartan-based regimen in clinical practice. the VIPE study. J. Renin Angiotensin Aldosterone Syst. 7, 236–242 (2006).
Khan, M. S. et al. Reverse cardiac remodeling following initiation of sacubitril/valsartan in patients with heart failure with and without diabetes. JACC Heart Fail. 9, 137–145 (2021).
Maizels, L. et al. Characterization of heart failure patients with reverse left ventricular remodelling post-angiotensin receptor blockers/neprilysin inhibitors therapy. Esc. Heart Fail. 9, 1682–1688 (2022).
Chau, K. H. et al. Regression of left ventricular mass after transcatheter aortic valve replacement: the PARTNER trials and registries. J. Am. Coll. Cardiol. 75, 2446–2458 (2020).
Algahim, M. F. et al. Progressive regression of left ventricular hypertrophy two years after bariatric surgery. Am. J. Med. 123, 549–555 (2010).
Ikonomidis, I. et al. Weight loss after bariatric surgery improves aortic elastic properties and left ventricular function in individuals with morbid obesity: a 3-year follow-up study. J. Hypertens. 25, 439–447 (2007).
Weiss, R. J. & Bent, B. Diltiazem-induced left ventricular mass regression in hypertensive patients. J. Clin. Hypertens. 3, 135–143 (1987).
Awata, N. et al. Regression of left ventricular hypertrophy in hypertensive patients treated with metoprolol. Curr. Ther. Res. Clin. Exp. 50, 175–182 (1991).
Cherchi, A., Sau, F. & Seguro, C. Possible regression of left ventricular hypertrophy during antihypertensive treatment with diuretics and/or beta blockers. J. Clin. Hypertens. 3, 216–225 (1987).
Islim, I. F., Watson, R. D., Ihenacho, H. N. C., Ebanks, M. & Singh, S. P. Amlodipine: effective for treatment of mild to moderate essential hypertension and left ventricular hypertrophy. Cardiology 96, 10–18 (2001).
Rosendorff, C., Dubiel, R., Xu, J. & Chavanu, K. J. Comparison of olmesartan medoxomil versus amlodipine besylate on regression of ventricular and vascular hypertrophy. Am. J. Cardiol. 104, 359–365 (2009).
Gu, M. et al. Clinical outcome of cardiac resynchronization therapy in dilated-phase hypertrophic cardiomyopathy. J. Geriatr. Cardiol. 14, 238–244 (2017).
Sugano, A. et al. Optimal cut-off value of reverse remodeling to predict long-term outcome after cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J. Cardiol. 69, 456–461 (2017).
Ocaranza, M. P. et al. Reverse remodeling in human heart failure after cardiac resynchronization therapy is associated with reduced RHO-kinase activation. Front. Pharmacol. 12, 565724 (2021).
Solomon, S. D. et al. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation 122, 985–992 (2010).
St John Sutton, M. G. et al. Sustained reverse left ventricular structural remodeling with cardiac resynchronization at one year is a function of etiology: quantitative Doppler echocardiographic evidence from the Multicenter InSync Randomized Clinical Evaluation (MIRACLE). Circulation 113, 266–272 (2006).
Drakos, S. G. et al. Reverse electrophysiologic remodeling after cardiac mechanical unloading for end-stage nonischemic cardiomyopathy. Ann. Thorac. Surg. 91, 764–769 (2011).
Muthiah, K. et al. Longitudinal structural, functional, and cellular myocardial alterations with chronic centrifugal continuous-flow left ventricular assist device support. J. Heart Lung Transplant. 36, 722–731 (2017).
Gupta, A. et al. Effect of spironolactone on diastolic function in hypertensive left ventricular hypertrophy. J. Hum. Hypertens. 29, 241–246 (2015).
Ori, Y. et al. Regression of left ventricular hypertrophy in patients with primary aldosteronism/low-renin hypertension on low-dose spironolactone. Nephrol. Dial. Transplant. 28, 1787–1793 (2013).
Kosugi, D. et al. Beneficial effects of sodium glucose cotransporter 2 inhibitors on left ventricular mass in patients with diabetes mellitus. J. Diabetes 13, 847–856 (2021).
Rørth, R. et al. Comparison of BNP and NT-proBNP in patients with heart failure and reduced ejection fraction. Circ. Heart Fail. 2, e006541 (2020).
Ichiki, T., Huntley, B. K. & Burnett, J. C. BNP molecular forms and processing by the cardiac serine protease corin. Adv. Clin. Chem. 61, 1–31 (2013).
Sutanto, H., Dobrev, D. & Heijman, J. Angiotensin receptor-neprilysin inhibitor (ARNI) and cardiac arrhythmias. Int. J. Mol. Sci. 22, 8994 (2021).
McKie, P. M. & Burnett, J. C. NT-proBNP: the gold standard biomarker in heart failure. J. Am. Coll. Cardiol. 68, 2437–2439 (2016).
Pascual-Figal, D. et al. Sacubitril-valsartan, clinical benefits and related mechanisms of action in heart failure with reduced ejection fraction. A review. Front. Cardiovasc. Med. 8, 754499 (2021).
Acknowledgements
The authors acknowledge support from the National Institutes of Health (T32 HL007822 to T.G.M., T32 GM142607 to M.A.J., and R01HL117138 and R01GM029090 to L.A.L.).
Author information
Authors and Affiliations
Contributions
All the authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
L.A.L. is a co-founder of MyoKardia, acquired by Bristol Myers Squib, and is a paid member of their Scientific Advisory Board. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Junichi Sadoshima, Junjie Xiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martin, T.G., Juarros, M.A. & Leinwand, L.A. Regression of cardiac hypertrophy in health and disease: mechanisms and therapeutic potential. Nat Rev Cardiol 20, 347–363 (2023). https://doi.org/10.1038/s41569-022-00806-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00806-6
This article is cited by
-
Regression of left ventricular hypertrophy
Hypertension Research (2024)
-
Exercise-induced cardiac mitochondrial reorganization and enhancement in spontaneously hypertensive rats
Pflügers Archiv - European Journal of Physiology (2024)
-
Astragaloside IV derivative HHQ16 ameliorates infarction-induced hypertrophy and heart failure through degradation of lncRNA4012/9456
Signal Transduction and Targeted Therapy (2023)
-
Echocardiographic Phenotypes of Subclinical Organ Damage: Clinical and Prognostic Value in the General Population. Findings from the Pamela Study
High Blood Pressure & Cardiovascular Prevention (2023)