Abstract
The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease.
Key points
-
The cardiac lymphatic vasculature appears during embryonic development and continues to mature structurally and functionally until late postnatal stages.
-
Both venous and non-venous sources contribute to the lymphatic endothelium of the heart, and the identity of the lymphatic endothelial cells of venous origin is defined very early in development during specification of the embryonic mesoderm.
-
In adult mice, the cardiac lymphatics respond to cardiac injury by sprouting within the damaged area in an attempt to clear the immune cells and excess tissue fluid (oedema).
-
Drug-induced augmentation of the lymphatic response to injury improves heart repair and function in adult mice, suggesting that the lymphatics could be a possible drug target for myocardial infarction and for other diseases.
-
The neonatal mouse heart has regenerative capacity that requires the presence of pro-reparative macrophages, which suggests an alternative function for the cardiac lymphatics during neonatal heart regeneration.
-
Adult zebrafish can regenerate their heart after cryoinjury, and the lymphatics respond to the site of injury to clear infiltrating immune cells, a process that is essential for complete regeneration.
Similar content being viewed by others
Introduction
The circulatory system of vertebrates is composed of two complementary vasculatures, the blood and lymphatic vascular systems1. The blood vasculature is a closed system responsible for transporting gases, fluids, nutrients, metabolites and cells to the tissues2. This extravasation of fluid and macromolecules results in a continuous accumulation of extracellular fluids and increased interstitial pressure2. Tissue fluid balance is maintained by the lymphatic vasculature, an open circulatory system that transports fluids and cells from organs back to the blood circulation3. In addition to regulating interstitial fluid homeostasis, lymphatic vessels have essential roles in the immune response through the uptake and transport of pathogens, antigens and immune cells from tissues to regional lymph nodes, before returning the extravasated fluid and solutes to the blood circulation.
In the heart, an extensive lymphatic network contributes to normal cardiac function in steady-state conditions and to myocardial healing after injury4. An increasing number of studies have determined the lineage heterogeneity of the cardiac lymphatics during development and their essential role in fibrotic repair after myocardial infarction (MI) in non-regenerative animal models, such as adult mice5,6,7,8,9, and regenerative models, such as zebrafish10,11,12,13. These studies hold great promise for ongoing efforts to develop therapies for cardiovascular diseases, highlighting the lymphatic vessels as a potential therapeutic target to reduce myocardial oedema and modulate the immune response after MI. In this Review, we summarize the current knowledge on the development, structure and function of the cardiac lymphatic vasculature, with an emphasis on breakthroughs over the past 5 years in the study of cardiac lymphatic heterogeneity in mice and zebrafish. We also discuss emerging findings on the immunomodulatory role of the cardiac lymphatics and their functional interaction with immune cells during the fibrotic repair process after injury in the adult mammalian heart, as well as during cardiovascular tissue restoration and regeneration in neonatal mice and in adult zebrafish. Finally, we describe ongoing preclinical studies investigating the lymphatic vessels as a potential therapeutic target in acute MI.
Cardiac lymphatic structure and function
The cardiac lymphatics run alongside the blood vessel network and have many of the functional features of the systemic lymphatic vasculature (Fig. 1), specifically the maintenance of homeostasis of interstitial fluid pressure14,15 and the modulation of the immune response16. Disruption of these processes can lead to severe health problems; for example, a 3.5% increase in myocardial fluids can lead to a 40% reduction in cardiac output17,18. Lymphatic vessels are lined by a monolayer of oak-leaf-shaped lymphatic endothelial cells (LECs) and are composed of three compartments: the initial lymphatics, the pre-collector lymphatics and the collector lymphatics1,19. Interestingly, the localization of the lymphatic capillaries and the routes of collector vessels in the heart are not fully conserved between species20.
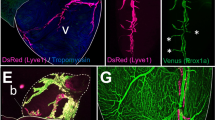
a | Schematic illustration of the mediastinal lymph nodes, where the cardiac lymphatics drain the lymph. b | Dorsal and ventral aspects of the adult mouse cardiac lymphatic network. c | The path of the lymph begins at the initial lymphatics, where lymphatic endothelial cells (LECs) connected by permeable button-like junctions drain immune cells, fluids, macromolecules and pathogens from the interstitial space, making up the lymph. The lymph is transported through the pre-collector and collector that are connected by impermeable zipper junctions to the mediastinal lymph nodes.
Interspecies differences
Initial lymphatics
The initial lymphatics or capillaries are thin, blind-ended and highly permeable vessels that are ideal for draining cells, fluid and macromolecules. In most mammals, such as humans, dogs and pigs, the initial lymphatics located in the area from the subepicardium to the subendocardium drain extracellular fluids, cells and macromolecules that make up the lymph21,22. However, in rabbit and mouse hearts, the lymphatics are absent from the endocardium23,24. The different mechanisms for immune cell uptake by the initial lymphatics have been described in detail previously16. Briefly, a primary valve system at the level of the LECs allows the entry of cells, fluids and macromolecules to the initial lymphatics and prevents their escape back to the interstitial space25,26,27,28. This primary valve system is created by flaps of adjacent LECs that interconnect and loosely overlap with one another25,29. These LECs have specialized cell–cell junctions, called buttons, which are discontinuous, thereby allowing fluid entry to the lymphatic vessels while also securing the structural integrity of the endothelium29. Furthermore, the abluminal side of the LECs is overlaid with extracellular-matrix-anchoring filaments that prevent the initial lymphatics from collapsing under increased interstitial pressure30. As pressure increases, the lymph is formed inside the initial lymphatics and is transported to the pre-collector and collector lymphatic vessels.
Pre-collector and collector lymphatics
When inside the lymphatic vessel, the lymph travels through the subepicardial pre-collectors and collectors to the mediastinal lymph nodes (MLNs) and then back to the systemic lymphatic circulation31. In the systemic lymphatic vasculature, the LECs have continuous seams of zipper-like cell–cell junctions that make the vessels impermeable to fluids and cells29. In the systemic lymphatics, each collecting vessel is arranged in a functional pumping unit called a lymphangion, which is defined as the section between two consecutive secondary intraluminal valves that is overlaid with contracting lymphatic smooth muscle cells14,15. Of note, the cardiac lymphatics do not have a lymphatic smooth muscle cell layer, and lymph flow is solely dependent on passive propulsion powered by the periodic motion of cardiac contraction5,6,32,33. As the lymph flows towards the MLNs, at each lymphangion the upstream valve closes preventing retrograde flow, while the downstream valve opens resulting in positive flow14,15.
In human, dog and pig hearts, collectors composing the left and right lymphatic trunks run along the major coronary arteries34,35. The left trunk collects lymph from the anterior and posterior interventricular branches and from the left marginal branch. The left trunk then passes behind the left atrial appendage and ascends onto the posterior surface of the pulmonary trunk and up to the pretracheal lymph nodes near the aortic arch. From the pretracheal lymph nodes, a single vessel travels behind the aorta to the cardiac lymph node, which lies between the superior vena cava and the right brachiocephalic artery, then a number of lymphatic vessels lead to the right lymphatic duct, which terminates in the right venous angle. The right trunk collects lymph from the right area of the heart and proceeds on the anterior surface of the aorta. The right trunk then enters the left anterior mediastinal chain and left paratracheal lymph nodes, from where it passes to the thoracic trunk and terminates in the left venous angle.
In contrast to the cardiac collector lymphatics of humans, pigs and dogs, which accompany coronary arteries, in mouse and rat hearts these vessels accompany cardiac veins20. Specifically, in mouse hearts, the left collector drains the paraconal interventricular sulcus (left and right ventricles) around the left conal vein towards the left side of the pulmonary trunk and upwards to the MLNs33. The second collector drains the lymph from the left ventricle running along the left cardiac vein followed by the coronary sinus and then upwards towards the MLNs33. The lymph then reaches the draining lymph node via the afferent collector lymphatics36. The unique functions and adaptations of lymphatic vessels in lymph nodes have been thoroughly reviewed previously37. In the MLNs, cells of the innate and adaptive immune system reside in specific locations, poised to be activated37,38. Following activation of adaptive immunity, lymphocytes enter the lymph and exit the lymph node via efferent lymphatic vessels39,40 until the lymph flow reaches either the right duct or the thoracic duct39. From the right duct or thoracic duct, the lymph eventually returns to the venous circulation at the level of the jugular and subclavian veins, where specialized lymphovenous valves ensure the unidirectional drainage of the lymph to the blood41,42,43,44.
Development of the cardiac lymphatics
Over the past two decades, our knowledge on the embryonic development of the lymphatic vasculature has increased substantially. During embryogenesis, the development of the lymphatic vessels takes place after the major blood vessels (the dorsal aorta and the cardinal vein) have been formed. Two distinct mechanisms have been proposed for the development of the lymphatic network: lymphvasculogenesis12,45,46 and lymphangiogenesis47,48,49,50. The origin of the lymphatic vasculature has been the subject of some controversy for many decades. An initial report by Sabin, dating back to the 1900s, suggested that the lymphatic endothelium buds directly from the venous endothelium51. By contrast, Huntington and McClure proposed that LECs originate from the mesoderm and subsequently form connections with the venous endothelium52.
Experimental work published in 2019 has shed some light on this historic debate. Stone and Stainier provided evidence that the fate of LECs in mice is hard-wired early on during embryogenesis at the level of the mesoderm53. Specifically, cell lineage tracing using Pax3–Cre and Myf5–Cre transgenic mice showed that, in addition to lateral plate mesoderm, the paraxial mesoderm also contributes endothelial cells during embryonic blood vessel development in mice53. Around embryonic day (E) 9.5, these paraxial mesoderm-derived endothelial cells selectively differentiate from the dorsolateral part of the anterior cardinal vein to form the first precursor LECs, characterized by the expression of the transcription factor prospero homeobox protein 1 (PROX1). These LECs subsequently give rise to the majority of the lymphatic endothelium, including systemic and organ-based (for example, the heart) lymphatics, with only limited contribution to the blood endothelium53. Prox1 expression is necessary and sufficient for LEC specification54,55 and, to be expressed, requires the transcription factors SOX18 and COUP transcription factor 2 (COUP-TF2)56,57. Both SOX18 and COUP-TF2 bind and activate Prox1 expression directly, whereas COUP-TF2 also promotes lymphatic differentiation indirectly by repressing the arterial fate driven by the Notch signalling pathway56,58. When Prox1 has been expressed, a positive feedback loop between PROX1 and vascular endothelial growth factor receptor 3 (VEGFR3) maintains the precursor LEC identity59. Concomitantly, precursor LECs start expressing lymphatic vessel endothelial hyaluronic acid receptor 1 (LYVE1), a protein that is essential for the lymphatic modulation of the immune response during inflammation60,61 but is redundant during embryogenesis62,63. At approximately E10.5, clusters of precursor LECs start aggregating and budding off along the length of the cardinal vein to form the lymph sacs, which are the lymphatic vessel precursors. For the budding process to take place, the VEGFR3–VEGFC signalling pathway is essential, although VEGFD also contributes to a lesser extent48. At around E12.5, adjacent LECs budding from the cardinal vein are connected by continuous, impermeable zipper junctions, providing strong structural integrity to the forming lymphatic vessels64,65. Starting at E16.5 and continuing during postnatal development, the junctions of the initial lymphatics transform into permeable buttons29,64,65.
The role of lymphatic junctions in health and disease has been reviewed previously66. Two different mechanisms maintain lymphovenous haemostasis, that is, prevent blood from flowing into the lymph sacs67,68. First, PROX1 activates the expression and production of podoplanin, which subsequently interacts with the platelet receptor C-type lectin domain family 2 (CLEC2)42, inducing platelet aggregation and thereby lymphovenous haemostasis42. Second, blood backflow can be prevented by the lymphovenous valves formed by specialized PROX1+ LECs residing in the cardinal vein, connecting the lymphatic vessels with the jugular and subclavian veins41,44. After the lymph sacs have been formed and separated from the cardinal vein, the lymph sacs start expanding into the developing embryonic tissues and organs by lymphangiogenesis, where the lymphatic vasculature continues to mature (Table 1). Detailed reviews of the formation of the systemic lymphatic network have been published previously69,70.
Mouse cardiac lymphatics
The cardiac lymphatic system has received substantial attention over the past 5 years. In particular, the development of the lymphatics in the forming heart, the characterization of the embryonic origin and the roles in adult heart disease have been summarized in a review published in 2019 (ref.4) (Fig. 2). In mice, the first cardiac LECs emerge at E12.5 from the extracardiac region near the outflow tract on the ventral side of the heart and from the sinus venosus on the dorsal side of the heart5. Whole-embryo staining at E10.5 and E12.5 identified a LEC population emerging from the cardinal vein and migrating towards the sinus venosus and outflow tract, suggesting that cardinal vein-derived endothelial cells might be the venous source of the coronary lymphatics5. By E14.5, the cardiac lymphatics extend along the base-to-apex axis on both sides of the heart, with the ventral side being slightly delayed compared with the dorsal side5. Interestingly, the epicardium and the outflow tract have been identified as sources of VEGFC for coronary vasculature development71, making these structures potential signalling hubs to control VEGFR3-dependent lymphatic sprouting. Studies investigating the spatiotemporal development by whole-mount and tissue section staining, as well as by Indian ink injection into the myocardium, suggest that during embryogenesis, lymphatic vessels appear only in the subepicardial layer5,32,33. From birth to approximately postnatal day (P) 15, lymphatic vessels continue to grow and branch laterally to adequately cover both the dorsal and the ventral surfaces of the mouse heart and also grow deeper into the underlying myocardium, without reaching the endocardium32,33. Although these studies are informative, they lack some of the imaging depth and detail that more modern approaches provide. The combination of tissue clearing, which renders large tissue samples transparent, with 3D imaging has proved to be a powerful tool for in-depth, multiview visualization of the whole vascular structure in the developing kidneys72 and injured heart73 of mice. Therefore, adopting similar methods in the future could provide more insight into the development of the cardiac lymphatic vasculature, for instance, to reveal how LECs of venous and non-venous origin integrate into a single vascular network (see below).
a | The cardiac lymphatic vasculature is visible at the dorsal and ventral side of the forming mouse heart from embryonic day (E) 14.5. b–d | Different cell lineages contribute to the generation of cardiac lymphatic endothelial cells. A subpopulation of paraxial mesoderm-derived venous endothelial cells bud from the cardinal vein to give rise to lymphatic endothelium (panel b). Other non-venous populations contribute to the cardiac lymphatics, such as the yolk sac-derived haemogenic endothelium (panel c) and an Isl1+ second heart field population that is found specifically on the ventral cardiac lymphatics (panel d). L, left; R, right.
Non-venous origin of the mouse cardiac lymphatics
The majority of cardiac LECs originate from the paraxial mesoderm-derived Tie2+ endothelium of the cardinal vein53, although recent studies have identified the contribution of non-venous sources5,8,9. Fate mapping using genetic drivers to trace Mesp1+ mesoderm, Nkx2-5+ cardiac mesoderm, Wt1+ epicardium and Wnt1+ neural crest cells excluded all of these as potential LEC sources. Further lineage tracing experiments using Cre recombinase (Cre)-driver lines under the control of the Vav1, Pdgfrb and Csf1r promoters identified the haemogenic endothelium of the yolk sac as a potential contributor of LECs5,74. Strikingly, two complementary studies have found that the ventral and dorsal lymphatic endothelia have distinct origins and develop through different mechanisms8,9. The first study used genetic lineage tracing to show the contribution of the Isl1+ lineage to cardiac LECs around the outflow tract and ventral side, suggesting a contribution from the second heart field (SHF)9. In the second study, a series of experiments proved that non-venous SHF-derived precursors contribute LECs exclusively to the ventral vascular network8. Initially, clonal analysis indicated that the origin of the cardiac lymphatic vasculature is different in the two sides of the heart8. Subsequently, genetic lineage tracing experiments showed that about half of the ventral lymphatic endothelium originates from Isl1+ and Mef2c+ precursors8. Moreover, the cardiac neural crest was excluded as a source, and the contribution of the Isl1+ lineage to the LECs of the cardinal vein was found to be minimal8. Finally, both constitutive deletion of Tbx1, which results in the lack of the SHF, and conditional deletion of Prox1 in the SHF in mice were found to lead to complete agenesis of the ventral lymphatics, with no effect on the dorsal lymphatics, supporting the notion that the SHF is an essential cellular source for the ventral lymphatic endothelium8.
Although lineage tracing experiments have proved valuable for determining the origin of LECs, issues around the specificity of the Cre drivers and incomplete or even ectopic recombination of Cre reporters have complicated data interpretation, leading to conflicting reports. Therefore, cross-validating new information with a combination of genetic knockout experiments, multiple lineage reporters and clonal analyses is crucial75. The relevance of each of the aforementioned origins of the cardiac lymphatics with respect to the role of the lymphatics in cardiac diseases is currently unclear. Further investigation of the molecular cues that drive specification of LECs from different sources will provide insight into the selective targeting of LEC subpopulations to invoke therapeutic lymphangiogenesis.
Zebrafish cardiac lymphatics
In zebrafish, the cardiac lymphatics are found only in the subepicardial layer and drain into large collecting vessels in the outflow tract, which connect to the facial lymphatic vasculature12. Similar to the mouse cardiac lymphatics, zebrafish cardiac lymphatic vessels derive from both venous and non-venous (angioblast) sources10,76. Specifically, the cardiac lymphatic endothelium is first established on the outflow tract, or bulbus arteriosus, from facial lymphatic vessels that originate from sprouts of the cardinal vein and primary head sinus (lymphangiogenesis), as well as from a population of lymphangioblasts (lymphvasculogenesis)12,76,77. This process takes place relatively late in development, but before the initiation of the coronary vasculature, at about 3–4 weeks post-fertilization (wpf)12,13. However, the expansion of cardiac lymphatic vessels over the ventricle takes place after the formation of the coronary vasculature in juvenile zebrafish, at approximately 12–16 wpf12,13. Over subsequent weeks, the bulbus arteriosus lymphatic vessels sprout towards the ventricle in close proximity to the major coronary vessels and continue to grow along the base-to-apex axis in a process analogous to the growth of the cardiac lymphatics in the mouse12,13. Interestingly, hearts with an under-developed coronary plexus caused by CXC-chemokine receptor type 4 (Cxcr4a) deficiency have severe ventricular lymphatic abnormalities, whereas the bulbus arteriosus lymphatics are normal12,13. This finding suggests that the presence of a mature coronary tree is required for the lymphatics to migrate down the ventricle, with potential coronary artery-derived signals (such as CXC-chemokine ligand 12 (Cxcl12a)) promoting this developmental process12,13. Apart from sprouting lymphatics, clusters of isolated LECs of unknown origin have been reported to connect and contribute to the cardiac lymphatic vasculature12. Similar to the systemic lymphatic vessels, the cardiac lymphatics require Vegfr3–Vegfc signalling to develop, with genetic models such as vegfr3−/−, vegfc+/− or hypomorphic vegfchy/hy zebrafish having a severe lymphangiogenic phenotype11,12,13.
Overall, the cardiac lymphatic system seems to be more heterogeneous than previously thought, with contributions from both venous and non-venous sources, such as the yolk sac haemogenic endothelium and SHF progenitors, and with contributions differing between the dorsal and ventral aspects of the forming heart. Understanding this diversity in more detail and assessing its implications in the adult heart and in response to pathological insult could reveal new therapeutic avenues for the treatment of cardiovascular diseases.
Cardiac lymphatic responses to injury
During adult homeostasis, the cardiac lymphatics have functions akin to those of the systemic lymphatics in modulating tissue fluid and immune surveillance (as discussed above). Following injury, such as MI, the cardiac lymphatics respond according to whether the default wound healing is via fibrotic repair, as in adult mammals including humans, or via a regenerative response, as occurs during neonatal stages in mammals and in adult zebrafish.
Cardiac fibrotic repair
Oedema after myocardial infarction
MI is a consequence of coronary artery occlusion caused, for example, by the formation of atherosclerotic plaques in the arterial wall78, which results in reduced blood flow to the heart and can lead to prolonged ischaemia and to cardiomyocyte death. The endothelium in the ischaemic region is also affected, leading to increased vascular permeability and a substantial loss of lymphatic vessels, causing poor myocardial fluid drainage and persistent oedema7,17,79. Adult humans and other adult mammals lack the ability to regenerate the heart80. Therefore, after myocardial ischaemia, the heart remodels in an attempt to compensate for the loss of cardiovascular tissue, and healing occurs by replacing the dead myocardium with scar tissue81 (see below). During the remodelling phase, the cardiac lymphatics undergo lymphangiogenesis by growing and expanding in the infarcted area7,60,82,83,84. However, despite the endogenous lymphatic response, the myocardial oedema and inflammation persist7,60,82,83,84. An important contributing factor to the insufficient drainage by the cardiac lymphatics after MI could be the reduced cardiac contractility caused by the death of cardiomyocytes, which acts as the major extrinsic force for lymph propulsion from the heart to the MLNs85,86. The increase in interstitial pressure, combined with the loss of myocardium, eventually leads to fibrosis, impaired heart function and ultimately heart failure17,18,87.
The immune response to heart injury
In adult mice, shortly after the induction of MI through surgical ligation of the left anterior descending coronary artery88, circulating pro-inflammatory stimuli, such as damage-associated molecular patterns and cytokines, activate and recruit innate immune cells to the injury site89. Neutrophils and monocytes are the first to infiltrate the infarcted myocardium to clear debris and dead cells by phagocytosis and efferocytosis, respectively90. In mice, neutrophil numbers peak 3 days after MI, followed by a biphasic response of monocytes and monocyte-derived macrophages up to 5 days post-injury (dpi), with gradual reduction to baseline levels thereafter91. During these phases, the embryonic-derived tissue-resident macrophages die and are replaced by monocytes and monocyte-derived macrophages92. The first phase (1–4 dpi) of the immune response after injury in the adult heart is an inflammatory phase, with an increase in the number of pro-inflammatory monocyte-derived CCR2+ macrophages84,93. These cells secrete inflammatory and proteolytic factors and have increased phagocytosis and efferocytosis84,93. By contrast, the second phase (≥5 dpi) is an anti-inflammatory phase, with pro-reparative, tissue-resident CCR2− macrophages contributing to angiogenesis and scar formation84,93,94. Interestingly, macrophages were previously thought to contribute only indirectly to scar formation by supporting the activation of cardiac fibroblasts into myofibroblasts84. However, a study published in 2020 demonstrated that macrophages can directly contribute to scar formation in the adult heart after MI by expressing and depositing collagen95. As in the mouse model, hearts from adult patients with heart failure are also populated by tissue-resident CCR2− macrophages and monocyte-derived CCR2+ macrophages96. After MI in mice, the epicardium and the pro-inflammatory macrophages secrete VEGFC, which drives lymphangiogenesis and the extensive remodelling of the cardiac lymphatic network5,7,60. This endogenous response of the cardiac lymphatics attempts to maintain an optimal immune cell load, which is necessary for effective tissue repair7,60,82,83,84. However, the response of the cardiac lymphatics is insufficient to clear the immune cells, which results in chronic inflammation and increased scar formation6,60. Apart from neutrophils, monocytes and macrophages, other leukocytes, such as T cells, infiltrate the heart during the first week after MI in adult mice91. Although the response of the adaptive immune system to MI has not been well studied, the current view is that regulatory T cells have a beneficial role in cardiac healing97,98. By contrast, CD4+ effector T cells produce pro-inflammatory cytokines and CD8+ T cells have direct cytotoxic effects99,100. The immune response to MI in adult mice has been described in detail previously89.
Augmentation of injury-induced cardiac lymphangiogenesis
The endogenous lymphangiogenic response is insufficient to clear the myocardial oedema and the infiltrated immune cells after MI. This inefficient endogenous response has prompted attempts to increase lymphangiogenesis and lymphatic function in the injured heart. Augmentation of the lymphangiogenic response with administration of recombinant VEGFC-C156S, which interacts specifically with VEGFR3, has been shown to improve cardiac function after MI in animal models, as assessed by echocardiography and cine-MRI6,7,60 (Fig. 3). Different routes of VEGFC-C156S administration, such as protein therapy7,60 or gene therapy with either adenovirus or adeno-associated virus (AAV) vectors6, have yielded inconsistent outcomes that could potentially be attributed to the short half-life of the VEGFC-C156S protein47. Injection of microparticles loaded with VEGFC-C156S into the left ventricle after induction of MI led to an increased clearance of myocardial oedema in rats7. Intraperitoneal injection of AAV–VEGFC-C156S at 7 days before MI induced an increased clearance of T cells in female mice and male rats6. Lastly, intraperitoneal injection of recombinant VEGFC-C156S after MI in mice increased macrophage clearance via a LYVE1-dependent mechanism compared with vehicle treatment60. LYVE1 is highly expressed at the surface of the initial lymphatics and interacts specifically with the ubiquitous glycosaminoglycan polymer hyaluronan that coats the surface of leukocytes101. Engagement of LYVE1 with hyaluronan promotes the docking and transmigration of human macrophages to lymphatic vessels in vitro102. In addition, dendritic cells dock and transmigrate to LECs in a LYVE1–hyaluronan-dependent manner in a mouse model of dermal injury103. Disruption of this interaction led to impaired migration of dendritic cells to draining lymph nodes and reduced the CD8+ T cell response to antigens103. Whereas administration of VEGFC-C156S has been shown to increase lymphangiogenesis and improve cardiac function after MI in animal models, trapping of VEGFC and VEGFD with the use of the soluble decoy VEGFR3 (sVEGFR3) has provided contradicting results. Initially, a study found extensive cardiac lymphatic defects, intramyocardial haemorrhage and higher mortality in sVEGFR3-transgenic mice compared with control littermate mice after MI104. However, intraperitoneal injection of AAV–sVEGFR3 at 7 days before MI in female mice and male rats6 did not affect lymphangiogenesis but led to reduced infarct region thinning and T cell infiltration to the heart at 7 dpi, as well as to improved cardiac function at 21 dpi6. Of note, surviving cardiomyocytes at the border zone and infarcted area expressed high levels of Vegfr3 and underwent hypertrophy in the first days after MI105,106. In the same regions, Vegfc and Vegfd were upregulated within 3 dpi, and in vitro studies showed that VEGFC contributes to cardiomyocyte hypertrophy and survival105,106. These studies collectively point to non-lymphangiogenic roles for VEGFC in the infarcted heart, which have to be taken into consideration when interpreting experimental outcomes following interference of the VEGFR3–VEGFC pathway. Furthermore, sex-dependent differences have been found in the cardiac lymphatic vasculature under normal and MI conditions107, highlighting another variable that needs to be accounted for when assessing the lymphatic responses after MI.
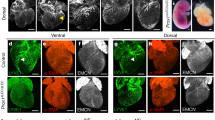
Schematic illustration of the endogenous response of the lymphatic vessels to myocardial infarction and the augmented response induced by administration of vascular endothelial growth factor C (VEGFC)-C156S. a | After induction of myocardial infarction through surgical ligation of the left anterior descending coronary artery, accumulation of fluids leads to oedema and infiltration of immune cells, such as macrophages, dendritic cells and T cells, into the myocardium. b | In response to myocardial infarction, cardiac lymphatic vessels undergo lymphangiogenesis in an attempt to clear the excessive tissue fluid and the inflammatory cells. However, this response is insufficient and the heart is repaired through fibrotic scar formation. c | Augmentation of the lymphangiogenic response through administration of VEGFC-C156S leads to decreased oedema and increased immune cell clearance and subsequently to improved cardiac healing and function.
Cardiac regeneration
Cardiac regeneration in neonatal mice
In contrast to adult mammalian hearts, which are incapable of functional recovery after injury, neonatal mammalian hearts have an evolutionarily conserved regenerative capacity80,108,109,110,111. The widely accepted notion is that in mice, the heart fully regenerates after left anterior descending coronary artery ligation at P1, whereas the same injury at P7 leads to fibrotic scarring112,113,114. Of note, anecdotal evidence from clinical case reports suggests that cardiac regeneration occurs in neonatal patients with MI caused by congenital heart disease115,116. In neonatal mice, the immune response triggered by MI is markedly different from that in adult animals, and these differences have been thoroughly reviewed previously117,118. Briefly, the macrophages found in normal hearts at early postnatal stages are primarily tissue-resident CCR2− macrophages that originate from embryonic sources and are maintained through local proliferation93,119,120. By contrast, circulating CCR2+ monocytes and monocyte-derived CCR2+ macrophages contribute little to the cardiac monocyte–macrophage population at these stages93,120. In response to cardiac injury in neonatal hearts, the number of tissue-resident CCR2− macrophages expands without additional infiltration of CCR2+ monocytes93.
Interestingly, general depletion of macrophages with the use of clodronate liposome treatment after MI at P1 inhibited cardiac regeneration and favoured fibrotic scar formation with significantly depressed cardiac function121. This lack of regeneration was attributed to impaired angiogenesis121, which is consistent with growing evidence supporting direct and indirect macrophage contributions to angiogenesis122. Although clodronate liposomes can specifically target macrophages123, they target phagocytic cells in general, such as dendritic cells, and these results therefore need to be interpreted with caution. Moreover, different macrophage depletion strategies can produce contrasting effects124. Therefore, examining the effects of depleting specific immune cell subpopulations on cardiac regeneration in neonatal mice with the use of genetic models is important. The essential function of macrophages in heart regeneration in neonatal mice121 together with the immunomodulatory role of lymphatic vessels in adult mouse hearts60 highlight an interesting area for further study as to how the cardiac lymphatics respond in a regenerative setting in mammals and to what extent the cardiac lymphatics interact with macrophages in neonatal hearts.
Cardiac regeneration in fish
Whereas the cardiac lymphatics have not been examined in the neonatal mouse model of heart regeneration, they have received attention in adult zebrafish models of cardiac injury during the past 2 years11,12,13. Zebrafish can fully regenerate their heart after apical resection without scar formation125 or via a temporary scar following cryoinjury126. During the first week after apical resection, vegfc expression increased transiently in the adult zebrafish, with no signs of cardiac lymphangiogenesis11,13. By contrast, elevated vegfc expression after cryoinjury lasted for weeks and was accompanied by enlargement and migration of lymphatic vessels into the wound site11,12,13. This observation suggests that different types of cardiac injury can initiate diverse healing responses. Similar to the mammalian immune response, macrophages and neutrophils have been reported to migrate to the injured site during the first week after cryoinjury in zebrafish13. The immune response and debris were cleared by the lymphatics from the wound area13. Moreover, disruption of the Vegfr3–Vegfc pathway blocked the lymphatic response to cryoinjury, which resulted in inefficient immune cell clearance and increased scar formation11,12,13. Surprisingly, the cardiac regenerative capacity was not completely lost in the absence of lymphatics, because a subset of zebrafish could fully recover after cryoinjury11,13. Nevertheless, data from RNA sequencing and immunostaining suggest that a lack of lymphatic response shifts the cardiac microenvironment from pro-regenerative to pro-inflammatory after cryoinjury in zebrafish, thereby affecting cardiac healing11,13.
In contrast to zebrafish, in Oryzias latipes (medaka), another teleost fish, the response to cardiac injury is excessive fibrosis and an unresolved scar127. Comparative analyses between the two species suggests that a reduced and delayed immune response impairs the regenerative ability of medaka after cryoinjury compared with that of zebrafish128. Astyanax mexicanus is a single fish species comprising surface-dwelling and cave-dwelling populations, which have altered physical and metabolic phenotypes while evolving independently in surface rivers versus caves in northern Mexico129. After surgical removal of the ventricular apex, surface-dwelling fish are able to fully regenerate their heart, whereas cavefish form a permanent fibrotic scar129. To date, the cardiac lymphatic vessels in medaka and A. mexicanus have not been investigated in terms of either their development or response to injury. Comparing the immune and lymphatic responses to cardiac injury between neonatal and adult mammals, between zebrafish and medaka, and between surface-dwelling and cave-dwelling A. mexicanus holds great promise for elucidating the mechanisms that lead to cardiac regeneration versus fibrotic repair after cardiac injury.
These studies raise important questions regarding the interaction of the cardiac lymphatics with immune cells and their contribution to heart repair after MI. For instance, is improved healing caused by the general clearance of immune cells or by selective clearance of subpopulations? How does alteration of lymphangiogenesis affect the clearance of adaptive immune cells, such as dendritic cells, and subsequently antigen presentation and recruitment of T cells to the infarcted region? In the future, it will be important to investigate in more detail the time-dependent interactions between lymphatic vessels and different immune cell types after MI. Finally, do the cardiac lymphatics in neonatal mammals respond and function differently from the cardiac lymphatics in adult mammals in order to retain pro-regenerative macrophages after MI? In summary, in adult rodents, endogenous lymphangiogenesis in response to cardiac injury seems to be insufficient to clear interstitial fluids and immune cell build-up, leading to chronic inflammation, myocardial oedema, fibrosis and impaired healing. However, this endogenous response can be augmented by increasing lymphangiogenesis, for instance, by VEGFC-C156S administration, as discussed above. Therefore, a better understanding of the molecular mechanisms by which lymphatic vessels respond to clear the oedema and infiltrated innate and adaptive immune cells after MI could provide the basis for developing therapies for patients with heart disease.
Clinical opportunities
MI is currently a major cause of mortality worldwide, and no treatments are currently available to revert the cardiac damage. Current treatments focus on early re-establishment of the blood flow to prevent further tissue damage and therapy with drugs such as angiotensin-converting enzyme (ACE) inhibitors and β-blockers to support the surviving myocardium130. Restoration of blood flow is initially accomplished by percutaneous coronary intervention or administration of thrombolytic drugs, which in severe cases involves invasive procedures, such as coronary artery bypass graft surgery or even heart transplantation130. Therefore, the development of new treatments to repair or regenerate the damaged myocardium continues to be of great interest. Initial studies focused on cell-based therapies involving the injection of cardiac or non-cardiac cells into the infarct area with the aim of replacing the lost cardiomyocytes and improving heart function after MI131,132. However, the evidence indicates that cell-based therapies alone are ineffective and require complementary approaches to make the cardiac microenvironment conducive to regeneration133,134,135.
A study published in 2020 showed that intracardiac injection of different types of adult stem and progenitor cells, dead cells or a chemical inducer of the innate immune response all improved heart function, which was attributed to an acute and beneficial immune response136. Therefore, early inflammation combined with a balanced innate and adaptive immune response seems to be crucial for optimal repair and potential regeneration of the infarcted heart, whereas broad immunosuppression has adverse effects137,138. As a result, a time-dependent, drug-mediated manipulation of the lymphatic response could help modulate the inflammatory content in the myocardium and promote both myocardial survival and restoration. Proof of principle is provided by the aforementioned studies targeting recombinant VEGFC-C156S to invoke increased lymphangiogenesis and improved outcome after MI5,6,7. However, VEGFC and its isoforms are not optimal drugs, given their very short half-life in serum47.
Currently, preclinical studies are investigating lymphangiogenesis as a potential drug target for immunomodulation after MI107,139,140,141. Most studies are focusing on the VEGFR3 signalling pathway, because the induction of this pathway has been shown to promote lymphangiogenesis and lead to better outcomes after MI in experimental animal models5,7,60,104. A phase I/IIa clinical trial assessing the efficacy and safety of intramyocardial adenovirus vector-mediated VEGFD-ΔNΔC gene therapy in patients with refractory angina showed promising results, with significant improvement in myocardial blood flow compared with placebo139. However, this positive finding is compromised by the need for repeat invasive administration of the gene therapy and the cost per patient. Therefore, exploring additional pathways that promote a cardiac lymphatic response is important. For instance, the epicardium-specific peptide adrenomedullin (encoded by Adm) has been identified as being cardioprotective through a beneficial effect on cardiac lymphatic permeability and lymphangiogenesis107,140. In a pilot clinical study, intravenous injection of adrenomedullin in patients with acute MI resulted in significantly improved cardiac structure and function, as evaluated by MRI, compared with baseline140. Additionally, overexpression of Adm in mice results in reduced oedema, dilated cardiac lymphatic vessels and improved cardiac function after MI107. In this study, adrenomedullin was found to regulate the gap junction protein connexin 43 in cardiac LECs, promoting their coupling and potentially increasing the permeability of the lymphatics107. This study highlights the importance of preclinical research focusing on inducing cardiac lymphatic growth by lymphangiogenesis and improving their functional maturation, and shows the therapeutic potential of this approach.
Targeting lymphangiogenesis could also have clinical applications beyond cardiovascular disease142. While this Review was in preparation, the global pandemic of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged. The vast majority of patients with COVID-19 have heart and lung complications, and post-mortem analyses have suggested that an excessive inflammatory response (known as a ‘cytokine storm’) and associated damage to organ microcirculation are major contributors to disease severity and mortality143. In addition, patients with pre-existing cardiovascular disease develop a more severe COVID-19 response to SARS-CoV-2 infection owing to direct viral effects on a compromised myocardium (myocarditis) and/or indirect effects via cardiac hyperinflammation and impaired coronary microvasculature144,145,146,147. Notably, children usually present with more mild COVID-19 symptoms than adult patients148. This difference has been hypothesized to be due to a more immature immune response at younger ages148. Considering this circumstantial evidence, one might speculate that augmentation of lymphatic growth and function could assist in clearing the oedema and immune cell accumulation in the hyperinflamed tissue environment in patients with COVID-19, thereby improving the disease outcome.
Conclusions
The development and function of the lymphatic vasculature, often described as the secondary circulatory system, have received increasing attention in the past decade. Studies using state-of-the-art imaging technologies and genetic models have focused on the lymphatic endothelium in more detail, revealing tissue-specific heterogeneity in origin, function and response to injury. In contrast to the pre-existing dogma, several non-venous sources have been shown to contribute to the cardiac lymphatics, indicating that further research is required to characterize fully the ontogeny of the cardiac lymphatics in different settings. In the context of adult cardiac injury, the lymphatics of the heart respond by growth and sprouting in an attempt to clear the oedema and immune cells from the damaged tissue. In animal models, augmentation of the cardiac lymphatic response after cardiac injury has proved to be beneficial for wound healing, whereas general immunosuppression leads to severe adverse effects. This finding supports the notion that controlled immunomodulation by lymphangiogenesis could be of great clinical value for treating patients with ischaemic heart disease and preventing or even reversing heart failure. Nevertheless, open questions remain regarding the response and function of the cardiac lymphatics in the disease setting, which requires close collaboration between basic and clinical researchers to deliver effective therapies. Overall, the focus needs to be on implementing a combinatorial approach to tackle the complexity of both restoring lost cardiovascular tissue and conditioning the local injury environment. Targeting the regulation of cardiac lymphangiogenesis to improve fluid balance and modulate the immune response and downstream fibrosis might emerge as a viable strategy to contribute to combined therapy for cardiac repair and regeneration.
References
Morfoisse, F. & Noel, A. Lymphatic and blood systems: identical or fraternal twins? Int. J. Biochem. Cell Biol. 114, 105562 (2019).
Potente, M. & Mäkinen, T. Vascular heterogeneity and specialization in development and disease. Nat. Rev. Mol. Cell Biol. 18, 477–494 (2017).
Petrova, T. V. & Koh, G. Y. Organ-specific lymphatic vasculature: from development to pathophysiology. J. Exp. Med. 215, 35–49 (2018).
Gancz, D., Perlmoter, G. & Yaniv, K. Formation and growth of cardiac lymphatics during embryonic development, heart regeneration, and disease. Cold Spring Harb. Perspect. Biol. 12, a037176 (2020).
Klotz, L. et al. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522, 62–67 (2015). This study shows that cardiac lymphatics have a unique ontology and that promoting lymphangiogenesis can improve cardiac function following MI in mice.
Houssari, M. et al. Lymphatic and immune cell cross-talk regulates cardiac recovery after experimental myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 40, 1722–1737 (2020).
Henri, O. et al. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation 133, 1484–1497 (2016). This study shows that remodelling of cardiac lymphatics leads to myocardial oedema following MI and promoting lymphangiogenesis can improve cardiac function by restoring interstitial fluid equilibrium in rats.
Lioux, G. et al. A second heart field-derived vasculogenic niche contributes to cardiac lymphatics. Dev. Cell 52, 350–363.e6 (2020). This study shows that the second heart field contributes to cardiac lymphatic endothelial cells through a local niche at the base of the great arteries.
Maruyama, K., Miyagawa-Tomita, S., Mizukami, K., Matsuzaki, F. & Kurihara, H. Isl1-expressing non-venous cell lineage contributes to cardiac lymphatic vessel development. Dev. Biol. 452, 134–143 (2019).
Nicenboim, J. et al. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature 522, 56–61 (2015).
Vivien, C. J. et al. Vegfc/d-dependent regulation of the lymphatic vasculature during cardiac regeneration is influenced by injury context. NPJ Regen. Med. 4, 18 (2019). Together with Gancz et al. (2019) and Harrison et al. (2019), this study shows that cardiac lymphatics are required for complete heart regeneration in adult zebrafish after cryosection injury.
Gancz, D. et al. Distinct origins and molecular mechanisms contribute to lymphatic formation during cardiac growth and regeneration. eLife 8, e44153 (2019).
Harrison, M. R. et al. Late developing cardiac lymphatic vasculature supports adult zebrafish heart function and regeneration. eLife 8, e42762 (2019).
Moore, J. E. & Bertram, C. D. Lymphatic system flows. Annu. Rev. Fluid Mech. 50, 459–482 (2018).
Scallan, J. P., Zawieja, S. D., Castorena-Gonzalez, J. A. & Davis, M. J. Lymphatic pumping: mechanics, mechanisms and malfunction. J. Physiol. 594, 5749–5768 (2016).
Jackson, D. G. Leucocyte trafficking via the lymphatic vasculature — mechanisms and consequences. Front. Immunol. 10, 471 (2019).
Dongaonkar, R. M., Stewart, R. H., Geissler, H. J. & Laine, G. A. Myocardial microvascular permeability, interstitial oedema, and compromised cardiac function. Cardiovasc. Res. 87, 331–339 (2010).
Laine, G. A. & Allen, S. J. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ. Res. 68, 1713–1721 (1991).
Ryan, T. J. Structure and function of lymphatics. J. Invest. Dermatol. 93, 18S–24S (1989).
Ratajska, A. et al. Comparative and developmental anatomy of cardiac lymphatics. Sci. World J. 2014, 183170 (2014).
Johnson, R. A. & Blake, T. M. Lymphatics of the heart. Circulation 33, 137–142 (1966).
Sacchi, G., Weber, E., Aglianò, M., Cavina, N. & Comparini, L. Lymphatic vessels of the human heart: precollectors and collecting vessels. A morpho-structural study. J. Submicrosc. Cytol. Pathol. 31, 515–525 (1999).
Böger, A. & Hort, W. Distribution of the lymph vessels in the mouse heart. A light and electron microscopic study [German]. Basic Res. Cardiol. 72, 510–529 (1977).
Marchetti, C., Poggi, P., Calligaro, A. & Casasco, A. Lymph vessels of the rabbit heart: distribution and fine structure in atria. Lymphology 19, 33–37 (1986).
Schmid-Schönbein, G. W. The second valve system in lymphatics. Lymphat. Res. Biol. 1, 25–31 (2003).
Bazigou, E., Wilson, J. T. & Moore, J. E. Primary and secondary lymphatic valve development: molecular, functional and mechanical insights. Microvasc. Res. 96, 38–45 (2014).
Trzewik, J. et al. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 15, 1711–1717 (2001).
Zhang, F. et al. Lacteal junction zippering protects against diet-induced obesity. Science 361, 599–603 (2018).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007).
Leak, L. V. & Burke, J. F. Ultrastructural studies on the lymphatic anchoring filaments. J. Cell Biol. 36, 129–149 (1968).
Sacchi, G., Weber, E., Aglianò, M., Raffaelli, N. & Comparini, L. The structure of superficial lymphatics in the human thigh: precollectors. Anat. Rec. 247, 53–62 (1997).
Juszyn´ski, M., Ciszek, B., Stachurska, E., Jabłon´ska, A. & Ratajska, A. Development of lymphatic vessels in mouse embryonic and early postnatal hearts. Dev. Dyn. 237, 2973–2986 (2008).
Flaht-Zabost, A. et al. Cardiac mouse lymphatics: developmental and anatomical update. Anat. Rec. 297, 1115–1130 (2014).
Eliska, O., Eliskova, M. & Miller, A. J. The absence of lymphatics in normal and atherosclerotic coronary arteries in man: a morphologic study. Lymphology 39, 76–83 (2006).
Shimada, T., Morita, T., Oya, M. & Kitamura, H. Morphological studies of the cardiac lymphatic system. Arch. Histol. Cytol. 53, 115–126 (1990).
Schineis, P., Runge, P. & Halin, C. Cellular traffic through afferent lymphatic vessels. Vascul. Pharmacol. 112, 31–41 (2019).
Jalkanen, S. & Salmi, M. Lymphatic endothelial cells of the lymph node. Nat. Rev. Immunol. 20, 566–578 (2020).
Liao, S. & von der Weid, P. Y. Lymphatic system: an active pathway for immune protection. Semin. Cell Dev. Biol. 38, 83–89 (2015).
Hunter, M. C., Teijeira, A. & Halin, C. T cell trafficking through lymphatic vessels. Front. Immunol. 7, 613 (2016).
Mandl, J. N. et al. Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naïve CD4+ and CD8+ T cells. Proc. Natl Acad. Sci. USA 109, 18036–18041 (2012).
Srinivasan, R. S. & Oliver, G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 25, 2187–2197 (2011).
Hess, P. R. et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J. Clin. Invest. 124, 273–284 (2014).
Turner, C. J., Badu-Nkansah, K., Crowley, D., van der Flier, A. & Hynes, R. O. Integrin-α5β1 is not required for mural cell functions during development of blood vessels but is required for lymphatic-blood vessel separation and lymphovenous valve formation. Dev. Biol. 392, 381–392 (2014).
Geng, X. et al. Multiple mouse models of primary lymphedema exhibit distinct defects in lymphovenous valve development. Dev. Biol. 409, 218–233 (2016).
Martinez-Corral, I. et al. Nonvenous origin of dermal lymphatic vasculature. Circ. Res. 116, 1649–1654 (2015).
Stanczuk, L. et al. CKit lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep. 10, 1708–1721 (2015).
Veikkola, T. et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 20, 1223–1231 (2001).
Karkkainen, M. J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Srinivasan, R. S. et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 21, 2422–2432 (2007). This study identified the venous origin of the lymphatic vasculature.
Rutkowski, J. M., Boardman, K. C. & Swartz, M. A. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am. J. Physiol. Heart Circ. Physiol. 291, H1402–H1410 (2006).
Sabin, F. R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am. J. Anat. 1, 367–389 (1902).
Huntington, G. S. & McClure, C. F. W. The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica). Am. J. Anat. 10, 177–312 (1910).
Stone, O. A. & Stainier, D. Y. R. Paraxial mesoderm is the major source of lymphatic endothelium. Dev. Cell 50, 247–255.e3 (2019). This study shows that the paraxial mesoderm is a major source of lymphatic endothelial cells, suggesting that lymphatic endothelial cell fate is imprinted earlier in development than previously thought.
Hong, Y. K. & Detmar, M. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 314, 85–92 (2003).
Wigle, J. T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
François, M. et al. Sox18 induces development of the lymphatic vasculature in mice. Nature 456, 643–647 (2008).
Srinivasan, R. S. et al. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev. 24, 696–707 (2010).
Murtomaki, A. et al. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development 140, 2365–2376 (2012).
Srinivasan, R. S. et al. The Prox1–Vegfr3 feedback loop maintains the identity and the number of lymphatic endothelial cell progenitors. Genes Dev. 28, 2175–2187 (2014).
Vieira, J. M. et al. The cardiac lymphatic system stimulates resolution of inflammation following myocardial infarction. J. Clin. Invest. 128, 3402–3412 (2018). This study shows that promoting lymphangiogenesis can improve cardiac function by increasing immune cell clearance through a LYVE1-dependent mechanism in mice.
Banerji, S. et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144, 789–801 (1999).
Gale, N. W. et al. Normal lymphatic development and function in mice deficient for the lymphatic hyaluronan receptor LYVE-1. Mol. Cell. Biol. 27, 595–604 (2007).
Luong, M. X. et al. Lack of lymphatic vessel phenotype in LYVE-1/CD44 double knockout mice. J. Cell. Physiol. 219, 430–437 (2009).
Yang, Y. et al. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120, 2340–2348 (2012).
Yao, L. C., Baluk, P., Srinivasan, R. S., Oliver, G. & McDonald, D. M. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. Am. J. Pathol. 180, 2561–2575 (2012).
Zhang, F., Zarkada, G., Yi, S. & Eichmann, A. Lymphatic endothelial cell junctions: molecular regulation in physiology and diseases. Front. Physiol. 11, 509 (2020).
Janardhan, H. P. & Trivedi, C. M. Establishment and maintenance of blood–lymph separation. Cell. Mol. Life Sci. 76, 1865–1876 (2019).
Welsh, J. D., Kahn, M. L. & Sweet, D. T. Lymphovenous hemostasis and the role of platelets in regulating lymphatic flow and lymphatic vessel maturation. Blood 128, 1169–1173 (2016).
Oliver, G., Kipnis, J., Randolph, G. J. & Harvey, N. L. The lymphatic vasculature in the 21st century: novel functional roles in homeostasis and disease. Cell 182, 270–296 (2020).
Petrova, T. V. & Koh, G. Y. Biological functions of lymphatic vessels. Science 369, eaax4063 (2020).
Chen, H. I. et al. The sinus venosus contributes to coronary vasculature through VEGFC-stimulated angiogenesis. Development 141, 4500–4512 (2014).
Jafree, D. J. et al. Spatiotemporal dynamics and heterogeneity of renal lymphatics in mammalian development and cystic kidney disease. eLife 8, e48183 (2019).
Merz, S. F. et al. Contemporaneous 3D characterization of acute and chronic myocardial I/R injury and response. Nat. Commun. 10, 2312 (2019).
Ulvmar, M. H., Martinez-Corral, I., Stanczuk, L. & Mäkinen, T. Pdgfrb-Cre targets lymphatic endothelial cells of both venous and non-venous origins. Genesis 54, 350–358 (2016).
Buckingham, M. E. & Meilhac, S. M. Tracing cells for tracking cell lineage and clonal behavior. Dev. Cell 21, 394–409 (2011).
Eng, T. C. et al. Zebrafish facial lymphatics develop through sequential addition of venous and non-venous progenitors. EMBO Rep. 20, e47079 (2019).
Okuda, K. S. et al. Lyve1 expression reveals novel lymphatic vessels and new mechanisms for lymphatic vessel development in zebrafish. Development 139, 2381–2391 (2012).
Frangogiannis, N. G. Pathophysiology of myocardial infarction. Compr. Physiol. 5, 1841–1875 (2015).
Weis, S. et al. Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction. J. Clin. Invest. 113, 885–894 (2004).
Price, E. L., Vieira, J. M. & Riley, P. R. Model organisms at the heart of regeneration. Dis. Model. Mech. 12, dmm040691 (2019).
Sam, F. et al. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am. J. Physiol. Heart Circ. Physiol. 279, H422–H428 (2000).
Van Amerongen, M. J., Harmsen, M. C., Van Rooijen, N., Petersen, A. H. & Van Luyn, M. J. A. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am. J. Pathol. 170, 818–829 (2007).
Frantz, S. et al. Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J. 27, 871–881 (2013).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Taira, A. et al. Flow velocity of cardiac lymph and contractility of the heart: an experimental study. Ann. Thorac. Surg. 23, 230–234 (1977).
Mehlhorn, U., Geissler, H. J., Laine, G. A. & Allen, S. J. Myocardial fluid balance. Eur. J. Cardiothorac. Surg. 20, 1220–1230 (2001).
Geissler, H. et al. Impact of cardiopulmonary bypass and cardioplegic arrest on myocardial efficiency. Crit. Care 3, P21 (1999).
Lugrin, J., Parapanov, R., Krueger, T. & Liaudet, L. Murine myocardial infarction model using permanent ligation of left anterior descending coronary artery. J. Vis. Exp. 150, e59591 (2019).
Adamo, L., Rocha-Resende, C., Prabhu, S. D. & Mann, D. L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 17, 269–285 (2020).
DeBerge, M. et al. Efferocytosis and outside-in signaling by cardiac phagocytes. Links to repair, cellular programming, and intercellular crosstalk in heart. Front. Immunol. 8, 1428 (2017).
Yan, X. et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 62, 24–35 (2013).
Bajpai, G. et al. Tissue resident CCR2− and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ. Res. 124, 263–278 (2019).
Lavine, K. J. et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. USA 111, 16029–16034 (2014). This study shows that neonatal hearts expand a population of embryonic-derived cardiac resident macrophages after MI, in contrast to adult hearts, which replace this population with monocyte-derived macrophages.
Dick, S. A. et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 20, 29–39 (2019).
Simões, F. C. et al. Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat. Commun. 11, 600 (2020).
Bajpai, G. et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 24, 1234–1245 (2018).
Tang, T. T. et al. Regulatory T cells ameliorate cardiac remodeling after myocardial infarction. Basic Res. Cardiol. 107, 232 (2012).
Matsumoto, K. et al. Regulatory T lymphocytes attenuate myocardial infarction-induced ventricular remodeling in mice. Int. Heart J. 52, 382–387 (2011).
Hofmann, U. et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation 125, 1652–1663 (2012).
Tae Yu, H. et al. Characterization of CD8+ CD57+ T cells in patients with acute myocardial infarction. Cell. Mol. Immunol. 12, 466–473 (2015).
Jackson, D. G. Hyaluronan in the lymphatics: the key role of the hyaluronan receptor LYVE-1 in leucocyte trafficking. Matrix Biol. 78–79, 219–235 (2019).
Lawrance, W., Banerji, S., Day, A. J., Bhattacharjee, S. & Jackson, D. G. Binding of hyaluronan to the native lymphatic vessel endothelial receptor LYVE-1 is critically dependent on receptor clustering and hyaluronan organization. J. Biol. Chem. 291, 8014–8030 (2016).
Johnson, L. A. et al. Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1. Nat. Immunol. 18, 762–770 (2017). This study shows that the interaction between hyaluronan and LYVE1 initiates the trafficking of dendritic cells through lymphatic vessels.
Vuorio, T. et al. Downregulation of VEGFR3 signaling alters cardiac lymphatic vessel organization and leads to a higher mortality after acute myocardial infarction. Sci. Rep. 8, 16709 (2018).
Zhao, T. et al. VEGF-C/VEGFR-3 pathway promotes myocyte hypertrophy and survival in the infarcted myocardium. Am. J. Transl. Res. 7, 697–709 (2015).
Zhao, T. et al. Differential expression of vascular endothelial growth factor isoforms and receptor subtypes in the infarcted heart. Int. J. Cardiol. 167, 2638–2645 (2013).
Trincot, C. E. et al. Adrenomedullin induces cardiac lymphangiogenesis after myocardial infarction and regulates cardiac edema via connexin 43. Circ. Res. 124, 101–113 (2019).
Lam, N. T. & Sadek, H. A. Neonatal heart regeneration comprehensive literature review. Circulation 138, 421–423 (2018).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011). This study was the first to demonstrate the cardiac regenerative window in neonatal mice, and the studies by Wang et al. (Cells, 2020) and Ye et al. (2018) demonstrate the cardiac regenerative window in rats and pigs, respectively.
Wang, H. et al. Natural heart regeneration in a neonatal rat myocardial infarction model. Cells 9, 229 (2020).
Ye, L. et al. Early regenerative capacity in the porcine heart. Circulation 138, 2798–2808 (2018).
Sereti, K. I. et al. Analysis of cardiomyocyte clonal expansion during mouse heart development and injury. Nat. Commun. 9, 754 (2018).
Gunadasa-Rohling, M. et al. Magnetic resonance imaging of the regenerating neonatal mouse heart. Circulation 138, 2439–2441 (2018).
Porrello, E. R. et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl Acad. Sci. USA 110, 187–192 (2013).
Saker, D. M., Walsh-Sukys, M., Spector, M. & Zahka, K. G. Cardiac recovery and survival after neonatal myocardial infarction. Pediatr. Cardiol. 18, 139–142 (1997).
Haubner, B. J. et al. Functional recovery of a human neonatal heart after severe myocardial infarction. Circ. Res. 118, 216–221 (2016).
Sattler, S. & Rosenthal, N. The neonate versus adult mammalian immune system in cardiac repair and regeneration. Biochim. Biophys. Acta Mol. Cell Res. 1863, 1813–1821 (2016).
Dittrich, A. & Lauridsen, H. Myocardial infarction and the immune response – scarring or regeneration? A comparative look at mammals and popular regenerating animal models. J. Immunol. Regen. Med. 4, 100016 (2019).
Wang, Z. et al. Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc. Natl Acad. Sci. USA 116, 18455–18465 (2019).
Molawi, K. et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 211, 2151–2158 (2014).
Aurora, A. B. et al. Macrophages are required for neonatal heart regeneration. J. Clin. Invest. 124, 1382–1392 (2014). This study shows that macrophages are necessary for the regeneration of the neonatal mouse heart after MI by providing signals to drive angiogenesis.
Du Cheyne, C., Tay, H. & De Spiegelaere, W. The complex TIE between macrophages and angiogenesis. Anat. Histol. Embryol. 49, 585–596 (2020).
van Rooijen, N. & Hendrikx, E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol. Biol. 605, 189–203 (2010).
Ferenbach, D. A. et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 82, 928–933 (2012).
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002).
González-Rosa, J. M., Martín, V., Peralta, M., Torres, M. & Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138, 1663–1674 (2011).
Ito, K. et al. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev. Dyn. 243, 1106–1115 (2014).
Lai, S. L. et al. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. eLife 6, e25605 (2017).
Stockdale, W. T. et al. Heart regeneration in the Mexican cavefish. Cell Rep. 25, 1997–2007.e7 (2018).
Reddy, K. Recent advances in the diagnosis and treatment of acute myocardial infarction. World J. Cardiol. 7, 243–276 (2015).
Orlic, D. et al. Bone marrow cells regenerate infarcted myocardium. Nature 410, 701–705 (2001).
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Eschenhagen, T. et al. Cardiomyocyte regeneration: a consensus statement. Circulation 136, 680–686 (2017).
Quyyumi, A. A. et al. PreSERVE-AMI: a randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ. Res. 120, 324–331 (2017).
Tompkins, B. A. et al. Preclinical studies of stem cell therapy for heart disease. Circ. Res. 122, 1006–1020 (2018).
Vagnozzi, R. J. et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577, 405–409 (2020). This study shows that cell death following stem-cell transplantation after MI improves outcome and function by inducing an acute immune-based wound-healing response.
Chung, E. S., Packer, M., Lo, K. H., Fasanmade, A. A. & Willerson, J. T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure: results of the Anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107, 3133–3140 (2003).
Roberts, R., DeMello, V. & Sobel, B. E. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation 53, I204–I206 (1976).
Hartikainen, J. et al. Adenoviral intramyocardial VEGF-DDNDC gene transfer increasesmyocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1-year follow-up. Eur. Heart J. 38, 2547–2555 (2017).
Kataoka, Y. et al. The first clinical pilot study of intravenous adrenomedullin administration in patients with acute myocardial infarction. J. Cardiovasc. Pharmacol. 56, 413–419 (2010).
Tatin, F. et al. Apelin modulates pathological remodeling of lymphatic endothelium after myocardial infarction. JCI Insight 2, e93887 (2017).
Maisel, K., Sasso, M. S., Potin, L. & Swartz, M. A. Exploiting lymphatic vessels for immunomodulation: RATIONALE, opportunities, and challenges. Adv. Drug Deliv. Rev. 114, 43–59 (2017).
Merad, M. & Martin, J. C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 20, 355–362 (2020).
Madjid, M., Safavi-Naeini, P., Solomon, S. D. & Vardeny, O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 5, 831–840 (2020).
Wang, L., Zhang, Y. & Zhang, S. Cardiovascular impairment in COVID-19: learning from current options for cardiovascular anti-inflammatory therapy. Front. Cardiovasc. Med. 7, 78 (2020).
Nishiga, M. et al. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 17, 543–558 (2020).
Xu, H. et al. Acute myocardial injury of patients with coronavirus disease 2019. Preprint at medRxiv https://doi.org/10.1101/2020.03.05.20031591 (2020).
Carsetti, R. et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child. Adolesc. Health 4, 414–416 (2020).
Ahn, J. H. et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 572, 62–66 (2019).
Antila, S. et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 214, 3645–3667 (2017).
Hsu, M. et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 10, 229 (2019).
Izen, R. M., Yamazaki, T., Nishinaka-Arai, Y., Hong, Y. K. & Mukouyama, Y. S. Postnatal development of lymphatic vasculature in the brain meninges. Dev. Dyn. 247, 741–753 (2018).
Nurmi, H. et al. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol. Med. 7, 1418–1425 (2015).
Bernier-Latmani, J. et al. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J. Clin. Invest. 125, 4572–4586 (2015).
Mahadevan, A. et al. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev. Cell 31, 690–706 (2014).
Lee, H. W. et al. Expression of lymphatic endothelium-specific hyaluronan receptor LYVE-1 in the developing mouse kidney. Cell Tissue Res. 343, 429–444 (2011).
Russell, P. S., Hong, J., Windsor, J. A., Itkin, M. & Phillips, A. R. J. Renal lymphatics: anatomy, physiology, and clinical implications. Front. Physiol. 10, 251 (2019).
Monroy, M., McCarter, A. L., Hominick, D., Cassidy, N. & Dellinger, M. T. Lymphatics in bone arise from pre-existing lymphatics. Development 147, dev184291 (2020).
Gur-Cohen, S. et al. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 366, 1218–1225 (2019).
Pichol-Thievend, C. et al. A blood capillary plexus-derived population of progenitor cells contributes to genesis of the dermal lymphatic vasculature during embryonic development. Development 145, dev160184 (2018).
Gordon, E. J. et al. Macrophages define dermal lymphatic vessel calibre during development by regulating lymphatic endothelial cell proliferation. Development 137, 3899–3910 (2010).
Sun, M. et al. Hyaluronan derived from the limbus is a key regulator of corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 60, 1050–1062 (2019).
Maruyama, K. et al. The maintenance of lymphatic vessels in the cornea is dependent on the presence of macrophages. Invest. Ophthalmol. Vis. Sci. 53, 3145–3153 (2012).
Maruyama, K. et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Invest. 115, 2363–2372 (2005).
Diamond, M. A. et al. Lymphatic vessels identified in failed corneal transplants with neovascularisation. Br. J. Ophthalmol. 103, 421–427 (2019).
Kelley, P. M., Steele, M. M. & Tempero, R. M. Regressed lymphatic vessels develop during corneal repair. Lab. Investig. 91, 1643–1651 (2011).
Hos, D. et al. Transient ingrowth of lymphatic vessels into the physiologically avascular cornea regulates corneal edema and transparency. Sci. Rep. 7, 7227 (2017).
Lee, H. S. et al. Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease. Investig. Ophthalmol. Vis. Sci. 56, 3140–3148 (2015).
Kiesewetter, A., Cursiefen, C., Eming, S. A. & Hos, D. Phase-specific functions of macrophages determine injury-mediated corneal hem- and lymphangiogenesis. Sci. Rep. 9, 308 (2019).
Acknowledgements
K.K. is funded by the Wellcome Trust Foundation (4-year PhD studentship 215103/Z/18/Z). J.M.V. is supported by a British Heart Foundation Intermediate Basic Science Research Fellowship (FS/19/31/34158). P.R.R. is supported by a British Heart Foundation chair award (CH/11/1/28798) and programme grant (RG/08/003/25264).
Author information
Authors and Affiliations
Contributions
K.K. researched data for the article and wrote the manuscript. J.M.V. and P.R.R. edited the manuscript before submission. All authors provided substantial contribution to the discussion of content.
Corresponding authors
Ethics declarations
Competing interests
P.R.R. is co-founder and equity holder in OxStem Cardio, an Oxford University spin-out that seeks to exploit therapeutic strategies stimulating endogenous repair in cardiovascular regenerative medicine. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks C.-L. Lien and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Cardinal vein
-
The basis for the intraembryonic venous circulation consisting of anterior and posterior cardinal veins, which drain blood from the head and body into a pair of common cardinal veins and subsequently empty into the sinus venosus of the primitive heart.
- Lymphvasculogenesis
-
De novo formation of new lymphatic vessels from progenitor cell clustering.
- Lymphangiogenesis
-
Formation of new lymphatic vessels arising from pre-existing ones, typically by sprouting.
- Paraxial mesoderm
-
Subpopulation of mesoderm-containing progenitor cells that give rise to somites, which form muscle, connective tissue and the dermis.
- Haemogenic endothelium
-
A special subset of vascular endothelium that acquires haematopoietic potential and can differentiate to haematopoietic stem and progenitor cells.
- Yolk sac
-
The first membranous sac that is attached to and envelopes the developing embryo, providing early nutrition and the first site of blood cell production.
- Second heart field
-
(SHF). Cardiac progenitor cells in splanchnic mesoderm that contribute myocardium and smooth muscle to the formed heart tube at either the arterial or the venous pole.
- Phagocytosis
-
The process by which a cell uses its plasma membrane to engulf a large particle or another cell.
- Efferocytosis
-
The process by which dying cells are removed by phagocytic cells, before their membrane integrity is breached.
Rights and permissions
About this article
Cite this article
Klaourakis, K., Vieira, J.M. & Riley, P.R. The evolving cardiac lymphatic vasculature in development, repair and regeneration. Nat Rev Cardiol 18, 368–379 (2021). https://doi.org/10.1038/s41569-020-00489-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-020-00489-x
This article is cited by
-
Animal models to study cardiac regeneration
Nature Reviews Cardiology (2024)
-
Progress of Mitochondrial Function Regulation in Cardiac Regeneration
Journal of Cardiovascular Translational Research (2024)
-
Clinical assessment and molecular mechanism of the upregulation of Toll-like receptor 2 (TLR2) in myocardial infarction
BMC Cardiovascular Disorders (2022)
-
Signaling cascades in the failing heart and emerging therapeutic strategies
Signal Transduction and Targeted Therapy (2022)
-
The Vasculature in Pulmonary Fibrosis
Current Tissue Microenvironment Reports (2022)