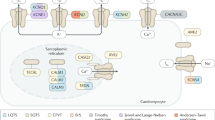
Abstract
A genetic risk of sudden cardiac arrest and sudden death due to an arrhythmic cause, known as sudden cardiac death (SCD), has become apparent from epidemiological studies in the general population and in patients with ischaemic heart disease. However, genetic susceptibility to sudden death is greatest in young people and is associated with uncommon, monogenic forms of heart disease. Despite comprehensive pathology and genetic evaluations, SCD remains unexplained in a proportion of young people and is termed sudden arrhythmic death syndrome, which poses challenges to the identification of relatives from affected families who might be at risk of SCD. In this Review, we assess the current understanding of the epidemiology and causes of SCD and evaluate both the monogenic and the polygenic contributions to the risk of SCD in the young and SCD associated with drug therapy. Finally, we analyse the potential clinical role of genomic testing in the prevention of SCD in the general population.
Key points
-
Sudden cardiac death (SCD) is a leading cause of death in Western countries, and a genetic risk of SCD is well established.
-
Rare variants in genes encoding proteins linked with cardiac structure or electrical activity have been associated with the risk of SCD in the young (aged <35 years).
-
Common genetic variants in the general population are increasingly recognized to contribute to the phenotypic expression of cardiac diseases and the risk of SCD.
-
A better understanding of the influence of genomic variation on the individual risk of SCD is needed to implement clinical strategies, such as polygenic risk scores, to predict and prevent SCD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Al-Khatib, S. M. et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardology/American Heart Association Task Force on Clinical Practice Guidelines and the Herat Rhythm Society. Circulation 138, e272–e391 (2018).
Priori, S. G. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 36, 2793–2867 (2015).
Myerburg, R. J. & Castellanos, A. in Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine Ch. 42 (eds Zipes, D. P. et al.) 865–908 (Elsevier, 2005).
Spooner, P. M. et al. Sudden cardiac death, genes, and arrhythmogenesis: consideration of new population and mechanistic approaches from a National Heart, Lung, and Blood Institute workshop, part I. Circulation 103, 2361–2364 (2001).
Priori, S. G. et al. Task Force on Sudden Cardiac Death of the European Society of Cardiology. Eur. Heart J. 22, 1374–1450 (2001).
Tseng, Z. H. et al. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation 137, 2689–2700 (2018).
Bjune, T. et al. Post-mortem toxicology in young sudden cardiac death victims: a nationwide cohort study. Europace 20, 614–621 (2018).
Finocchiaro, G. et al. Obesity and sudden cardiac death in the young: clinical and pathological insights from a large national registry. Eur. J. Prev. Cardiol. 25, 395–401 (2018).
Spooner, P. M. Sudden cardiac death: influence of diabetes. Diabetes Obes. Metab. 10, 523–532 (2008).
Lynge, T. H. et al. Sudden cardiac death among persons with diabetes aged 1-49 years: a 10-year nationwide study of 14 294 deaths in Denmark. Eur. Heart J. 41, 2699–2706 (2020).
Yankelson, L. et al. Life-threatening events during endurance sports: is heat stroke more prevalent than arrhythmic death? J. Am. Coll. Cardiol. 64, 463–469 (2014).
Blom, M. T. et al. Improved survival after out-of-hospital cardiac arrest and use of automated external defibrillators. Circulation 130, 1868–1875 (2014).
Kong, M. H. et al. Systematic review of the incidence of sudden cardiac death in the United States. J. Am. Coll. Cardiol. 57, 794–801 (2011).
De Vreede-Swagemakers, J. J. M. et al. Out-of-hospital cardiac arrest in the 1990s: a population-based study in the Maastricht area on incidence, characteristics and survival. J. Am. Coll. Cardiol. 30, 1500–1505 (1997).
Driscoll, D. J. & Edwards, W. D. Sudden unexpected death in children and adolescents. J. Am. Coll. Cardiol. 5, 118B–121B (1985).
Meyer, L. et al. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age: a 30-year review. Circulation 126, 1363–1372 (2012).
Ackerman, M., Atkins, D. L. & Triedman, J. K. Sudden cardiac death in the young. Circulation 133, 1006–1026 (2016).
Winkel, B. G. et al. Nationwide study of sudden cardiac death in persons aged 1-35 years. Eur. Heart J. 32, 983–990 (2011).
Bowker, T. J. et al. Sudden, unexpected cardiac or unexplained death in England: a national survey. QJM 96, 269–279 (2003).
Huikuri, H. V., Castellanos, A. & Myerburg, R. J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 345, 1473–1482 (2001).
Bagnall, R. D. et al. A prospective study of sudden cardiac death among children and young adults. N. Engl. J. Med. 374, 2441–2452 (2016).
Priori, S. G. et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10, 1932–1963 (2013).
Friedlander, Y. et al. Family history as a risk factor for primary cardiac arrest. Circulation 97, 155–160 (1998).
Friedlander, Y. et al. Sudden death and myocardial infarction in first degree relatives as predictors of primary cardiac arrest. Atherosclerosis 162, 211–216 (2002).
Jouven, X., Desnos, M., Guerot, C. & Ducimetière, P. Predicting sudden death in the population: the Paris prospective study I. Circulation 99, 1978–1983 (1999).
Kaikkonen, K. S., Kortelainen, M. L., Linna, E. & Huikuri, H. V. Family history and the risk of sudden cardiac death as a manifestation of an acute coronary event. Circulation 114, 1462–1467 (2006).
Dekker, L. R. C. et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case-control study in acute myocardial infarction patients. Circulation 114, 1140–1145 (2006).
Corrado, D., Basso, C. & Thiene, G. Sudden cardiac death in young people with apparently normal heart. Cardiovasc. Res. 50, 399–408 (2001).
Papadakis, M. et al. The magnitude of sudden cardiac death in the young: a death certificate-based review in England and Wales. Europace 11, 1353–1358 (2009).
Pilmer, C. M., Kirsh, J. A., Hildebrandt, D., Krahn, A. D. & Gow, R. M. Sudden cardiac death in children and adolescents between 1 and 19 years of age. Heart Rhythm 11, 239–245 (2014).
Lahrouchi, N. et al. The yield of postmortem genetic testing in sudden death cases with structural findings at autopsy. Eur. J. Hum. Genet. 28, 17–22 (2020).
Nunn, L. M. et al. Diagnostic yield of molecular autopsy in patients with sudden arrhythmic death syndrome using targeted exome sequencing. Europace 18, 888–896 (2016).
Kumar, S. et al. Familial cardiological and targeted genetic evaluation: low yield in sudden unexplained death and high yield in unexplained cardiac arrest syndromes. Heart Rhythm 10, 1653–1660 (2013).
Hansen, B. L. et al. Diagnostic yield in victims of sudden cardiac death and their relatives. Europace 22, 964–971 (2020).
Basso, C. et al. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 471, 691–705 (2017).
De Noronha, S. V. et al. The importance of specialist cardiac histopathological examination in the investigation of young sudden cardiac deaths. Europace 16, 899–907 (2014).
Stiles, M. K. et al. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm 18, e1–e50 (2021).
Schwartz, P. J. et al. Inherited cardiac arrhythmias. Nat. Rev. Dis. Prim. 6, 58 (2020).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Whiffin, N. et al. Using high-resolution variant frequencies to empower clinical genome interpretation. Genet. Med. 19, 1151–1158 (2017).
Behr, E. et al. Cardiological assessment of first-degree relatives in sudden arrhythmic death syndrome. Lancet 362, 1457–1459 (2003).
Behr, E. R. et al. Sudden arrhythmic death syndrome: familial evaluation identifies inheritable heart disease in the majority of families. Eur. Heart J. 29, 1670–1680 (2008).
Papadakis, M. et al. The diagnostic yield of Brugada syndrome after sudden death with normal autopsy. J. Am. Coll. Cardiol. 71, 1204–1214 (2018).
Ackerman, M. J., Tester, D. J. & Driscoll, D. J. Molecular autopsy of sudden unexplained death in the young. Am. J. Forensic Med. Pathol. 22, 105–111 (2001).
Ackerman, M. J., Tester, D. J., Porter, J. & Edwards, W. D. Molecular diagnosis of the inherited long-QT syndrome in a woman who died after near-drowning. N. Engl. J. Med. 341, 1121–1125 (1999).
Bagnall, R. D., Das, K. J., Duflou, J. & Semsarian, C. Exome analysis-based molecular autopsy in cases of sudden unexplained death in the young. Heart Rhythm 11, 655–662 (2014).
Hertz, C. L. et al. Next-generation sequencing of 100 candidate genes in young victims of suspected sudden cardiac death with structural abnormalities of the heart. Int. J. Leg. Med. 130, 91–102 (2016).
Christiansen, S. L. et al. Genetic investigation of 100 heart genes in sudden unexplained death victims in a forensic setting. Eur. J. Hum. Genet. 24, 1797–1802 (2016).
Lahrouchi, N. et al. Utility of post-mortem genetic testing in cases of sudden arrhythmic death syndrome. J. Am. Coll. Cardiol. 69, 2134–2145 (2017).
Tester, D. J., Medeiros-Domingo, A., Will, M. L., Haglund, C. M. & Ackerman, M. J. Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin. Proc. 87, 524–539 (2012).
Hofman, N. et al. Contribution of inherited heart disease to sudden cardiac death in childhood. Pediatrics 120, e967–e973 (2007).
van der Werf, C. et al. Diagnostic yield in sudden unexplained death and aborted cardiac arrest in the young: the experience of a tertiary referral center in the Netherlands. Heart Rhythm 7, 1383–1389 (2010).
Tester, D. J. et al. Cardiac genetic predisposition in sudden infant death syndrome. J. Am. Coll. Cardiol. 71, 1217–1227 (2018).
Shanks, G. W., Tester, D. J., Nishtala, S., Evans, J. M. & Ackerman, M. J. Genomic triangulation and coverage analysis in whole-exome sequencing-based molecular autopsies. Circ. Cardiovasc. Genet. 10, e001828 (2017).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Walsh, R. et al. Enhancing rare variant interpretation in inherited arrhythmias through quantitative analysis of consortium disease cohorts and population controls. Genet. Med. 23, 47–58 (2021).
Fellmann, F. et al. European recommendations integrating genetic testing into multidisciplinary management of sudden cardiac death. Eur. J. Hum. Genet. 27, 1763–1773 (2019).
Krahn, A. D. et al. Systematic assessment of patients with unexplained cardiac arrest: cardiac arrest survivors with preserved ejection fraction registry (CASPER). Circulation 120, 278–285 (2009).
Vittoria Matassini, M. et al. Evolution of clinical diagnosis in patients presenting with unexplained cardiac arrest or syncope due to polymorphic ventricular tachycardia. Heart Rhythm 11, 274–281 (2014).
Visser, M. et al. Long-term outcome of patients initially diagnosed with idiopathic ventricular fibrillation. Circ. Arrhythm. Electrophysiol. 9, e004258 (2016).
Mellor, G. et al. Genetic testing in the evaluation of unexplained cardiac arrest: from the CASPER (Cardiac Arrest Survivors with Preserved Ejection Fraction Registry). Circ. Cardiovasc. Genet. 10, e001686 (2017).
Visser, M. et al. Next-generation sequencing of a large gene panel in patients initially diagnosed with idiopathic ventricular fibrillation. Heart Rhythm 14, 1035–1040 (2017).
Leinonen, J. T. et al. The genetics underlying idiopathic ventricular fibrillation: a special role for catecholaminergic polymorphic ventricular tachycardia? Int. J. Cardiol. 250, 139–145 (2018).
Giudicessi, J. R. & Ackerman, M. J. Role of genetic heart disease in sentinel sudden cardiac arrest survivors across the age spectrum. Int. J. Cardiol. 270, 214–220 (2018).
Asatryan, B. et al. Usefulness of genetic testing in sudden cardiac arrest survivors with or without previous clinical evidence of heart disease. Am. J. Cardiol. 123, 2031–2038 (2019).
Isbister, J. C. et al. “Concealed cardiomyopathy” as a cause of previously unexplained sudden cardiac arrest. Int. J. Cardiol. 324, 96–101 (2021).
Ackerman, M. J. et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm 8, 1308–1339 (2011).
Viskin, S. & Belhassen, B. Idiopathic ventricular fibrillation. Am. Heart J. 120, 661–671 (1990).
Haïssaguerre, M. et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation 106, 962–967 (2002).
Noda, T. et al. Malignant entity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract. J. Am. Coll. Cardiol. 46, 1288–1294 (2005).
Alders, M. et al. Haplotype-sharing analysis implicates chromosome 7q36 harboring DPP6 in familial idiopathic ventricular fibrillation. Am. J. Hum. Genet. 84, 468–476 (2009).
Sande, J. N. T. et al. Detailed characterization of familial idiopathic ventricular fibrillation linked to the DPP6 locus. Heart Rhythm 13, 905–912 (2016).
Blom, M. T. et al. Genetic, clinical and pharmacological determinants of out-of-hospital cardiac arrest: rationale and outline of the AmsteRdam Resuscitation Studies (ARREST) registry. Open Heart 1, e000112 (2014).
Milano, A. et al. Sudden cardiac arrest and rare genetic variants in the community. Circ. Cardiovasc. Genet. 9, 147–153 (2016).
Altshuler, D. M. et al. Integrating common and rare genetic variation in diverse human populations. Nature 467, 52–58 (2010).
Khera, A. V. et al. Rare genetic variants associated with sudden cardiac death in adults. J. Am. Coll. Cardiol. 74, 2623–2634 (2019).
Van Driest, S. L. et al. Association of arrhythmia-related genetic variants with phenotypes documented in electronic medical records. JAMA 315, 47–57 (2016).
Wang, Q. et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell 80, 805–811 (1995).
Kapplinger, J. D. et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 7, 33–46 (2010).
McNair, W. P. et al. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation 110, 2163–2167 (2004).
Groenewegen, W. A. et al. A cardiac sodium channel mutation cosegregates with a rare connexin40 genotype in familial atrial standstill. Circ. Res. 92, 14–22 (2003).
Benson, D. W. et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J. Clin. Invest. 112, 1019–1028 (2003).
Magnani, J. W. et al. Sequencing of SCN5A identifies rare and common variants associated with cardiac conduction: cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium. Circ. Cardiovasc. Genet. 7, 365–373 (2014).
Lin, H. et al. Common and rare coding genetic variation underlying the electrocardiographic PR interval. Circ. Genom. Precis. Med. 11, e002037 (2018).
Abdellah, Z. et al. Finishing the euchromatic sequence of the human genome. Nature 431, 931–945 (2004).
Belmont, J. W. et al. The international HapMap project. Nature 426, 789–796 (2003).
Splawski, I. et al. Variant of SCN5A sodium channel implicated in risk of cardiac arrhythmia. Science 297, 1333–1336 (2002).
Burke, A. et al. Role of SCN5A Y1102 polymorphism in sudden cardiac death in blacks. Circulation 112, 798–802 (2005).
Bezzina, C. R. et al. Genome-wide association study identifies a susceptibility locus at 21q21 for ventricular fibrillation in acute myocardial infarction. Nat. Genet. 42, 688–691 (2010).
Arking, D. E. et al. Identification of a sudden cardiac death susceptibility locus at 2q24.2 through genome-wide association in European ancestry individuals. PLoS Genet. 7, e1002158 (2011).
Christophersen, I. E. et al. Fifteen genetic loci associated with the electrocardiographic P wave. Circ. Cardiovasc. Genet. 10, e001667 (2017).
Pfeufer, A. et al. Genome-wide association study of PR interval. Nat. Genet. 42, 153–159 (2010).
Ilkhanoff, L. et al. A common SCN5A variant is associated with PR interval and atrial fibrillation among African Americans. J. Cardiovasc. Electrophysiol. 25, 1150–1157 (2014).
Ritchie, M. D. et al. Genome- and phenome-wide analyses of cardiac conduction identifies markers of arrhythmia risk. Circulation 127, 1377–1385 (2013).
Sotoodehnia, N. et al. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat. Genet. 42, 1068–1076 (2010).
Arking, D. E. et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat. Genet. 38, 644–651 (2006).
Jamshidi, Y. et al. Common variation in the NOS1AP gene is associated with drug-induced QT prolongation and ventricular arrhythmia. J. Am. Coll. Cardiol. 60, 841–850 (2012).
Arking, D. E. et al. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat. Genet. 46, 826–836 (2014).
Teumer, A. et al. KCND3 potassium channel gene variant confers susceptibility to electrocardiographic early repolarization pattern. JCI Insight 4, e131156 (2019).
Goldenberg, I. et al. Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J. Am. Coll. Cardiol. 57, 51–59 (2011).
Priori, S. G., Napolitano, C. & Schwartz, P. J. Low penetrance in the long-QT syndrome clinical impact. Circulation 99, 529–533 (1999).
Schwartz, P. J., Crotti, L. & George, A. L. Modifier genes for sudden cardiac death. Eur. Heart J. 39, 3925–3931 (2018).
Crotti, L. et al. KCNH2-K897T is a genetic modifier of latent congenital long-QT syndrome. Circulation 112, 1251–1258 (2005).
Crotti, L. et al. NOS1AP is a genetic modifier of the long-QT syndrome. Circulation 120, 1657–1663 (2009).
Lahrouchi, N. et al. Transethnic genome-wide association study provides insights in the genetic architecture and heritability of long QT syndrome. Circulation 142, 324–338 (2020).
Giudicessi, J. R., Roden, D. M., Wilde, A. A. M. & Ackerman, M. J. Classification and reporting of potentially proarrhythmic common genetic variation in long QT syndrome genetic testing. Circulation 137, 619–630 (2018).
Hosseini, S. M. et al. Reappraisal of reported genes for sudden arrhythmic death: evidence-based evaluation of gene validity for Brugada syndrome. Circulation 138, 1195–1205 (2018).
Walsh, R. et al. Defining the genetic architecture of hypertrophic cardiomyopathy: re-evaluating the role of non-sarcomeric genes. Eur. Heart J. 38, 3461–3468 (2017).
Walsh, R. et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet. Med. 19, 192–203 (2017).
Walsh, R., Tadros, R. & Bezzina, C. R. When genetic burden reaches threshold. Eur. Heart J. 41, 3849–3855 (2020).
Bezzina, C. R. et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 45, 1044–1049 (2013).
Ripatti, S. et al. A multilocus genetic risk score for coronary heart disease: case-control and prospective cohort analyses. Lancet 376, 1393–1400 (2010).
Abraham, G. et al. Genomic prediction of coronary heart disease. Eur. Heart J. 37, 3267–3278 (2016).
Khera, A. V. et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N. Engl. J. Med. 375, 2349–2358 (2016).
Inouye, M. et al. Genomic risk prediction of coronary artery disease in 480,000 adults: implications for primary prevention. J. Am. Coll. Cardiol. 72, 1883–1893 (2018).
Noseworthy, P. A. et al. Common genetic variants, QT interval, and sudden cardiac death in a Finnish population-based study. Circ. Cardiovasc. Genet. 4, 305–311 (2011).
Rosenberg, M. A. et al. Validation of polygenic scores for QT interval in clinical populations. Circ. Cardiovasc. Genet. 10, e001724 (2017).
Turkowski, K. L. et al. Corrected QT interval-polygenic risk score and its contribution to type 1, type 2, and type 3 long-QT syndrome in probands and genotype-positive family members. Circ. Genom. Precis. Med. 13, e002922 (2020).
Wijeyeratne, Y. D. et al. SCN5A mutation type and a genetic risk score associate variably with Brugada syndrome phenotype in SCN5A families. Circ. Genom. Precis. Med. 13, e002911 (2020).
Juang, J.-M. J. et al. Validation and disease risk assessment of previously reported genome-wide genetic variants associated with Brugada syndrome: SADS-TW BrS registry. Circ. Genom. Precis. Med. 13, e002797 (2020).
Makarawate, P. et al. Common and rare susceptibility genetic variants predisposing to Brugada syndrome in Thailand. Heart Rhythm 17, 2145–2153 (2020).
Tadros, R. et al. Predicting cardiac electrical response to sodium-channel blockade and Brugada syndrome using polygenic risk scores. Eur. Heart J. 40, 3097–3107 (2019).
Roden, D. M. Drug-induced prolongation of the QT interval. N. Engl. J. Med. 10, 1013–1035 (2004).
Straus, S. M. J. M. et al. Non-cardiac QTc-prolonging drugs and the risk of sudden cardiac death. Eur. Heart J. 26, 2007–2012 (2005).
Behr, E. R. & Roden, D. Drug-induced arrhythmia: pharmacogenomic prescribing? Eur. Heart J. 34, 89–95 (2013).
Zeltser, D. et al. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine 82, 282–290 (2003).
Roden, D. M. Long QT syndrome: reduced repolarization reserve and the genetic link. J. Intern. Med. 259, 59–69 (2006).
Itoh, H. et al. Latent genetic backgrounds and molecular pathogenesis in drug-induced long-QT syndrome. Circ. Arrhythm. Electrophysiol. 2, 511–523 (2009).
Paulussen, A. D. C. et al. Genetic variations of HCNQ1, KCNH2, SCN5A, KCNE1, and KCNE2 in drug-induced long QT syndrome patients. J. Mol. Med. 82, 182–188 (2004).
Yang, P. et al. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation 105, 1943–1948 (2002).
Ramirez, A. H. et al. Novel rare variants in congenital cardiac arrhythmia genes are frequent in drug-induced torsades de pointes. Pharmacogenomics J. 13, 325–329 (2013).
Kääb, S. et al. A large candidate gene survey identifies the KCNE1 D85N polymorphism as a possible modulator of drug-induced torsades de pointes. Circ. Cardiovasc. Genet. 5, 91–99 (2012).
Becker, M. L. et al. A common NOS1AP genetic polymorphism is associated with increased cardiovascular mortality in users of dihydropyridine calcium channel blockers. Br. J. Clin. Pharmacol. 67, 61–67 (2009).
Kao, W. H. L. et al. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation 119, 940–951 (2009).
Behr, E. R. et al. Genome wide analysis of drug-induced torsades de pointes: lack of common variants with large effect sizes. PLoS ONE 8, e78511 (2013).
Strauss, D. G. et al. Common genetic variant risk score is associated with drug-induced QT prolongation and torsade de pointes risk. Circulation 135, 1300–1310 (2017).
Torkamani, A., Wineinger, N. E. & Topol, E. J. The personal and clinical utility of polygenic risk scores. Nat. Rev. Genet. 19, 581–590 (2018).
Glinge, C., Lahrouchi, N., Jabbari, R., Tfelt-Hansen, J. & Bezzina, C. R. Genome-wide association studies of cardiac electrical phenotypes. Cardiovasc. Res. 116, 1620–1634 (2020).
Holm, I. A. et al. The BabySeq project: implementing genomic sequencing in newborns. BMC Pediatr. 18, 1–10 (2018).
Kaltman, J. R. et al. Screening for sudden cardiac death in the young: report from a National Heart, Lung, and Blood Institute working group. Circulation 123, 1911–1918 (2011).
Margey, R. et al. Sudden cardiac death in 14- to 35-year olds in Ireland from 2005 to 2007: a retrospective registry. Europace 13, 1411–1418 (2011).
Acknowledgements
C.S. and E.R.B. are supported by the Robert Lancaster Memorial Fund sponsored by McColl’s Retail Group Ltd. C.R.B. is supported by the Dutch Heart Foundation (CVON Predict2 project), the Netherlands Organization for Scientific Research (VICI fellowship, 016.150.610) and Fondation Leducq (17CVD02). M.J.A. is supported by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program.
Author information
Authors and Affiliations
Contributions
C.S. researched data for and wrote the article. All the authors contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks C. Napolitano, C. Semsarian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
gnomAD: https://gnomad.broadinstitute.org/
HapMap: https://www.genome.gov/10001688/international-hapmap-project
Human Genome Project: https://www.genome.gov/human-genome-project
Glossary
- Alleles
-
An allele is one of several alternative forms of a gene occupying a specific locus on a chromosome.
- Minor allele frequency
-
The frequency at which the second most common allele occurs in a given population.
- Founder variants
-
Disease-causing genetic variants that are found repeatedly in a given population and that are derived from a shared ancestor who harboured that variant.
- Primary arrhythmia syndromes
-
Inheritable cardiac conditions predisposing individuals to arrhythmias in the absence of structural heart disease.
- Exonic
-
Relating to a coding segment of DNA.
- Single-nucleotide polymorphisms
-
Substitutions of individual nucleotides that occur with a measurable frequency.
- Risk alleles
-
Alleles that confer a risk of developing a disease.
- Linkage disequilibrium
-
Non-random association between particular DNA variants at two sites that are physically close to one another on the chromosome (that is, the frequency is significantly greater than that expected from the product of the observed allelic frequencies at each site independently).
- Intronic
-
Relating to a non-coding segment of DNA.
Rights and permissions
About this article
Cite this article
Scrocco, C., Bezzina, C.R., Ackerman, M.J. et al. Genetics and genomics of arrhythmic risk: current and future strategies to prevent sudden cardiac death. Nat Rev Cardiol 18, 774–784 (2021). https://doi.org/10.1038/s41569-021-00555-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-021-00555-y
This article is cited by
-
Dosage of the pseudoautosomal gene SLC25A6 is implicated in QTc interval duration
Scientific Reports (2023)