Abstract
In the absence of contemporary, population-based epidemiological studies, estimates of the incidence and prevalence of the inherited cardiomyopathies have been derived from screening studies, most often of young adult populations, to assess cardiovascular risk or to detect the presence of disease in athletes or military recruits. The global estimates for hypertrophic cardiomyopathy (1/500 individuals), dilated cardiomyopathy (1/250) and arrhythmogenic right ventricular cardiomyopathy (1/5,000) are probably conservative given that only individuals who fulfil diagnostic criteria would have been included. This caveat is highly relevant because a substantial minority or even a majority of individuals who carry disease-causing genetic variants and are at risk of disease complications have incomplete and/or late-onset disease expression. The genetic literature on cardiomyopathy, which is often focused on the identification of genetic variants, has been biased in favour of pedigrees with higher penetrance. In clinical practice, an abnormal electrocardiogram with normal or non-diagnostic imaging results is a common finding for the sarcomere variants that cause hypertrophic cardiomyopathy, the titin and sarcomere variants that cause dilated cardiomyopathy and the desmosomal variants that cause either arrhythmogenic right ventricular cardiomyopathy or dilated cardiomyopathy. Therefore, defining the genetic epidemiology is also challenging given the overlapping phenotypes, incomplete and age-related expression, and highly variable penetrance even within individual families carrying the same genetic variant.
Key points
-
Contemporary age-adjusted and sex-adjusted, population-based epidemiological studies of the incidence and prevalence of the inherited cardiomyopathies are lacking.
-
Contemporary understanding of the genetic epidemiology of the inherited cardiomyopathies reveals incomplete and age-related disease expression and low penetrance for the majority of the genetic variants associated with these conditions.
-
Current prevalence estimates for hypertrophic cardiomyopathy (1/500) and dilated cardiomyopathy (1/250) are on the basis of screening studies, mainly in healthy young adults, and probably underestimate the true prevalence of disease and the risk of disease-related complications.
-
Prevalence estimates for arrhythmogenic cardiomyopathy (1/2,000 to 1/5,000) are on the basis of extrapolation from allele frequencies of genetic variants and the generally low penetrance of most of these variants.
-
Inherited restrictive cardiomyopathy is rare and usually associated with hypertrophic, dilated, infiltrative or arrhythmic cardiomyopathy, sometimes with musculoskeletal abnormalities in other members of the family.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
McKenna, W. & Elliott, P. in Goldman–Cecil Medicine 26th edn Ch. 54 (eds Goldman, L. & Schaffer, A. I.) 297–314 (Elsevier, 2019).
Marian, A. J. & Braunwald, E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 121, 749–770 (2017).
Maron, B. J. Clinical course and management of hypertrophic cardiomyopathy. N. Engl. J. Med. 379, 655–668 (2018).
Hershberger, R. E., Hedges, D. J. & Morales, A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat. Rev. Cardiol. 10, 531–547 (2013).
Peters, S., Kumar, S., Elliott, P., Kalman, J. M. & Fatkin, D. Arrhythmic genotypes in familial dilated cardiomyopathy: implications for genetic testing and clinical management. Heart Lung Circ. 28, 31–38 (2019).
Towbin, J. A. et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 16, e301–e372 (2019).
Elliott, P. M. et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 35, 2733–2779 (2014).
Redfield, M. M. et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 289, 194–202 (2003).
Wang, T. J. et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 108, 977–982 (2003).
Yeboah, J. et al. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the multi-ethnic study of atherosclerosis (MESA). Circulation 126, 2713–2719 (2012).
Fitzpatrick, A. P., Shapiro, L. M., Rickards, A. F. & Poole-Wilson, P. A. Familial restrictive cardiomyopathy with atrioventricular block and skeletal myopathy. Br. Heart J. 63, 114–118 (1990).
Coats, C. J. & Hollman, A. Hypertrophic cardiomyopathy: lessons from history. Heart 94, 1258–1263 (2008).
[No authors listed]. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br. Heart J. 44, 672–673 (1980).
Richardson, P. et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology task force on the definition and classification of cardiomyopathies. Circulation 93, 841–842 (1996).
Elliott, P. et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology working group on myocardial and pericardial diseases. Eur. Heart J. 29, 270–276 (2008).
van Waning, J. I. et al. Genetics, clinical features, and long-term outcome of noncompaction cardiomyopathy. J. Am. Coll. Cardiol. 71, 711–722 (2018).
Maron, B. J. & Epstein, S. E. Hypertrophic cardiomyopathy: a discussion of nomenclature. Am. J. Cardiol. 43, 1242–1244 (1979).
McLeod, C. J. et al. Outcome of patients with hypertrophic cardiomyopathy and a normal electrocardiogram. J. Am. Coll. Cardiol. 54, 229–233 (2009).
Gersh, B. J. et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation 124, 2761–2796 (2011).
Ingles, J. et al. Nonfamilial hypertrophic cardiomyopathy: prevalence, natural history, and clinical implications. Circ. Cardiovasc. Genet. 10, e001620 (2017).
Lewis, J. F. & Maron, B. J. Diversity of patterns of hypertrophy in patients with systemic hypertension and marked left ventricular wall thickening. Am. J. Cardiol. 65, 874–881 (1990).
Dungu, J. et al. The electrocardiographic features associated with cardiac amyloidosis of variant transthyretin isoleucine 122 type in Afro-Caribbean patients. Am. Heart J. 164, 72–79 (2012).
Hill, M. N. et al. Hypertension care and control in underserved urban African American men: behavioral and physiologic outcomes at 36 months. Am. J. Hypertens. 16, 906–913 (2003).
Braunwald, E., Brockenbrough, E. C. & Morrow, A. G. Hypertrophic subaortic stenosis — a broadened concept. Circulation 26, 161–165 (1962).
Ho, C. Y. et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the sarcomeric human cardiomyopathy registry (SHaRe). Circulation 138, 1387–1398 (2018).
Maron, B. J. et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation 102, 858–864 (2000).
Finocchiaro, G. et al. Sudden death can be the first manifestation of hypertrophic cardiomyopathy: data from a United Kingdom pathology registry. JACC Clin. Electrophysiol. 5, 252–254 (2019).
Hada, Y. et al. Prevalence of hypertrophic cardiomyopathy in a population of adult Japanese workers as detected by echocardiographic screening. Am. J. Cardiol. 59, 183–184 (1987).
Codd, M. B., Sugrue, D. D., Gersh, B. J. & Melton, L. J. III. Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation 80, 564–572 (1989).
Maron, B. J. et al. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA study. Coronary artery risk development in (young) adults. Circulation 92, 785–789 (1995).
Maron, B. J., Mathenge, R., Casey, S. A., Poliac, L. C. & Longe, T. F. Clinical profile of hypertrophic cardiomyopathy identified de novo in rural communities. J. Am. Coll. Cardiol. 33, 1590–1595 (1999).
Corrado, D., Basso, C., Schiavon, M. & Thiene, G. Screening for hypertrophic cardiomyopathy in young athletes. N. Engl. J. Med. 339, 364–369 (1998).
Miura, K. et al. Epidemiology of idiopathic cardiomyopathy in Japan: results from a nationwide survey. Heart 87, 126–130 (2002).
Nistri, S. et al. Screening for hypertrophic cardiomyopathy in a young male military population. Am. J. Cardiol. 91, 1021–1023 (2003).
Maron, B. J. et al. Prevalence of hypertrophic cardiomyopathy in a population-based sample of American Indians aged 51 to 77 years (the Strong Heart study). Am. J. Cardiol. 93, 1510–1514 (2004).
Zou, Y. et al. Prevalence of idiopathic hypertrophic cardiomyopathy in China: a population-based echocardiographic analysis of 8080 adults. Am. J. Med. 116, 14–18 (2004).
Maro, E. E., Janabi, M. & Kaushik, R. Clinical and echocardiographic study of hypertrophic cardiomyopathy in Tanzania. Trop. Doct. 36, 225–227 (2006).
Morita, H. et al. Single-gene mutations and increased left ventricular wall thickness in the community: the Framingham Heart study. Circulation 113, 2697–2705 (2006).
Ma, J. Z. et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death in China. J. Sci. Med. Sport 10, 227–233 (2007).
Ng, C. T. et al. Prevalence of hypertrophic cardiomyopathy on an electrocardiogram-based pre-participation screening programme in a young male south-east Asian population: results from the Singapore armed forces electrocardiogram and echocardiogram screening protocol. Europace 13, 883–888 (2011).
Eberly, L. A. et al. Association of race with disease expression and clinical outcomes among patients with hypertrophic cardiomyopathy. JAMA Cardiol. 5, 83–91 (2019).
Semsarian, C., Ingles, J., Maron, M. S. & Maron, B. J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 65, 1249–1254 (2015).
Lipshultz, S. E. et al. The incidence of pediatric cardiomyopathy in two regions of the United States. N. Engl. J. Med. 348, 1647–1655 (2003).
Nugent, A. W. et al. The epidemiology of childhood cardiomyopathy in Australia. N. Engl. J. Med. 348, 1639–1646 (2003).
Towbin, J. A. et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA 296, 1867–1876 (2006).
Arola, A. et al. Epidemiology of idiopathic cardiomyopathies in children and adolescents. A nationwide study in Finland. Am. J. Epidemiol. 146, 385–393 (1997).
Cirino, A. L. et al. Hypertrophic Cardiomyopathy Overview (University of Washington, 1993).
Alfares, A. A. et al. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet. Med. 17, 880–888 (2015).
Sharma, S. et al. Physiologic limits of left ventricular hypertrophy in elite junior athletes: relevance to differential diagnosis of athlete’s heart and hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 40, 1431–1436 (2002).
Niimura, H. et al. Mutations in the gene for cardiac myosin-binding protein C and late-onset familial hypertrophic cardiomyopathy. N. Engl. J. Med. 338, 1248–1257 (1998).
Mogensen, J. et al. Frequency and clinical expression of cardiac troponin I mutations in 748 consecutive families with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 44, 2315–2325 (2004).
Rapezzi, C. et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 34, 1448–1458 (2013).
Waller, B. F. Hearts of the “oldest old”. Mayo Clin. Proc. 63, 625–627 (1988).
Neubauer, S. et al. Distinct subgroups in hypertrophic cardiomyopathy in the NHLBI HCM registry. J. Am. Coll. Cardiol. 74, 2333–2345 (2019).
Mestroni, L. et al. Familial dilated cardiomyopathy: evidence for genetic and phenotypic heterogeneity. Heart muscle disease study group. J. Am. Coll. Cardiol. 34, 181–190 (1999).
Michels, V. V. et al. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N. Engl. J. Med. 326, 77–82 (1992).
Schultheiss, H. P. et al. Dilated cardiomyopathy. Nat. Rev. Dis. Prim. 5, 32 (2019).
Henry, W. L., Gardin, J. M. & Ware, J. H. Echocardiographic measurements in normal subjects from infancy to old age. Circulation 62, 1054–1061 (1980).
Mahon, N. G. et al. Echocardiographic evaluation in asymptomatic relatives of patients with dilated cardiomyopathy reveals preclinical disease. Ann. Intern. Med. 143, 108–115 (2005).
Davies, M. et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet 358, 439–444 (2001).
Wang, T. J., Levy, D., Benjamin, E. J. & Vasan, R. S. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann. Intern. Med. 138, 907–916 (2003).
Seidelmann, S. B. et al. Familial dilated cardiomyopathy diagnosis is commonly overlooked at the time of transplant listing. J. Heart Lung Transplant. 35, 474–480 (2016).
Rusconi, P. et al. Differences in presentation and outcomes between children with familial dilated cardiomyopathy and children with idiopathic dilated cardiomyopathy: a report from the pediatric cardiomyopathy registry study group. Circ. Heart Fail. 10, e002637 (2017).
Norton, N. et al. Evaluating pathogenicity of rare variants from dilated cardiomyopathy in the exome era. Circ. Cardiovasc. Genet. 5, 167–174 (2012).
Muntoni, F. et al. Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N. Engl. J. Med. 329, 921–925 (1993).
Towbin, J. A. et al. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation 87, 1854–1865 (1993).
Hearn, T. et al. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alstrom syndrome. Nat. Genet. 31, 79–83 (2002).
Santorelli, F. M. et al. Maternally inherited cardiomyopathy and hearing loss associated with a novel mutation in the mitochondrial tRNA(Lys) gene (G8363A). Am. J. Hum. Genet. 58, 933–939 (1996).
Bonne, G. et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 21, 285–288 (1999).
Peretto, G. et al. Cardiac and neuromuscular features of patients with LMNA-related cardiomyopathy. Ann. Intern. Med. 171, 458–463 (2019).
Marcus, F. I. et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation 65, 384–398 (1982).
Basso, C. et al. Arrhythmogenic right ventricular cardiomyopathy. Circulation 94, 983–991 (1996).
Basso, C., Corrado, D. & Thiene, G. Arrhythmogenic right ventricular cardiomyopathy: what’s in a name? From a congenital defect (dysplasia) to a genetically determined cardiomyopathy (dystrophy). Am. J. Cardiol. 106, 275–277 (2010).
Awad, M. M., Calkins, H. & Judge, D. P. Mechanisms of disease: molecular genetics of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Nat. Clin. Pract. Cardiovasc. Med. 5, 258–267 (2008).
Norman, M. et al. Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 112, 636–642 (2005).
McKenna, W. J. et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task force of the working group myocardial and pericardial disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br. Heart J. 71, 215–218 (1994).
Marcus, F. I. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur. Heart J. 31, 806–814 (2010).
Platonov, P. G. et al. High interobserver variability in the assessment of epsilon waves: implications for diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm 13, 208–216 (2016).
Gerull, B. et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat. Genet. 36, 1162–1164 (2004).
Landrum, M. J. et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 42, D980–D985 (2013).
McKenna, W. J., Maron, B. J. & Thiene, G. Classification, epidemiology, and global burden of cardiomyopathies. Circ. Res. 121, 722–730 (2017).
Thiene, G., Nava, A., Corrado, D., Rossi, L. & Pennelli, N. Right ventricular cardiomyopathy and sudden death in young people. N. Engl. J. Med. 318, 129–133 (1988).
Peters, S., Trümmel, M. & Meyners, W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int. J. Cardiol. 97, 499–501 (2004).
Merner, N. D. et al. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am. J. Hum. Genet. 82, 809–821 (2008).
Sliwa, K., Damasceno, A. & Mayosi, B. M. Epidemiology and etiology of cardiomyopathy in Africa. Circulation 112, 3577–3583 (2005).
Munclinger, M. J., Patel, J. J. & Mitha, A. S. Follow-up of patients with arrhythmogenic right ventricular cardiomyopathy dysplasia. S. Afr. Med. J. 90, 61–68 (2000).
Hendricks, N., Watkins, D. A. & Mayosi, B. M. Lessons from the first report of the arrhythmogenic right ventricular cardiomyopathy registry of South Africa. Cardiovasc. J. Afr. 21, 129–130 (2010).
Protonotarios, N. et al. Cardiac abnormalities in familial palmoplantar keratosis. Br. Heart J. 56, 321–326 (1986).
Coonar, A. S. et al. Gene for arrhythmogenic right ventricular cardiomyopathy with diffuse nonepidermolytic palmoplantar keratoderma and woolly hair (Naxos disease) maps to 17q21. Circulation 97, 2049–2058 (1998).
McKoy, G. et al. Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355, 2119–2124 (2000).
Norgett, E. E. et al. Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum. Mol. Genet. 9, 2761–2766 (2000).
Awad, M. M. et al. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am. J. Hum. Genet. 79, 136–142 (2006).
Syrris, P. et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am. J. Hum. Genet. 79, 978–984 (2006).
Pilichou, K. et al. Mutations in desmoglein-2 gene are associated with arrhythmogenic right ventricular cardiomyopathy. Circulation 113, 1171–1179 (2006).
Ahmad, F. et al. Localization of a gene responsible for arrhythmogenic right ventricular dysplasia to chromosome 3p23. Circulation 98, 2791–2795 (1998).
Milting, H. et al. The TMEM43 Newfoundland mutation p.S358L causing ARVC-5 was imported from Europe and increases the stiffness of the cell nucleus. Eur. Heart J. 36, 872–881 (2014).
Capell, B. C. & Collins, F. S. Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet. 7, 940–952 (2006).
Fatkin, D. et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 341, 1715–1724 (1999).
Hasselberg, N. E. et al. Lamin A/C cardiomyopathy: young onset, high penetrance, and frequent need for heart transplantation. Eur. Heart J. 39, 853–860 (2017).
van Tintelen, J. P. et al. High yield of LMNA mutations in patients with dilated cardiomyopathy and/or conduction disease referred to cardiogenetics outpatient clinics. Am. Heart J. 154, 1130–1139 (2007).
Quarta, G. et al. Mutations in the lamin A/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 33, 1128–1136 (2012).
Tiso, N. et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum. Mol. Genet. 10, 189–194 (2001).
Priori, S. G. et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 106, 69–74 (2002).
Schmitt, J. P. et al. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299, 1410–1413 (2003).
Haghighi, K. et al. Human phospholamban null results in lethal dilated cardiomyopathy revealing a critical difference between mouse and human. J. Clin. Invest. 111, 869–876 (2003).
Haghighi, K. et al. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc. Natl Acad. Sci. USA 103, 1388–1393 (2006).
van der Zwaag, P. A. et al. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur. J. Heart Fail. 14, 1199–1207 (2012).
Groeneweg, J. A. et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ. Cardiovasc. Genet. 8, 437–446 (2015).
van Spaendonck-Zwarts, K. Y. et al. Recurrent and founder mutations in the Netherlands: the cardiac phenotype of DES founder mutations p.S13F and p.N342D. Neth. Heart J. 20, 219–228 (2012).
Lorenzon, A. et al. Desmin mutations and arrhythmogenic right ventricular cardiomyopathy. Am. J. Cardiol. 111, 400–405 (2013).
Remme, C. A. Cardiac sodium channelopathy associated with SCN5A mutations: electrophysiological, molecular and genetic aspects. J. Physiol. 591, 4099–4116 (2013).
Olson, T. M. et al. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA 293, 447–454 (2005).
te Riele, A. S. J. M. et al. Multilevel analyses of SCN5A mutations in arrhythmogenic right ventricular dysplasia/cardiomyopathy suggest non-canonical mechanisms for disease pathogenesis. Cardiovasc. Res. 113, 102–111 (2017).
Dalal, D. et al. Penetrance of mutations in plakophilin-2 among families with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J. Am. Coll. Cardiol. 48, 1416–1424 (2006).
Quarta, G. et al. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy. Circulation 123, 2701–2709 (2011).
Dalal, D. et al. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation 112, 3823–3832 (2005).
Xu, T. et al. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J. Am. Coll. Cardiol. 55, 587–597 (2010).
Gifford, C. A. et al. Oligogenic inheritance of a human heart disease involving a genetic modifier. Science 364, 865–870 (2019).
James, C. A. et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J. Am. Coll. Cardiol. 62, 1290–1297 (2013).
Chelko, S. P. et al. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight 1, e85923 (2016).
Martherus, R. et al. Accelerated cardiac remodeling in desmoplakin transgenic mice in response to endurance exercise is associated with perturbed Wnt/β-catenin signaling. Am. J. Physiol. Heart Circ. Physiol. 310, H174–H187 (2016).
Carruth, E. D. et al. Prevalence and electronic health record-based phenotype of loss-of-function genetic variants in arrhythmogenic right ventricular cardiomyopathy-associated genes. Circ. Genom. Precis. Med. 12, e002579 (2019).
van Lint, F. H. M. et al. Arrhythmogenic right ventricular cardiomyopathy-associated desmosomal variants are rarely de novo. Circ. Genom. Precis. Med. 12, e002467 (2019).
Qiu, X. et al. Mutations of plakophilin-2 in Chinese with arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am. J. Cardiol. 103, 1439–1444 (2009).
Chen, L. et al. A founder homozygous DSG2 variant in east Asia results in ARVC with full penetrance and heart failure phenotype. Int. J. Cardiol. 274, 263–270 (2019).
Kubo, T. et al. Prevalence, clinical significance, and genetic basis of hypertrophic cardiomyopathy with restrictive phenotype. J. Am. Coll. Cardiol. 49, 2419–2426 (2007).
Mogensen, J. et al. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J. Clin. Invest. 111, 209–216 (2003).
Kittleson, M. M. et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation 142, e7–e22 (2020).
Brodehl, A. et al. Mutations in FLNC are associated with familial restrictive cardiomyopathy. Hum. Mutat. 37, 269–279 (2016).
Tucker, N. R. et al. Novel mutation in FLNC (filamin C) causes familial restrictive cardiomyopathy. Circ. Cardiovasc. Genet. 10, e001780 (2017).
Bermudez-Jimenez, F. J. et al. Novel desmin mutation p.Glu401Asp impairs filament formation, disrupts cell membrane integrity, and causes severe arrhythmogenic left ventricular cardiomyopathy/dysplasia. Circulation 137, 1595–1610 (2018).
Brodehl, A. et al. Restrictive cardiomyopathy is caused by a novel homozygous desmin (DES) mutation p.Y122H leading to a severe filament assembly defect. Genes 10, 918 (2019).
Muchtar, E., Blauwet, L. A. & Gertz, M. A. Restrictive cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 121, 819–837 (2017).
Jenni, R. et al. Persisting myocardial sinusoids of both ventricles as an isolated anomaly: echocardiographic, angiographic, and pathologic anatomical findings. Cardiovasc. Intervent. Radiol. 9, 127–131 (1986).
Kawel, N. et al. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ. Cardiovasc. Imaging 5, 357–366 (2012).
Petersen, S. E. et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 46, 101–105 (2005).
Weir-McCall, J. R. et al. Left ventricular noncompaction: anatomical phenotype or distinct cardiomyopathy? J. Am. Coll. Cardiol. 68, 2157–2165 (2016).
Ross, S. B. et al. A systematic review and meta-analysis of the prevalence of left ventricular non-compaction in adults. Eur. Heart J. 41, 1428–1436 (2020).
Hershberger, R. E. et al. Genetic evaluation of cardiomyopathy — a Heart Failure Society of America practice guideline. J. Card. Fail. 24, 281–302 (2018).
Jarcho, J. A. et al. Mapping a gene for familial hypertrophic cardiomyopathy to chromosome 14q1. N. Engl. J. Med. 321, 1372–1378 (1989).
Geisterfer-Lowrance, A. A. et al. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell 62, 999–1006 (1990).
Epstein, N. D., Cohn, G. M., Cyran, F. & Fananapazir, L. Differences in clinical expression of hypertrophic cardiomyopathy associated with two distinct mutations in the beta-myosin heavy chain gene. A 908Leu–Val mutation and a 403Arg–Gln mutation. Circulation 86, 345–352 (1992).
Fananapazir, L. & Epstein, N. D. Genotype-phenotype correlations in hypertrophic cardiomyopathy. Insights provided by comparisons of kindreds with distinct and identical beta-myosin heavy chain gene mutations. Circulation 89, 22–32 (1994).
Karibe, A. et al. Hypertrophic cardiomyopathy caused by a novel alpha-tropomyosin mutation (V95A) is associated with mild cardiac phenotype, abnormal calcium binding to troponin, abnormal myosin cycling, and poor prognosis. Circulation 103, 65–71 (2001).
Sheikh, N. et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation 129, 1637–1649 (2014).
Maron, B. J., Spirito, P., Wesley, Y. & Arce, J. Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N. Engl. J. Med. 315, 610–614 (1986).
Baig, M. K. et al. Familial dilated cardiomyopathy: cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J. Am. Coll. Cardiol. 31, 195–201 (1998).
Parks, S. B. et al. Lamin A/C mutation analysis in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. Am. Heart J. 156, 161–169 (2008).
Acknowledgements
The authors are supported by a grant from the Leducq Foundation (W.J.M. and D.P.J.).
Author information
Authors and Affiliations
Contributions
Both authors contributed to all aspects of preparing the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.P.J. has received payments as a consultant from 4D Molecular Therapeutics, ADRx Pharma and Pfizer, and has received research funding from Array Biopharma and Eidos Therapeutics. W.J.M. declares no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks L. Mestroni, C. Semsarian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Supplementary information
Glossary
- Mendelian pattern
-
Inheritance pattern of single-gene disorders with high penetrance, including autosomal dominant, autosomal recessive and X-linked.
- Incomplete disease expression
-
A genetic predisposition to a disease that does not cause manifestations that are sufficient to meet the criteria for diagnosis of the condition.
- Age-related penetrance
-
A genetic predisposition that does not cause phenotypic manifestations until later in life.
- Epsilon waves
-
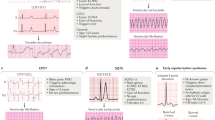
A feature of the electrocardiogram defined as a reproducible low-amplitude signal between the end of the QRS complex and the onset of the T wave in the right precordial leads (V1 to V3).
- Variants of uncertain significance
-
DNA variants that do not meet criteria for being classified as pathogenic, likely pathogenic, benign or likely benign, according to the joint recommendation of the American College of Medical Genetics and the Association for Molecular Pathology.
- Desmosome
-
Subcellular structures mediating intercellular junctions for many types of cell, particularly cardiomyocytes.
- Haplotype
-
Sets of chromosomal markers for sections of DNA that segregate in families.
- Digenic heterozygosity
-
The presence of one pathogenic variant in each of two different genes, each of which contributes to the phenotype.
- Oligogenic inheritance
-
A constellation of more than two DNA variants, each of which contributes to the phenotype.
- Allele frequency
-
The number of times that a variant is present in a large population divided by the total number of copies of all variants at that particular locus.
- Hypertrabeculation
-
An increased ratio of non-compacted to compacted areas of the left ventricle; also referred to as non-compaction.
Rights and permissions
About this article
Cite this article
McKenna, W.J., Judge, D.P. Epidemiology of the inherited cardiomyopathies. Nat Rev Cardiol 18, 22–36 (2021). https://doi.org/10.1038/s41569-020-0428-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-020-0428-2
This article is cited by
-
CRYAB stop-loss variant causes rare syndromic dilated cardiomyopathy with congenital cataract: expanding the phenotypic and mutational spectrum of alpha-B crystallinopathy
Journal of Human Genetics (2024)
-
Exploring TTN variants as genetic insights into cardiomyopathy pathogenesis and potential emerging clues to molecular mechanisms in cardiomyopathies
Scientific Reports (2024)
-
Diagnostic yield from cardiac gene testing for inherited cardiac conditions and re-evaluation of pre-ACMG variants of uncertain significance
Irish Journal of Medical Science (1971 -) (2024)
-
Late gadolinium enhancement entropy as a new measure of myocardial tissue heterogeneity for prediction of adverse cardiac events in patients with hypertrophic cardiomyopathy
Insights into Imaging (2023)
-
A systematic literature review of economic evaluations and cost-of-illness studies of inherited cardiomyopathies
Netherlands Heart Journal (2023)