Abstract
Sirtuins are NAD+-dependent deacetylase and deacylase enzymes that control important cellular processes, including DNA damage repair, cellular metabolism, mitochondrial function and inflammation. Consequently, mammalian sirtuins are regarded as crucial regulators of cellular function and organism healthspan. Sirtuin activity and NAD+ levels decrease with age in many tissues, and reduced sirtuin expression is associated with several cardiovascular diseases. By contrast, increased sirtuin expression and activity slows disease progression and improves cardiovascular function in preclinical models and delays various features of cellular ageing. The potential cardiometabolic benefits of sirtuins have resulted in clinical trials with sirtuin-modulating agents; although expectations are high, these drugs have not yet been proven to improve healthspan. In this Review, we examine the role of sirtuins in atherosclerosis, summarize advances in the development of compounds that activate or inhibit sirtuin activity and critically evaluate the therapeutic potential of these agents.
Key points
-
Sirtuins protect against many processes associated with ageing and atherosclerosis, but only SIRT6 has been shown to extend lifespan in mice.
-
Natural compounds with sirtuin-modulating activity can delay or prevent the development of atherosclerosis in preclinical models, but have low bioavailability and off-target effects.
-
Small-molecule activators of sirtuins and NAD+ boosters have shown promising cardiometabolic effects in animal models and are well tolerated in healthy volunteers, but clinical trials showing prevention of atherosclerosis or clinical events are lacking.
-
Drug discovery has shifted from SIRT1 to SIRT6, because the lifespan-extending and chromatin-remodelling effects of SIRT6 make it an attractive therapeutic target.
-
Unravelling the multiple enzymatic activities of the sirtuins and their targets will facilitate drug discovery of specific sirtuin-activating compounds to prevent or treat atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
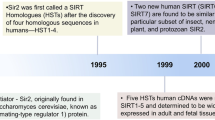
Imai, S., Armstrong, C. M., Kaeberlein, M. & Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403, 795–800 (2000).
Haigis, M. C. & Sinclair, D. A. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 5, 253–295 (2010).
Imai, S. & Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 24, 464–471 (2014).
Imai, S. I. & Guarente, L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech. Dis. 2, 16017 (2016).
Revollo, J. R., Grimm, A. A. & Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 279, 50754–50763 (2004).
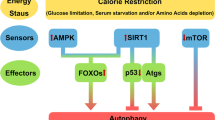
Guarente, L. Calorie restriction and sirtuins revisited. Genes Dev. 27, 2072–2085 (2013).
Kane, A. E. & Sinclair, D. A. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ. Res. 123, 868–885 (2018).
You, W. et al. Structural basis of sirtuin 6 activation by synthetic small molecules. Angew. Chem. Int. Ed. Engl. 56, 1007–1011 (2017).
You, W., Zheng, W., Weiss, S., Chua, K. F. & Steegborn, C. Structural basis for the activation and inhibition of sirtuin 6 by quercetin and its derivatives. Sci. Rep. 9, 19176 (2019).
Tenhunen, J. et al. Screening of SIRT6 inhibitors and activators: a novel activator has an impact on breast cancer cells. Biomed. Pharmacother. 138, 111452 (2021).
Huang, Z. et al. Identification of a cellularly active SIRT6 allosteric activator. Nat. Chem. Biol. 14, 1118–1126 (2018).
Guo, J. et al. Endothelial SIRT6 is vital to prevent hypertension and associated cardiorenal injury through targeting Nkx3.2-GATA5 signaling. Circ. Res. 124, 1448–1461 (2019).
Grootaert, M. O. J., Finigan, A., Figg, N. L., Uryga, A. K. & Bennett, M. R. SIRT6 protects smooth muscle cells from senescence and reduces atherosclerosis. Circ. Res. 128, 474–491 (2021).
Arsiwala, T. et al. Sirt6 deletion in bone marrow-derived cells increases atherosclerosis–central role of macrophage scavenger receptor 1. J. Mol. Cell Cardiol. 139, 24–32 (2020).
Zhang, X., Ameer, F. S., Azhar, G. & Wei, J. Y. Alternative splicing increases sirtuin gene family diversity and modulates their subcellular localization and function. Int. J. Mol. Sci. 22, 473 (2021).
Tanno, M., Sakamoto, J., Miura, T., Shimamoto, K. & Horio, Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 282, 6823–6832 (2007).
Wang, R. H. et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 14, 312–323 (2008).
Martinez-Pastor, B. & Mostoslavsky, R. Sirtuins, metabolism, and cancer. Front. Pharmacol. 3, 22 (2012).
Brunet, A. et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303, 2011–2015 (2004).
Houtkooper, R. H., Pirinen, E. & Auwerx, J. Sirtuins as regulators of metabolism and healthspan. Nat. Rev. Mol. Cell Biol. 13, 225–238 (2012).
Yeung, F. et al. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 (2004).
Michishita, E. et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452, 492–496 (2008).
Onn, L. et al. SIRT6 is a DNA double-strand break sensor. eLife 9, e51636 (2020).
Toiber, D. et al. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Mol. Cell 51, 454–468 (2013).
Hou, T. et al. SIRT6 coordinates with CHD4 to promote chromatin relaxation and DNA repair. Nucleic Acids Res. 48, 2982–3000 (2020).
Kanfi, Y. et al. The sirtuin SIRT6 regulates lifespan in male mice. Nature 483, 218–221 (2012).
Roichman, A. et al. Restoration of energy homeostasis by SIRT6 extends healthy lifespan. Nat. Commun. 12, 3208 (2021).
Tian, X. et al. SIRT6 is responsible for more efficient DNA double-strand break repair in long-lived species. Cell 177, 622–638 (2019).
Meng, F. et al. Synergy between SIRT1 and SIRT6 helps recognize DNA breaks and potentiates the DNA damage response and repair in humans and mice. eLife 9, e55828 (2020).
Mostoslavsky, R. et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124, 315–329 (2006).
Chang, A. R., Ferrer, C. M. & Mostoslavsky, R. SIRT6, a mammalian deacylase with multitasking abilities. Physiol. Rev. 100, 145–169 (2020).
Sociali, G. et al. SIRT6 deacetylase activity regulates NAMPT activity and NAD(P)(H) pools in cancer cells. FASEB J. 33, 3704–3717 (2019).
Pan, H. et al. SIRT6 safeguards human mesenchymal stem cells from oxidative stress by coactivating NRF2. Cell Res. 26, 190–205 (2016).
Rezazadeh, S. et al. SIRT6 promotes transcription of a subset of NRF2 targets by mono-ADP-ribosylating BAF170. Nucleic Acids Res. 47, 7914–7928 (2019).
Jiang, H. et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496, 110–113 (2013).
Ford, E. et al. Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 20, 1075–1080 (2006).
Vazquez, B. N. et al. SIRT7 promotes genome integrity and modulates non-homologous end joining DNA repair. EMBO J. 35, 1488–1503 (2016).
Li, L. et al. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat. Commun. 7, 12235 (2016).
Tang, M. et al. SIRT7-mediated ATM deacetylation is essential for its deactivation and DNA damage repair. Sci. Adv. 5, eaav1118 (2019).
Vakhrusheva, O. et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 102, 703–710 (2008).
Skoge, R. H., Dolle, C. & Ziegler, M. Regulation of SIRT2-dependent α-tubulin deacetylation by cellular NAD levels. DNA Repair. 23, 33–38 (2014).
Serrano, L. et al. The tumor suppressor SirT2 regulates cell cycle progression and genome stability by modulating the mitotic deposition of H4K20 methylation. Genes Dev. 27, 639–653 (2013).
Zhang, H., Head, P. E. & Yu, D. S. SIRT2 orchestrates the DNA damage response. Cell Cycle 15, 2089–2090 (2016).
Wang, F., Nguyen, M., Qin, F. X. & Tong, Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 6, 505–514 (2007).
Liu, G. et al. Loss of NAD-dependent protein deacetylase sirtuin-2 alters mitochondrial protein acetylation and dysregulates mitophagy. Antioxid. Redox Signal. 26, 849–863 (2017).
Lantier, L. et al. SIRT2 knockout exacerbates insulin resistance in high fat-fed mice. PLoS ONE 13, e0208634 (2018).
Chen, Y. et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 12, 534–541 (2011).
Sundaresan, N. R. et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest. 119, 2758–2771 (2009).
Someya, S. et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143, 802–812 (2010).
van de Ven, R. A. H., Santos, D. & Haigis, M. C. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol. Med. 23, 320–331 (2017).
Hirschey, M. D. et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 (2010).
Tseng, A. H., Shieh, S. S. & Wang, D. L. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic. Biol. Med. 63, 222–234 (2013).
Haigis, M. C. et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell 126, 941–954 (2006).
Laurent, G. et al. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell 50, 686–698 (2013).
Anderson, K. A. et al. SIRT4 Is a lysine deacylase that controls leucine metabolism and insulin secretion. Cell Metab. 25, 838–855 (2017).
Mathias, R. A. et al. Sirtuin 4 is a lipoamidase regulating pyruvate dehydrogenase complex activity. Cell 159, 1615–1625 (2014).
Jeong, S. M. et al. SIRT4 has tumor-suppressive activity and regulates the cellular metabolic response to DNA damage by inhibiting mitochondrial glutamine metabolism. Cancer Cell 23, 450–463 (2013).
Laurent, G. et al. SIRT4 represses peroxisome proliferator-activated receptor α activity to suppress hepatic fat oxidation. Mol. Cell. Biol. 33, 4552–4561 (2013).
Luo, Y. X. et al. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur. Heart J. 38, 1389–1398 (2017).
Du, J. et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science 334, 806–809 (2011).
Zhou, L. et al. SIRT5 promotes IDH2 desuccinylation and G6PD deglutarylation to enhance cellular antioxidant defense. EMBO Rep. 17, 811–822 (2016).
Balestrieri, M. L. et al. Sirtuin 6 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of incretin treatment. Diabetes 64, 1395–1406 (2015).
Donato, A. J. et al. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J. Physiol. 589, 4545–4554 (2011).
Gao, P. et al. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J. Mol. Med. 92, 347–357 (2014).
Gorenne, I. et al. Vascular smooth muscle cell sirtuin 1 protects against DNA damage and inhibits atherosclerosis. Circulation 127, 386–396 (2013).
Orimo, M. et al. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler. Thromb. Vasc. Biol. 29, 889–894 (2009).
Mattagajasingh, I. et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl Acad. Sci. USA 104, 14855–14860 (2007).
Zhang, Q. J. et al. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc. Res. 80, 191–199 (2008).
Guo, Y. et al. Endothelial SIRT1 prevents age-induced impairment of vasodilator responses by enhancing the expression and activity of soluble guanylyl cyclase in smooth muscle cells. Cardiovasc. Res. 115, 678–690 (2019).
Ota, H. et al. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J. Mol. Cell. Cardiol. 43, 571–579 (2007).
Stein, S. et al. SIRT1 reduces endothelial activation without affecting vascular function in ApoE-/- mice. Aging 2, 353–360 (2010).
Zu, Y. et al. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ. Res. 106, 1384–1393 (2010).
Breitenstein, A. et al. Sirt1 inhibition promotes in vivo arterial thrombosis and tissue factor expression in stimulated cells. Cardiovasc. Res. 89, 464–472 (2011).
Xu, S. et al. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. Aging 8, 1064–1082 (2016).
Liu, Z., Wang, J., Huang, X., Li, Z. & Liu, P. Deletion of sirtuin 6 accelerates endothelial dysfunction and atherosclerosis in apolipoprotein E-deficient mice. Transl. Res. 172, 18–29 (2016).
Lee, O. H. et al. Sirtuin 6 deficiency induces endothelial cell senescence via downregulation of forkhead box M1 expression. Aging 12, 20946–20967 (2020).
Cardus, A., Uryga, A. K., Walters, G. & Erusalimsky, J. D. SIRT6 protects human endothelial cells from DNA damage, telomere dysfunction, and senescence. Cardiovasc. Res. 97, 571–579 (2013).
He, Y. et al. SIRT6 inhibits inflammatory response through regulation of NRF2 in vascular endothelial cells. Int. Immunopharmacol. 99, 107926 (2021).
Wang, T. et al. Sirt6 stabilizes atherosclerosis plaques by promoting macrophage autophagy and reducing contact with endothelial cells. Biochem. Cell Biol. 98, 120–129 (2020).
Yang, Z. et al. SIRT6 promotes angiogenesis and hemorrhage of carotid plaque via regulating HIF-1α and reactive oxygen species. Cell Death Dis. 12, 77 (2021).
Zhong, L. et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell 140, 280–293 (2010).
Winnik, S. et al. Mild endothelial dysfunction in Sirt3 knockout mice fed a high-cholesterol diet: protective role of a novel C/EBP-β-dependent feedback regulation of SOD2. Basic Res. Cardiol. 111, 33 (2016).
Winnik, S. et al. Deletion of Sirt3 does not affect atherosclerosis but accelerates weight gain and impairs rapid metabolic adaptation in LDL receptor knockout mice: implications for cardiovascular risk factor development. Basic Res. Cardiol. 109, 399 (2014).
Dikalova, A. E. et al. Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while Sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ. Res. 126, 439–452 (2020).
Wang, J. et al. Vascular smooth muscle cell senescence promotes atherosclerosis and features of plaque vulnerability. Circulation 132, 1909–1919 (2015).
Ho, C., van der Veer, E., Akawi, O. & Pickering, J. G. SIRT1 markedly extends replicative lifespan if the NAD+ salvage pathway is enhanced. FEBS Lett. 583, 3081–3085 (2009).
van der Veer, E. et al. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J. Biol. Chem. 282, 10841–10845 (2007).
Li, L. et al. SIRT1 acts as a modulator of neointima formation following vascular injury in mice. Circ. Res. 108, 1180–1189 (2011).
Fry, J. L. et al. Vascular smooth muscle sirtuin-1 protects against diet-induced aortic stiffness. Hypertension 68, 775–784 (2016).
Grootaert, M. O. J. & Bennett, M. R. Vascular smooth muscle cells in atherosclerosis: time for a reassessment. Cardiovasc. Res. 117, 2326–2339 (2021).
Ma, S. et al. Precise theranostic nanomedicines for inhibiting vulnerable atherosclerotic plaque progression through regulation of vascular smooth muscle cell phenotype switching. Theranostics 8, 3693–3706 (2018).
Wan, W. et al. PDGFR-β modulates vascular smooth muscle cell phenotype via IRF-9/SIRT-1/NF-κB pathway in subarachnoid hemorrhage rats. J. Cereb. Blood Flow. Metab. 39, 1369–1380 (2019).
Bartoli-Leonard, F. et al. Loss of SIRT1 in diabetes accelerates DNA damage-induced vascular calcification. Cardiovasc. Res. 117, 836–849 (2021).
Qiu, L., Yi, S., Yu, T. & Hao, Y. Sirt3 protects against thoracic aortic dissection formation by reducing reactive oxygen species, vascular inflammation, and apoptosis of smooth muscle cells. Front. Cardiovasc. Med. 8, 675647 (2021).
Yang, X. et al. SIRT1 inhibition promotes atherosclerosis through impaired autophagy. Oncotarget 8, 51447–51461 (2017).
He, J. et al. SIRT6 reduces macrophage foam cell formation by inducing autophagy and cholesterol efflux under ox-LDL condition. FEBS J. 284, 1324–1337 (2017).
Lee, S. J., Baek, S. E., Jang, M. A. & Kim, C. D. SIRT1 inhibits monocyte adhesion to the vascular endothelium by suppressing Mac-1 expression on monocytes. Exp. Mol. Med. 51, 1–12 (2019).
Stein, S. et al. SIRT1 decreases Lox-1-mediated foam cell formation in atherogenesis. Eur. Heart J. 31, 2301–2309 (2010).
Schug, T. T. et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell Biol. 30, 4712–4721 (2010).
Lee, Y. et al. Myeloid sirtuin 6 deficiency causes insulin resistance in high-fat diet-fed mice by eliciting macrophage polarization toward an M1 phenotype. Diabetes 66, 2659–2668 (2017).
Zhang, B., Ma, Y. & Xiang, C. SIRT2 decreases atherosclerotic plaque formation in low-density lipoprotein receptor-deficient mice by modulating macrophage polarization. Biomed. Pharmacother. 97, 1238–1242 (2018).
Wei, T. et al. SIRT3 (Sirtuin-3) prevents Ang II (angiotensin II)-induced macrophage metabolic switch improving perivascular adipose tissue function. Arterioscler. Thromb. Vasc. Biol. 41, 714–730 (2021).
Ding, Y. et al. Protective role of sirtuin3 against oxidative stress and NLRP3 inflammasome in cholesterol accumulation and foam cell formation of macrophages with ox-LDL-stimulation. Biochem. Pharmacol. 192, 114665 (2021).
Liu, P. et al. Sirtuin 3-induced macrophage autophagy in regulating NLRP3 inflammasome activation. Biochim. Biophys. Acta Mol. Basis Dis. 1864, 764–777 (2018).
Legutko, A. et al. Sirtuin 1 promotes Th2 responses and airway allergy by repressing peroxisome proliferator-activated receptor-γ activity in dendritic cells. J. Immunol. 187, 4517–4529 (2011).
Audrito, V. et al. NAD-biosynthetic and consuming enzymes as central players of metabolic regulation of innate and adaptive immune responses in cancer. Front. Immunol. 10, 1720 (2019).
Bonkowski, M. S. & Sinclair, D. A. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 17, 679–690 (2016).
Howitz, K. T. et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425, 191–196 (2003).
Iside, C., Scafuro, M., Nebbioso, A. & Altucci, L. SIRT1 activation by natural phytochemicals: an overview. Front. Pharmacol. 11, 1225 (2020).
Yang, H. et al. Design and synthesis of compounds that extend yeast replicative lifespan. Aging Cell 6, 35–43 (2007).
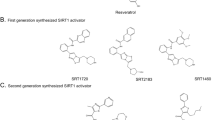
Milne, J. C. et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 450, 712–716 (2007).
Hubbard, B. P. et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science 339, 1216–1219 (2013).
Sinclair, D. A. & Guarente, L. Small-molecule allosteric activators of sirtuins. Annu. Rev. Pharmacol. Toxicol. 54, 363–380 (2014).
Dai, H., Sinclair, D. A., Ellis, J. L. & Steegborn, C. Sirtuin activators and inhibitors: promises, achievements, and challenges. Pharmacol. Ther. 188, 140–154 (2018).
Klein, M. A. & Denu, J. M. Biological and catalytic functions of sirtuin 6 as targets for small-molecule modulators. J. Biol. Chem. 295, 11021–11041 (2020).
Baur, J. A. et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444, 337–342 (2006).
Pearson, K. J. et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8, 157–168 (2008).
Minor, R. K. et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 1, 70 (2011).
Feige, J. N. et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 8, 347–358 (2008).
Norata, G. D. et al. Anti-inflammatory and anti-atherogenic effects of cathechin, caffeic acid and trans-resveratrol in apolipoprotein E deficient mice. Atherosclerosis 191, 265–271 (2007).
Do, G. M. et al. Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem. Biophys. Res. Commun. 374, 55–59 (2008).
Li, J. et al. Resveratrol improves endothelial dysfunction and attenuates atherogenesis in apolipoprotein E-deficient mice. J. Nutr. Biochem. 67, 63–71 (2019).
Seo, Y. et al. Antiatherogenic effect of resveratrol attributed to decreased expression of ICAM-1 (intercellular adhesion molecule-1). Arterioscler. Thromb. Vasc. Biol. 39, 675–684 (2019).
Berbee, J. F. et al. Resveratrol protects against atherosclerosis, but does not add to the antiatherogenic effect of atorvastatin, in APOE*3-Leiden.CETP mice. J. Nutr. Biochem. 24, 1423–1430 (2013).
Chen, Y. X., Zhang, M., Cai, Y., Zhao, Q. & Dai, W. The Sirt1 activator SRT1720 attenuates angiotensin II-induced atherosclerosis in apoE-/- mice through inhibiting vascular inflammatory response. Biochem. Biophys. Res. Commun. 465, 732–738 (2015).
Gano, L. B. et al. The SIRT1 activator SRT1720 reverses vascular endothelial dysfunction, excessive superoxide production, and inflammation with aging in mice. Am. J. Physiol. Heart Circ. Physiol. 307, H1754–H1763 (2014).
Feng, T. et al. SIRT1 activator E1231 protects from experimental atherosclerosis and lowers plasma cholesterol and triglycerides by enhancing ABCA1 expression. Atherosclerosis 274, 172–181 (2018).
Miranda, M. X. et al. The Sirt1 activator SRT3025 provides atheroprotection in Apoe-/- mice by reducing hepatic Pcsk9 secretion and enhancing Ldlr expression. Eur. Heart J. 36, 51–59 (2015).
Gertz, M. et al. A molecular mechanism for direct sirtuin activation by resveratrol. PLoS ONE 7, e49761 (2012).
Baur, J. A. & Sinclair, D. A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506 (2006).
Park, S. J. et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433 (2012).
Huang, P. et al. A critical role of nicotinamide phosphoribosyltransferase in human telomerase reverse transcriptase induction by resveratrol in aortic smooth muscle cells. Oncotarget 6, 10812–10824 (2015).
You, W. & Steegborn, C. Binding site for activator MDL-801 on SIRT6. Nat. Chem. Biol. 17, 519–521 (2021).
Donmez, G. & Outeiro, T. F. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol. Med. 5, 344–352 (2013).
Heltweg, B. et al. Antitumor activity of a small-molecule inhibitor of human silent information regulator 2 enzymes. Cancer Res. 66, 4368–4377 (2006).
Sociali, G. et al. Quinazolinedione SIRT6 inhibitors sensitize cancer cells to chemotherapeutics. Eur. J. Med. Chem. 102, 530–539 (2015).
Damonte, P. et al. SIRT6 inhibitors with salicylate-like structure show immunosuppressive and chemosensitizing effects. Bioorg. Med. Chem. 25, 5849–5858 (2017).
Ferrara, G. et al. Sirt6 inhibition delays the onset of experimental autoimmune encephalomyelitis by reducing dendritic cell migration. J. Neuroinflammation 17, 228 (2020).
Mendez-Lara, K. A. et al. Nicotinamide prevents apolipoprotein B-containing lipoprotein oxidation, inflammation and atherosclerosis in apolipoprotein E-deficient mice. Antioxidants 9, 1162 (2020).
de Picciotto, N. E. et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell 15, 522–530 (2016).
Mills, K. F. et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 24, 795–806 (2016).
Kuhnast, S. et al. Niacin reduces atherosclerosis development in APOE*3Leiden.CETP mice mainly by reducing non-HDL-cholesterol. PLoS ONE 8, e66467 (2013).
Canto, C. et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 15, 838–847 (2012).
Gardell, S. J. et al. Boosting NAD+ with a small molecule that activates NAMPT. Nat. Commun. 10, 3241 (2019).
Camacho-Pereira, J. et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 23, 1127–1139 (2016).
Gan, L. et al. CD38 deficiency alleviates Ang II-induced vascular remodeling by inhibiting small extracellular vesicle-mediated vascular smooth muscle cell senescence in mice. Signal. Transduct. Target. Ther. 6, 223 (2021).
Henning, R. J., Bourgeois, M. & Harbison, R. D. Poly (ADP-ribose) polymerase (PARP) and PARP inhibitors: mechanisms of action and role in cardiovascular disorders. Cardiovasc. Toxicol. 18, 493–506 (2018).
Berman, A. Y., Motechin, R. A., Wiesenfeld, M. Y. & Holz, M. K. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis. Oncol. 1, 35 (2017).
Magyar, K. et al. Cardioprotection by resveratrol: a human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 50, 179–187 (2012).
Agarwal, B. et al. Resveratrol for primary prevention of atherosclerosis: clinical trial evidence for improved gene expression in vascular endothelium. Int. J. Cardiol. 166, 246–248 (2013).
Tome-Carneiro, J. et al. One-year consumption of a grape nutraceutical containing resveratrol improves the inflammatory and fibrinolytic status of patients in primary prevention of cardiovascular disease. Am. J. Cardiol. 110, 356–363 (2012).
Hoffmann, E. et al. Pharmacokinetics and tolerability of SRT2104, a first-in-class small molecule activator of SIRT1, after single and repeated oral administration in man. Br. J. Clin. Pharmacol. 75, 186–196 (2013).
Libri, V. et al. A pilot randomized, placebo controlled, double blind phase I trial of the novel SIRT1 activator SRT2104 in elderly volunteers. PLoS ONE 7, e51395 (2012).
Baksi, A. et al. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br. J. Clin. Pharmacol. 78, 69–77 (2014).
Venkatasubramanian, S. et al. Effects of the small molecule SIRT1 activator, SRT2104 on arterial stiffness in otherwise healthy cigarette smokers and subjects with type 2 diabetes mellitus. Open. Heart 3, e000402 (2016).
Wiewel, M. A. et al. SRT2379, a small-molecule SIRT1 activator, fails to reduce cytokine release in a human endotoxemia model. Crit. Care 17 (Suppl 4), P8 (2013).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01340911 (2017).
D’Andrea, E., Hey, S. P., Ramirez, C. L. & Kesselheim, A. S. Assessment of the role of niacin in managing cardiovascular disease outcomes: a systematic review and meta-analysis. JAMA Netw. Open 2, e192224 (2019).
Investigators, A.-H. et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
Martens, C. R. et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults. Nat. Commun. 9, 1286 (2018).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT03821623 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04040959 (2022).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04112043 (2021).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT04903210 (2022).
Zwaans, B. M. & Lombard, D. B. Interplay between sirtuins, MYC and hypoxia-inducible factor in cancer-associated metabolic reprogramming. Dis. Model. Mech. 7, 1023–1032 (2014).
Yang, Y. et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic. Biol. Med. 65, 667–679 (2013).
Singh, L., Sharma, S., Xu, S., Tewari, D. & Fang, J. Curcumin as a natural remedy for atherosclerosis: a pharmacological review. Molecules 26, 4036 (2021).
Zhang, F., Feng, J., Zhang, J., Kang, X. & Qian, D. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 20, 280 (2020).
Deng, Q., Li, X. X., Fang, Y., Chen, X. & Xue, J. Therapeutic potential of quercetin as an antiatherosclerotic agent in atherosclerotic cardiovascular disease: a review. Evid. Based Complement. Altern. Med. 2020, 5926381 (2020).
Chuengsamarn, S., Rattanamongkolgul, S., Phonrat, B., Tungtrongchitr, R. & Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J. Nutr. Biochem. 25, 144–150 (2014).
Na, L. X. et al. Curcuminoids exert glucose-lowering effect in type 2 diabetes by decreasing serum free fatty acids: a double-blind, placebo-controlled trial. Mol. Nutr. Food Res. 57, 1569–1577 (2013).
Takahashi, M. et al. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int. J. Sports Med. 35, 469–475 (2014).
Zahedi, M., Ghiasvand, R., Feizi, A., Asgari, G. & Darvish, L. Does quercetin improve cardiovascular risk factors and inflammatory biomarkers in women with type 2 diabetes: a double-blind randomized controlled clinical trial. Int. J. Prev. Med. 4, 777–785 (2013).
Venkatasubramanian, S. et al. Cardiovascular effects of a novel SIRT1 activator, SRT2104, in otherwise healthy cigarette smokers. J. Am. Heart Assoc. 2, e000042 (2013).
Kiss, T. et al. Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Geroscience 41, 419–439 (2019).
Ruparelia, N., Digby, J. E. & Choudhury, R. P. Effects of niacin on atherosclerosis and vascular function. Curr. Opin. Cardiol. 26, 66–70 (2011).
Acknowledgements
The authors are funded by British Heart Foundation (BHF) grants RG/13/14/30314, RG/20/2/34763, PG/6/24/32090, PG/16/11/32021, PG/13/14/30314 and CH/2000003, the National Institute of Health Research (NIHR) Cambridge Biomedical Research Centre, NIHR Senior Investigator NF-SI-0616-10036, and the BHF Centre for Research Excellence RE/18/1/34212. The authors thank A. K. Uryga (NovoNordisk) for careful reviewing of our manuscript before submission.
Author information
Authors and Affiliations
Contributions
M.O.J.G. researched data for the article. Both authors discussed its content and wrote the manuscript. M.R.B. reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Cao Feng, Hua Li and Nagalingam Sundaresan for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Apoptosis
-
A distinctive and important mode of ‘programmed’ cell death, characterized by cell shrinkage, chromatin condensation, DNA fragmentation and cell death.
- Autophagy
-
A natural process in which unnecessary or damaged cellular components are removed by intrinsic mechanisms, thereby balancing energy sources at crucial times in development and in response to cellular stress.
- Cell senescence
-
A stable cell cycle arrest that can be triggered in normal cells in response to intrinsic or extrinsic stimuli, particularly after DNA damage.
- DNA damage response
-
(DDR). A complex network of genes and intracellular signalling pathways responsible for sensing and responding to DNA damage, regulating DNA repair, cell cycle regulation, replication stress responses and apoptosis.
- Chromatin remodelling
-
Dynamic modification of the chromatin architecture to allow condensed genomic DNA to access the regulatory transcription machinery proteins, thereby controlling gene expression.
Rights and permissions
About this article
Cite this article
Grootaert, M.O.J., Bennett, M.R. Sirtuins in atherosclerosis: guardians of healthspan and therapeutic targets. Nat Rev Cardiol 19, 668–683 (2022). https://doi.org/10.1038/s41569-022-00685-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-022-00685-x
This article is cited by
-
Sirtuins in kidney diseases: potential mechanism and therapeutic targets
Cell Communication and Signaling (2024)
-
DDX3X interacts with SIRT7 to promote PD-L1 expression to facilitate PDAC progression
Oncogenesis (2024)
-
Pathogenetic mechanisms and treatment targets in cerebral malaria
Nature Reviews Neurology (2023)
-
SIRT4 in ageing
Biogerontology (2023)
-
Platelet factor 4 (PF4) induces cluster of differentiation 40 (CD40) expression in human aortic endothelial cells (HAECs) through the SIRT1/NF-κB/p65 signaling pathway
In Vitro Cellular & Developmental Biology - Animal (2023)