Abstract
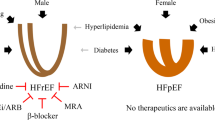
Heart failure with preserved ejection fraction (HFpEF) affects half of all patients with heart failure worldwide, is increasing in prevalence, confers substantial morbidity and mortality, and has very few effective treatments. HFpEF is arguably the greatest unmet medical need in cardiovascular disease. Although HFpEF was initially considered to be a haemodynamic disorder characterized by hypertension, cardiac hypertrophy and diastolic dysfunction, the pandemics of obesity and diabetes mellitus have modified the HFpEF syndrome, which is now recognized to be a multisystem disorder involving the heart, lungs, kidneys, skeletal muscle, adipose tissue, vascular system, and immune and inflammatory signalling. This multiorgan involvement makes HFpEF difficult to model in experimental animals because the condition is not simply cardiac hypertrophy and hypertension with abnormal myocardial relaxation. However, new animal models involving both haemodynamic and metabolic disease, and increasing efforts to examine human pathophysiology, are revealing new signalling pathways and potential therapeutic targets. In this Review, we discuss the cellular and molecular pathobiology of HFpEF, with the major focus being on mechanisms relevant to the heart, because most research has focused on this organ. We also highlight the involvement of other important organ systems, including the lungs, kidneys and skeletal muscle, efforts to characterize patients with the use of systemic biomarkers, and ongoing therapeutic efforts. Our objective is to provide a roadmap of the signalling pathways and mechanisms of HFpEF that are being characterized and which might lead to more patient-specific therapies and improved clinical outcomes.
Key points
-
The historical focus of studies into the pathophysiology of heart failure with preserved ejection fraction (HFpEF) has been on diastolic dysfunction, cardiac hypertrophy and myocardial fibrosis.
-
However, HFpEF actually involves many different components affecting both systolic and diastolic heart function and also other organs and systems, including the lungs, kidneys, vasculature, adipose tissue and skeletal muscle.
-
Preclinical studies, particularly those combining obesity and metabolic defects with haemodynamic and cardiac disease as occurs in the majority of patients with HFpEF, are beginning to reveal novel molecular mechanisms and therapeutic targets.
-
The proposed molecular and cellular abnormalities in HFpEF and those observed in diabetes mellitus and obesity overlap substantially, including metabolic defects in fuel utilization and efficiency, inflammatory responses, lipotoxicity, pathological growth of myocytes and loss of cytoprotective signalling.
-
In addition to exploring novel haemodynamic interventions with drugs and devices, new therapies are targeting pleiotropic signalling cascades to counteract changes in metabolic, inflammatory and pathological stress pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
21 January 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41569-021-00516-5
References
Gladden, J. D., Chaanine, A. H. & Redfield, M. M. Heart failure with preserved ejection fraction. Ann. Rev. Med. 69, 65–79 (2018).
Lam, C. S. P. et al. Mortality associated with heart failure with preserved vs. reduced ejection fraction in a prospective international multi-ethnic cohort study. Eur. Heart J. 39, 1770–1780 (2018).
Zakeri, R. & Cowie, M. R. Heart failure with preserved ejection fraction: controversies, challenges and future directions. Heart 104, 377–384 (2018).
Shah, S. J. et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute working group summary. Circulation 141, 1001–1026 (2020).
Dodek, A., Kassebaum, D. G. & Bristow, J. D. Pulmonary edema in coronary-artery disease without cardiomegaly. Paradox of the stiff heart. N. Engl. J. Med. 286, 1347–1350 (1972).
Dougherty, A. H., Naccarelli, G. V., Gray, E. L., Hicks, C. H. & Goldstein, R. A. Congestive heart failure with normal systolic function. Am. J. Cardiol. 54, 778–782 (1984).
Soufer, R. et al. Intact systolic left ventricular function in clinical congestive heart failure. Am. J. Cardiol. 55, 1082–1086 (1985).
Topol, E. J., Traill, T. A. & Fortuin, N. J. Hypertensive hypertrophic cardiomyopathy of the elderly. N. Engl. J. Med. 312, 277–283 (1985).
Vasan, R. S. et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J. Am. Coll. Cardiol. 33, 1948–1955 (1999).
Owan, T. E. et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 355, 251–259 (2006).
Melenovsky, V. et al. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J. Am. Coll. Cardiol. 49, 198–207 (2007).
Kawaguchi, M., Hay, I., Fetics, B. & Kass, D. A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation 107, 714–720 (2003).
Borlaug, B. A., Lam, C. S., Roger, V. L., Rodeheffer, R. J. & Redfield, M. M. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 54, 410–418 (2009).
Borlaug, B. A. et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114, 2138–2147 (2006).
Phan, T. T. et al. Impaired heart rate recovery and chronotropic incompetence in patients with heart failure with preserved ejection fraction. Circ. Heart Fail. 3, 29–34 (2010).
Dominguez, E. et al. Heart rate response and functional capacity in patients with chronic heart failure with preserved ejection fraction. ESC. Heart Fail. 5, 579–585 (2018).
Lam, C. S. et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J. Am. Coll. Cardiol. 53, 1119–1126 (2009).
Thenappan, T., Prins, K. W., Cogswell, R. & Shah, S. J. Pulmonary hypertension secondary to heart failure with preserved ejection fraction. Can. J. Cardiol. 31, 430–439 (2015).
Borlaug, B. A., Kane, G. C., Melenovsky, V. & Olson, T. P. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur. Heart J. 37, 3293–3302 (2016).
Gorter, T. M. et al. Right heart dysfunction and failure in heart failure with preserved ejection fraction: mechanisms and management. Position statement on behalf of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 20, 16–37 (2018).
Levine, A. R., Simon, M. A. & Gladwin, M. T. Pulmonary vascular disease in the setting of heart failure with preserved ejection fraction. Trends Cardiovasc. Med. 29, 207–217 (2019).
Ghio, S. et al. Pulmonary hypertension and right ventricular remodeling in HFpEF and HFrEF. Heart Fail. Rev. 25, 85–91 (2020).
Norman, H. S. et al. Decreased cardiac functional reserve in heart failure with preserved systolic function. J. Card. Fail. 17, 301–308 (2011).
Borlaug, B. A. et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 56, 845–854 (2010).
Borlaug, B. A. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circ. J. 78, 20–32 (2013).
Gorter, T. M., Obokata, M., Reddy, Y. N. V., Melenovsky, V. & Borlaug, B. A. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur. Heart J. 39, 2825–2835 (2018).
Del Buono, M. G. et al. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 2209–2225 (2019).
Kitzman, D. W. & Shah, S. J. The HFpEF obesity phenotype: the elephant in the room. J. Am. Coll. Cardiol. 68, 200–203 (2016).
Paulus, W. J. & Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
Obokata, M., Reddy, Y. N. V., Pislaru, S. V., Melenovsky, V. & Borlaug, B. A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 136, 6–19 (2017).
Forman, D. E. & Goodpaster, B. H. Weighty matters in HFpEF and aging. JACC Heart Fail. 6, 650–652 (2018).
Westermann, D. et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ. Heart Fail. 4, 44–52 (2011).
D’Elia, E. et al. Role of biomarkers in cardiac structure phenotyping in heart failure with preserved ejection fraction: critical appraisal and practical use. Eur. J. Heart Fail. 17, 1231–1239 (2015).
Abernethy, A. et al. Pro-inflammatory biomarkers in stable versus acutely decompensated heart failure with preserved ejection fraction. J. Am. Heart Assoc. 7, e007385 (2018).
Chirinos, J. A. et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 75, 1281–1295 (2020).
DuBrock, H. M., AbouEzzeddine, O. F. & Redfield, M. M. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS ONE 13, e0201836 (2018).
Mohammed, S. F. et al. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131, 550–559 (2015).
Lee, J. F. et al. Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart 102, 278–284 (2016).
Franssen, C. et al. Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC Heart Fail. 4, 312–324 (2016).
Zeng, H. & Chen, J. X. Microvascular rarefaction and heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 6, 15 (2019).
D’Amario, D. et al. Microvascular dysfunction in heart failure with preserved ejection fraction. Front. Physiol. 10, 1347 (2019).
Haykowsky, M. J. et al. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: role of lean body mass. J. Gerontol. A Biol. Sci. Med. Sci. 68, 968–975 (2013).
Kitzman, D. W. et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 306, H1364–H1370 (2014).
Haykowsky, M. J. et al. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am. J. Cardiol. 113, 1211–1216 (2014).
Dhakal, B. P. et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ. Heart Fail. 8, 286–294 (2015).
Hirai, D. M., Musch, T. I. & Poole, D. C. Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am. J. Physiol. Heart Circ. Physiol. 309, H1419–H1439 (2015).
Tromp, J. et al. Heart failure with preserved ejection fraction in Asia. Eur. J. Heart Fail. 21, 23–36 (2019).
McHugh, K. et al. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 602–611 (2019).
Shear, F. E. Novel paradigms in the therapeutic management of heart failure with preserved ejection fraction: clinical perspectives. Am. J. Cardiovasc. Dis. 9, 91–108 (2019).
Wintrich, J. et al. Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future. Clin. Res. Cardiol. 109, 1079–1098 (2020).
Paulus, W. J. Unfolding discoveries in heart failure. N. Engl. J. Med. 382, 679–682 (2020).
Okayama, H. et al. Alterations in expression of sarcoplasmic reticulum gene in Dahl rats during the transition from compensatory myocardial hypertrophy to heart failure. J. Hypertens. 15, 1767–1774 (1997).
Qu, P. et al. Time-course changes in left ventricular geometry and function during the development of hypertension in Dahl salt-sensitive rats. Hypertens. Res. 23, 613–623 (2000).
Chen-Izu, Y. et al. Hypertension-induced remodeling of cardiac excitation-contraction coupling in ventricular myocytes occurs prior to hypertrophy development. Am. J. Physiol. Heart Circ. Physiol. 293, H3301–H3310 (2007).
Weber, K. T., Janicki, J. S., Pick, R., Capasso, J. & Anversa, P. Myocardial fibrosis and pathologic hypertrophy in the rat with renovascular hypertension. Am. J. Cardiol. 65, 1G–7G (1990).
Fillmore, N. et al. Uncoupling of glycolysis from glucose oxidation accompanies the development of heart failure with preserved ejection fraction. Mol. Med. 24, 3 (2018).
Nagata, K. et al. Early changes in excitation-contraction coupling: transition from compensated hypertrophy to failure in Dahl salt-sensitive rat myocytes. Cardiovasc. Res. 37, 467–477 (1998).
Capasso, J. M., Palackal, T., Olivetti, G. & Anversa, P. Left ventricular failure induced by long-term hypertension in rats. Circ. Res. 66, 1400–1412 (1990).
Lupon, J. et al. Heart failure with preserved ejection fraction infrequently evolves toward a reduced phenotype in long-term survivors. Circ. Heart Fail. 12, e005652 (2019).
Nishio, M. et al. Therapeutic effects of angiotensin II type 1 receptor blocker at an advanced stage of hypertensive diastolic heart failure. J. Hypertens. 25, 455–461 (2007).
Yamamoto, K. et al. Roles of renin-angiotensin and endothelin systems in development of diastolic heart failure in hypertensive hearts. Cardiovasc. Res. 47, 274–283 (2000).
Wake, R. et al. Beneficial effect of candesartan on rat diastolic heart failure. J. Pharmacol. Sci. 98, 372–379 (2005).
Yoshida, J. et al. AT1 receptor blocker added to ACE inhibitor provides benefits at advanced stage of hypertensive diastolic heart failure. Hypertension 43, 686–691 (2004).
Jeong, M. Y. et al. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci. Transl. Med. 10, eaao0144 (2018).
Wallner, M. et al. HDAC inhibition improves cardiopulmonary function in a feline model of diastolic dysfunction. Sci. Transl. Med. 12, eaay7205 (2020).
Gallet, R. et al. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction (HFpEF) in rats by decreasing fibrosis and inflammation. JACC Basic Transl. Sci. 1, 14–28 (2016).
Fein, F. S. & Sonnenblick, E. H. Diabetic cardiomyopathy. Prog. Cardiovasc. Dis. 27, 255–270 (1985).
Jia, G., Hill, M. A. & Sowers, J. R. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ. Res. 122, 624–638 (2018).
Tofovic, S. P., Kusaka, H., Kost, C. K. Jr & Bastacky, S. Renal function and structure in diabetic, hypertensive, obese ZDFxSHHF-hybrid rats. Ren. Fail. 22, 387–406 (2000).
Leite, S. et al. Arterial remodeling and dysfunction in the ZSF1 rat model of heart failure with preserved ejection fraction. Circ. Heart Fail. 12, e005596 (2019).
Boustany-Kari, C. M. et al. A soluble guanylate cyclase activator inhibits the progression of diabetic nephropathy in the ZSF1 rat. J. Pharmacol. Exp. Ther. 356, 712–719 (2016).
Lai, Y. C. et al. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation 133, 717–731 (2016).
Hopf, A. E. et al. Diabetes-induced cardiomyocyte passive stiffening is caused by impaired insulin-dependent titin modification and can be modulated by neuregulin-1. Circ. Res. 123, 342–355 (2018).
Salah, E. M., Bastacky, S. I., Jackson, E. K. & Tofovic, S. P. Captopril attenuates cardiovascular and renal disease in a rat model of heart failure with preserved ejection fraction. J. Cardiovasc. Pharmacol. 71, 205–214 (2018).
Schiattarella, G. G. et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568, 351–356 (2019).
Tong, D. et al. Female sex is protective in a preclinical model of heart failure with preserved ejection fraction. Circulation 140, 1769–1771 (2019).
Munagala, V. K., Hart, C. Y., Burnett, J. C. Jr., Meyer, D. M. & Redfield, M. M. Ventricular structure and function in aged dogs with renal hypertension: a model of experimental diastolic heart failure. Circulation 111, 1128–1135 (2005).
Reiter, U. et al. Early-stage heart failure with preserved ejection fraction in the pig: a cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 18, 63 (2016).
Sorop, O. et al. Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc. Res. 114, 954–964 (2018).
Schwarzl, M. et al. A porcine model of hypertensive cardiomyopathy: implications for heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 309, H1407–H1418 (2015).
Redfield, M. M. et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA 309, 1268–1277 (2013).
Reddy, Y. N. V., Carter, R. E., Obokata, M., Redfield, M. M. & Borlaug, B. A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 138, 861–870 (2018).
Zile, M. R. et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation 124, 2491–2501 (2011).
Lindman, B. R. et al. Cardiovascular phenotype in HFpEF patients with or without diabetes: a RELAX trial ancillary study. J. Am. Coll. Cardiol. 64, 541–549 (2014).
Armstrong, A. C. et al. Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: the multi-ethnic study of atherosclerosis. Echocardiography 31, 12–20 (2014).
Nakamura, M. & Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 15, 387–407 (2018).
Wettschureck, N. et al. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat. Med. 7, 1236–1240 (2001).
Zhang, W. et al. Selective loss of fine tuning of Gq/11 signaling by RGS2 protein exacerbates cardiomyocyte hypertrophy. J. Biol. Chem. 281, 5811–5820 (2006).
Takimoto, E. et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J. Clin. Invest. 119, 408–420 (2009).
Bishu, K. et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am. Heart J. 164, 763–770.e3 (2012).
Tschope, C., Van Linthout, S. & Kherad, B. Heart failure with preserved ejection fraction and future pharmacological strategies: a glance in the crystal ball. Curr. Cardiol. Rep. 19, 70 (2017).
Sztechman, D., Czarzasta, K., Cudnoch-Jedrzejewska, A., Szczepanska-Sadowska, E. & Zera, T. Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J. Physiol. Pharmacol. 69, 829–845 (2018).
Fraccarollo, D. et al. Deletion of cardiomyocyte mineralocorticoid receptor ameliorates adverse remodeling after myocardial infarction. Circulation 123, 400–408 (2011).
Rickard, A. J. et al. Cardiomyocyte mineralocorticoid receptors are essential for deoxycorticosterone/salt-mediated inflammation and cardiac fibrosis. Hypertension 60, 1443–1450 (2012).
Cohen, J. B. et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 8, 172–184 (2020).
Pitt, B. et al. Spironolactone for heart failure with preserved ejection fraction. N. Engl. J. Med. 370, 1383–1392 (2014).
de Denus, S. et al. Spironolactone metabolites in TOPCAT – new insights into regional variation. N. Engl. J. Med. 376, 1690–1692 (2017).
Bristow, M. R. et al. Detection and management of geographic disparities in the TOPCAT trial: lessons learned and derivative recommendations. JACC Basic Transl. Sci. 1, 180–189 (2016).
Ravassa, S. et al. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo-DHF trial. Eur. J. Heart Fail. 20, 1290–1299 (2018).
Maron, M. S. et al. Effect of spironolactone on myocardial fibrosis and other clinical variables in patients with hypertrophic cardiomyopathy. Am. J. Med. 131, 837–841 (2018).
Myhre, P. L. et al. Mechanistic effects of spironolactone on cardiovascular and renal biomarkers in heart failure with preserved ejection fraction: a TOPCAT biorepository study. Circ. Heart Fail. 13, e006638 (2020).
Yamamoto, K., Origasa, H. & Hori, M. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur. J. Heart Fail. 15, 110–118 (2013).
Pal, N. et al. Effect of selective heart rate slowing in heart failure with preserved ejection fraction. Circulation 132, 1719–1725 (2015).
Komajda, M. et al. Effect of ivabradine in patients with heart failure with preserved ejection fraction: the EDIFY randomized placebo-controlled trial. Eur. J. Heart Fail. 19, 1495–1503 (2017).
Mesubi, O. O. & Anderson, M. E. Atrial remodelling in atrial fibrillation: CaMKII as a nodal proarrhythmic signal. Cardiovasc. Res. 109, 542–557 (2016).
Anderson, M. E., Brown, J. H. & Bers, D. M. CaMKII in myocardial hypertrophy and heart failure. J. Mol. Cell. Cardiol. 51, 468–473 (2011).
Joiner, M. L. et al. CaMKII determines mitochondrial stress responses in heart. Nature 491, 269–273 (2012).
Suetomi, T., Miyamoto, S. & Brown, J. H. Inflammation in nonischemic heart disease: initiation by cardiomyocyte CaMKII and NLRP3 inflammasome signaling. Am. J. Physiol. Heart Circ. Physiol. 317, H877–H890 (2019).
Rusciano, M. R. et al. CaMKII activity in the inflammatory response of cardiac diseases. Int. J. Mol. Sci. 20, 4374 (2019).
Hegyi, B., Bers, D. M. & Bossuyt, J. CaMKII signaling in heart diseases: emerging role in diabetic cardiomyopathy. J. Mol. Cell. Cardiol. 127, 246–259 (2019).
Shi, J. et al. Molecular determinants for cardiovascular TRPC6 channel regulation by Ca2+/calmodulin-dependent kinase II. J. Physiol. 591, 2851–2866 (2013).
Kuwahara, K. et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J. Clin. Invest. 116, 3114–3126 (2006).
Lin, B. L. et al. In vivo selective inhibition of TRPC6 by antagonist BI 749327 ameliorates fibrosis and dysfunction in cardiac and renal disease. Proc. Natl Acad. Sci. USA 116, 10156–10161 (2019).
Davis, J., Burr, A. R., Davis, G. F., Birnbaumer, L. & Molkentin, J. D. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev. Cell 23, 705–715 (2012).
Zhang, C. L. et al. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110, 479–488 (2002).
Kong, Y. et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation 113, 2579–2588 (2006).
Ago, T. et al. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133, 978–993 (2008).
Doi, R. et al. Development of different phenotypes of hypertensive heart failure: systolic versus diastolic failure in Dahl salt-sensitive rats. J. Hypertens. 18, 111–120 (2000).
Yamamoto, K. et al. Myocardial stiffness is determined by ventricular fibrosis, but not by compensatory or excessive hypertrophy in hypertensive heart. Cardiovasc. Res. 55, 76–82 (2002).
van Heerebeek, L. et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117, 43–51 (2008).
Fukui, S. et al. Diabetes mellitus accelerates left ventricular diastolic dysfunction through activation of the renin-angiotensin system in hypertensive rats. Hypertens. Res. 32, 472–480 (2009).
Liu, F. et al. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor α. Circulation 131, 795–804 (2015).
Cavalera, M., Wang, J. & Frangogiannis, N. G. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl. Res. 164, 323–335 (2014).
Alex, L., Russo, I., Holoborodko, V. & Frangogiannis, N. G. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 315, H934–H949 (2018).
Panchal, S. K. et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 57, 611–624 (2011).
Hahn, V. S. et al. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail. 8, 712–724 (2020).
Fu, X. et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J. Clin. Invest. 128, 2127–2143 (2018).
Xiao, Y. et al. Hippo pathway deletion in adult resting cardiac fibroblasts initiates a cell state transition with spontaneous and self-sustaining fibrosis. Genes Dev. 33, 1491–1505 (2019).
DeLeon-Pennell, K. Y., Meschiari, C. A., Jung, M. & Lindsey, M. L. Matrix metalloproteinases in myocardial infarction and heart failure. Prog. Mol. Biol. Transl. Sci. 147, 75–100 (2017).
Gonzalez, A. et al. Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension 55, 1418–1424 (2010).
Kasner, M. et al. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J. Am. Coll. Cardiol. 57, 977–985 (2011).
Lopez, B., Querejeta, R., Gonzalez, A., Larman, M. & Diez, J. Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential role of lysyl oxidase. Hypertension 60, 677–683 (2012).
Kanagala, P. et al. Relationship between focal and diffuse fibrosis assessed by CMR and clinical outcomes in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging 12, 2291–2301 (2019).
Zile, M. R. et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation 131, 1247–1259 (2015).
Lopez, B. et al. Is plasma cardiotrophin-1 a marker of hypertensive heart disease? J. Hypertens. 23, 625–632 (2005).
de Boer, R. A. et al. Galectin-3 in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 15, 1095–1101 (2013).
Polat, V., Bozcali, E., Uygun, T., Opan, S. & Karakaya, O. Diagnostic significance of serum galectin-3 levels in heart failure with preserved ejection fraction. Acta Cardiol. 71, 191–197 (2016).
Corden, B., Adami, E., Sweeney, M., Schafer, S. & Cook, S. A. IL-11 in cardiac and renal fibrosis: late to the party but a central player. Br. J. Pharmacol. 177, 1695–1708 (2020).
Chen, W. Y., Hong, J., Gannon, J., Kakkar, R. & Lee, R. T. Myocardial pressure overload induces systemic inflammation through endothelial cell IL-33. Proc. Natl Acad. Sci. USA 112, 7249–7254 (2015).
Hedman, A. K. et al. Identification of novel pheno-groups in heart failure with preserved ejection fraction using machine learning. Heart 106, 342–349 (2020).
Du, W. et al. Plasma levels of heart failure biomarkers are primarily a reflection of extracardiac production. Theranostics 8, 4155–4169 (2018).
Luo, M. & Anderson, M. E. Mechanisms of altered Ca2+ handling in heart failure. Circ. Res. 113, 690–708 (2013).
Nagayama, T. et al. Control of in vivo left ventricular contraction/relaxation kinetics by myosin binding protein C: protein kinase A phosphorylation dependent and independent regulation. Circulation 116, 2399–2408 (2007).
Kapur, S. et al. Early development of intracellular calcium cycling defects in intact hearts of spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 299, H1843–H1853 (2010).
Primessnig, U. et al. Novel pathomechanisms of cardiomyocyte dysfunction in a model of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 18, 987–997 (2016).
Borbely, A. et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation 111, 774–781 (2005).
van Heerebeek, L. et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113, 1966–1973 (2006).
van Heerebeek, L. et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126, 830–839 (2012).
Kruger, M. et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ. Res. 104, 87–94 (2009).
Asram, M. I. et al. Reduced right ventricular sarcomere contractility in HFpEF with severe obesity. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.052414 (2020).
Hamdani, N. et al. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ. Heart Fail. 6, 1239–1249 (2013).
Perreault, C. L., Bing, O. H., Brooks, W. W., Ransil, B. J. & Morgan, J. P. Differential effects of cardiac hypertrophy and failure on right versus left ventricular calcium activation. Circ. Res. 67, 707–712 (1990).
Akella, A. B., Ding, X. L., Cheng, R. & Gulati, J. Diminished Ca2+ sensitivity of skinned cardiac muscle contractility coincident with troponin T-band shifts in the diabetic rat. Circ. Res. 76, 600–606 (1995).
Papadaki, M. et al. Diabetes with heart failure increases methylglyoxal modifications in the sarcomere, which inhibit function. JCI Insight 3, e121264 (2018).
Lin, Y. H. et al. Site-specific acetyl-mimetic modification of cardiac troponin I modulates myofilament relaxation and calcium sensitivity. J. Mol. Cell. Cardiol. 139, 135–147 (2020).
Foster, D. B. et al. The cardiac acetyl-lysine proteome. PLoS ONE 8, e67513 (2013).
Samant, S. A. et al. HDAC3-dependent reversible lysine acetylation of cardiac myosin heavy chain isoforms modulates their enzymatic and motor activity. J. Biol. Chem. 286, 5567–5577 (2011).
Francis, S. H., Busch, J. L., Corbin, J. D. & Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 62, 525–563 (2010).
Surks, H. K. et al. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Iα. Science 286, 1583–1587 (1999).
Sawada, N. et al. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem. Biophys. Res. Commun. 280, 798–805 (2001).
Sawada, N. et al. Cyclic GMP kinase and RhoA Ser188 phosphorylation integrate pro- and antifibrotic signals in blood vessels. Mol.Cell. Biol. 29, 6018–6032 (2009).
Farah, C., Michel, L. Y. M. & Balligand, J. L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 15, 292–316 (2018).
Lau, K. S. et al. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol. Genomics 2, 21–27 (2000).
Stamler, J. S. & Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 81, 209–237 (2001).
Holtwick, R. et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J. Clin. Invest. 111, 1399–1407 (2003).
Takimoto, E. et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat. Med. 11, 214–222 (2005).
Lee, D. I. et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 519, 472–476 (2015).
Koitabashi, N. et al. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation: novel mechanism of cardiac stress modulation by PDE5 inhibition. J. Mol. Cell. Cardiol. 48, 713–724 (2010).
Kinoshita, H. et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ. Res. 106, 1849–1860 (2010).
Tokudome, T. et al. Regulator of G-protein signaling subtype 4 mediates antihypertrophic effect of locally secreted natriuretic peptides in the heart. Circulation 117, 2329–2339 (2008).
Ranek, M. J. et al. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature 566, 264–269 (2019).
Ranek, M. J., Terpstra, E. J., Li, J., Kass, D. A. & Wang, X. Protein kinase G positively regulates proteasome-mediated degradation of misfolded proteins. Circulation 128, 365–376 (2013).
Wang, W. Z., Jones, A. W., Wang, M., Durante, W. & Korthuis, R. J. Preconditioning with soluble guanylate cyclase activation prevents postischemic inflammation and reduces nitrate tolerance in heme oxygenase-1 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 305, H521–H532 (2013).
Inserte, J. & Garcia-Dorado, D. The cGMP/PKG pathway as a common mediator of cardioprotection: translatability and mechanism. Br. J. Pharmacol. 172, 1996–2009 (2015).
Kokkonen-Simon, K. M. et al. Marked disparity of microRNA modulation by cGMP-selective PDE5 versus PDE9 inhibitors in heart disease. JCI Insight 3, e121739 (2018).
Collins, S. A heart–adipose tissue connection in the regulation of energy metabolism. Nat. Rev. Endocrinol. 10, 157–163 (2014).
Mitschke, M. M. et al. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 27, 1621–1630 (2013).
Layland, J., Li, J. M. & Shah, A. M. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 540, 457–467 (2002).
Dunkerly-Eyring, B. & Kass, D. A. Myocardial phosphodiesterases and their role in cGMP regulation. J. Cardiovasc. Pharmacol. 75, 483–493 (2019).
Sasaki, H. et al. PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J. Clin. Invest. 124, 2464–2471 (2014).
Fukuma, N. et al. Estrogen receptor-α non-nuclear signaling confers cardioprotection and is essential to cGMP-PDE5 inhibition efficacy. JACC Basic. Transl. Sci. 5, 282–295 (2020).
Chirinos, J. A. & Zamani, P. The nitrate-nitrite-NO pathway and its implications for heart failure and preserved ejection fraction. Curr. Heart Fail. Rep. 13, 47–59 (2016).
Scott, N. J. A., Rademaker, M. T., Charles, C. J., Espiner, E. A. & Richards, A. M. Hemodynamic, hormonal, and renal actions of phosphodiesterase-9 inhibition in experimental heart failure. J. Am. Coll. Cardiol. 74, 889–901 (2019).
Santos, C. X., Raza, S. & Shah, A. M. Redox signaling in the cardiomyocyte: from physiology to failure. Int. J. Biochem. Cell Biol. 74, 145–151 (2016).
Faria, A. & Persaud, S. J. Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacol. Ther. 172, 50–62 (2017).
Takimoto, E. & Kass, D. A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49, 241–248 (2007).
Kaludercic, N. et al. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ. Res. 106, 193–202 (2010).
Takimoto, E. et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J. Clin. Invest. 115, 1221–1231 (2005).
Noordali, H., Loudon, B. L., Frenneaux, M. P. & Madhani, M. Cardiac metabolism – a promising therapeutic target for heart failure. Pharmacol. Ther. 182, 95–114 (2018).
Karwi, Q. G., Uddin, G. M., Ho, K. L. & Lopaschuk, G. D. Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 5, 68 (2018).
Lopaschuk, G. D., Ussher, J. R., Folmes, C. D., Jaswal, J. S. & Stanley, W. C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 90, 207–258 (2010).
Beer, M. et al. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with 31P-SLOOP magnetic resonance spectroscopy. J. Am. Coll. Cardiol. 40, 1267–1274 (2002).
Conway, M. A. et al. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 338, 973–976 (1991).
Kato, T. et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ. Heart Fail. 3, 420–430 (2010).
Nascimben, L. et al. Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation 91, 1824–1833 (1995).
Neubauer, S. et al. Downregulation of the Na+-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation 100, 1847–1850 (1999).
Fillmore, N. & Lopaschuk, G. D. Targeting mitochondrial oxidative metabolism as an approach to treat heart failure. Biochim. Biophys. Acta 1833, 857–865 (2013).
Phan, T. T. et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J. Am. Coll. Cardiol. 54, 402–409 (2009).
Neubauer, S. et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 86, 1810–1818 (1992).
Arany, Z. et al. Transcriptional coactivator PGC-1α controls the energy state and contractile function of cardiac muscle. Cell Metab. 1, 259–271 (2005).
Berthiaume, J. M., Kurdys, J. G., Muntean, D. M. & Rosca, M. G. Mitochondrial NAD+/NADH redox state and diabetic cardiomyopathy. Antioxid. Redox Signal. 30, 375–398 (2019).
Pillai, V. B. et al. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J. Biol. Chem. 285, 3133–3144 (2010).
Horton, J. L. et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 2, e84897 (2016).
Lee, C. F. et al. Normalization of NAD+ redox balance as a therapy for heart failure. Circulation 134, 883–894 (2016).
Diguet, N. et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137, 2256–2273 (2018).
Karamanlidis, G. et al. Mitochondrial complex I deficiency increases protein acetylation and accelerates heart failure. Cell Metab. 18, 239–250 (2013).
Diakos, N. A. et al. Evidence of glycolysis up-regulation and pyruvate mitochondrial oxidation mismatch during mechanical unloading of the failing human heart: implications for cardiac reloading and conditioning. JACC Basic. Transl. Sci. 1, 432–444 (2016).
Doenst, T., Nguyen, T. D. & Abel, E. D. Cardiac metabolism in heart failure: implications beyond ATP production. Circ. Res. 113, 709–724 (2013).
Lei, B. et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J. Mol. Cell. Cardiol. 36, 567–576 (2004).
Tumova, J., Andel, M. & Trnka, J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol. Res. 65, 193–207 (2015).
Lauzier, B. et al. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J. Mol. Cell. Cardiol. 55, 92–100 (2013).
Sowton, A. P., Griffin, J. L. & Murray, A. J. Metabolic profiling of the diabetic heart: toward a richer picture. Front. Physiol. 10, 639 (2019).
Davila-Roman, V. G. et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J. Am. Coll. Cardiol. 40, 271–277 (2002).
Mahmod, M. et al. The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Res. 20, 88 (2018).
Wei, J. et al. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am. J. Physiol. Heart Circ. Physiol. 310, H14–H19 (2016).
Djousse, L. et al. Plasma free fatty acids and risk of heart failure: the Cardiovascular Health Study. Circ. Heart Fail. 6, 964–969 (2013).
Hunter, W. G. et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J. Am. Heart Assoc. 5, e003190 (2016).
Hage, C. et al. Inflammatory biomarkers predict heart failure severity and prognosis in patients with heart failure with preserved ejection fraction: a holistic proteomic approach. Circ. Cardiovasc. Genet. 10, e001633 (2017).
Zordoky, B. N. et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE 10, e0124844 (2015).
Aubert, G. et al. The failing heart relies on ketone bodies as a fuel. Circulation 133, 698–705 (2016).
Mizuno, Y. et al. The diabetic heart utilizes ketone bodies as an energy source. Metabolism 77, 65–72 (2017).
Ho, K. L. et al. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc. Res. 115, 1606–1616 (2019).
Newman, J. C. & Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 25, 42–52 (2014).
Ferrannini, E., Mark, M. & Mayoux, E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 39, 1108–1114 (2016).
Xia, Y. et al. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem. Cell Biol. 131, 471–481 (2009).
Suetomi, T. et al. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca2+/calmodulin-dependent protein kinase IIδ signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 138, 2530–2544 (2018).
Patel, B. et al. CCR2+ monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic. Transl. Sci. 3, 230–244 (2018).
Laroumanie, F. et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 129, 2111–2124 (2014).
Kallikourdis, M. et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat. Commun. 8, 14680 (2017).
Sanders-van Wijk, S. et al. Proteomic evaluation of the comorbidity–inflammation paradigm in heart failure with preserved ejection fraction: results from the PROMIS-HFpEF study. Circulation 142, 2029–2044 (2020).
Tartiere-Kesri, L., Tartiere, J. M., Logeart, D., Beauvais, F. & Cohen Solal, A. Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 59, 455–461 (2012).
Borlaug, B. A. et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J. Am. Coll. Cardiol. 50, 1570–1577 (2007).
Mohammed, S. F. et al. Resting ventricular-vascular function and exercise capacity in heart failure with preserved ejection fraction: a RELAX trial ancillary study. Circ. Heart Fail. 7, 580–589 (2014).
Kass, D. A. & Kelly, R. P. Ventriculo-arterial coupling: concepts, assumptions, and applications. Ann. Biomed. Eng. 20, 41–62 (1992).
Kelly, R. P., Tunin, R. & Kass, D. A. Effect of reduced aortic compliance on cardiac efficiency and contractile function of in situ canine left ventricle. Circ. Res. 71, 490–502 (1992).
Chirinos, J. A. et al. Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 8, e011457 (2019).
Bache, R. J., Arentzen, C. E., Simon, A. B. & Vrobel, T. R. Abnormalities in myocardial perfusion during tachycardia in dogs with left ventricular hypertrophy: metabolic evidence for myocardial ischemia. Circulation 69, 409–417 (1984).
Bache, R. J., Dai, X. Z., Alyono, D., Vrobel, T. R. & Homans, D. C. Myocardial blood flow during exercise in dogs with left ventricular hypertrophy produced by aortic banding and perinephritic hypertension. Circulation 76, 835–842 (1987).
Toyota, E. et al. Dynamics of flow velocities in endocardial and epicardial coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 288, H1598–H1603 (2005).
Wei, T. et al. Sirtuin 3 deficiency accelerates hypertensive cardiac remodeling by impairing angiogenesis. J. Am. Heart Assoc. 6, e006114 (2017).
Sundaresan, N. R. et al. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Invest. 119, 2758–2771 (2009).
He, X. et al. Endothelial specific SIRT3 deletion impairs glycolysis and angiogenesis and causes diastolic dysfunction. J. Mol. Cell. Cardiol. 112, 104–113 (2017).
Marechaux, S. et al. Vascular and microvascular endothelial function in heart failure with preserved ejection fraction. J. Card. Fail. 22, 3–11 (2016).
Yang, J. H. et al. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 22, 432–441 (2020).
Taqueti, V. R. et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur. Heart J. 39, 840–849 (2018).
AbouEzzeddine, O. F. et al. Myocardial energetics in heart failure with preserved ejection fraction. Circ. Heart Fail. 12, e006240 (2019).
Haykowsky, M. J., Tomczak, C. R., Scott, J. M., Paterson, D. I. & Kitzman, D. W. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J. Appl. Physiol. 119, 739–744 (2015).
Wong, L. L. et al. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur. J. Heart Fail. 17, 393–404 (2015).
Yan, H. et al. miRNAs as biomarkers for diagnosis of heart failure: a systematic review and meta-analysis. Medicine 96, e6825 (2017).
Wong, L. L. et al. Combining circulating microRNA and NT-proBNP to detect and categorize heart failure subtypes. J. Am. Coll. Cardiol. 73, 1300–1313 (2019).
Chen, Y. T., Wong, L. L., Liew, O. W. & Richards, A. M. Heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF): the diagnostic value of circulating microRNAs. Cells 8, 1651 (2019).
Hahn, V. S. et al. Myocardial gene expression signatures in human heart failure with preserved ejection fraction. Circulation https://doi.org/10.1161/CIRCULATIONAHA.120.050498 (2020).
Obokata, M., Reddy, Y. N. V., Melenovsky, V., Pislaru, S. & Borlaug, B. A. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur. Heart J. 40, 689–697 (2019).
Mohammed, S. F. et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 130, 2310–2320 (2014).
Melenovsky, V., Hwang, S. J., Lin, G., Redfield, M. M. & Borlaug, B. A. Right heart dysfunction in heart failure with preserved ejection fraction. Eur. Heart J. 35, 3452–3462 (2014).
Zakeri, R. & Mohammed, S. F. Epidemiology of right ventricular dysfunction in heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 12, 295–301 (2015).
Kanjanahattakij, N. et al. High right ventricular stroke work index is associated with worse kidney function in patients with heart failure with preserved ejection fraction. Cardiorenal Med. 8, 123–129 (2018).
Patel, R. B. et al. Diffuse right ventricular fibrosis in heart failure with preserved ejection fraction and pulmonary hypertension. ESC. Heart Fail. 7, 253–263 (2020).
Williams, J. L. et al. Defining the molecular signatures of human right heart failure. Life Sci. 196, 118–126 (2018).
Mancini, D. M., Davis, L., Wexler, J. P., Chadwick, B. & LeJemtel, T. H. Dependence of enhanced maximal exercise performance on increased peak skeletal muscle perfusion during long-term captopril therapy in heart failure. J. Am. Coll. Cardiol. 10, 845–850 (1987).
Sullivan, M. J., Knight, J. D., Higginbotham, M. B. & Cobb, F. R. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation 80, 769–781 (1989).
LeJemtel, T. H., Maskin, C. S., Lucido, D. & Chadwick, B. J. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation 74, 245–251 (1986).
Adams, V., Linke, A. & Winzer, E. Skeletal muscle alterations in HFrEF vs. HFpEF. Curr. Heart Fail. Rep. 14, 489–497 (2017).
Tucker, W. J., Haykowsky, M. J., Seo, Y., Stehling, E. & Forman, D. E. Impaired exercise tolerance in heart failure: role of skeletal muscle morphology and function. Curr. Heart Fail. Rep. 15, 323–331 (2018).
Weiss, K. et al. Fatigability, exercise intolerance, and abnormal skeletal muscle energetics in heart failure. Circ. Heart Fail. 10, e004129 (2017).
Molina, A. J. et al. Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC Heart Fail. 4, 636–645 (2016).
Tucker, W. J. et al. Impact of exercise training on peak oxygen uptake and its determinants in heart failure with preserved ejection fraction. Card. Fail. Rev. 2, 95–101 (2016).
Wu, H. & Ballantyne, C. M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Invest. 127, 43–54 (2017).
Zamani, P. et al. Peripheral determinants of oxygen utilization in heart failure with preserved ejection fraction: central role of adiposity. JACC Basic. Transl. Sci. 5, 211–225 (2020).
Bowen, T. S. et al. Effects of endurance training on detrimental structural, cellular, and functional alterations in skeletal muscles of heart failure with preserved ejection fraction. J. Card. Fail. 24, 603–613 (2018).
Olson, T. P., Johnson, B. D. & Borlaug, B. A. Impaired pulmonary diffusion in heart failure with preserved ejection fraction. JACC Heart Fail. 4, 490–498 (2016).
Hoeper, M. M. et al. Diffusion capacity and mortality in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. JACC Heart Fail. 4, 441–449 (2016).
Fayyaz, A. U. et al. Global pulmonary vascular remodeling in pulmonary hypertension associated with heart failure and preserved or reduced ejection fraction. Circulation 137, 1796–1810 (2018).
Wang, L. et al. Treatment with treprostinil and metformin normalizes hyperglycemia and improves cardiac function in pulmonary hypertension associated with heart failure with preserved ejection fraction. Arterioscler. Thromb. Vasc. Biol. 40, 1543–1558 (2020).
Agrawal, V. et al. Natriuretic peptide receptor C contributes to disproportionate right ventricular hypertrophy in a rodent model of obesity-induced heart failure with preserved ejection fraction with pulmonary hypertension. Pulm. Circ. 9, 2045894019878599 (2019).
Shah, K. S. & Fang, J. C. Is heart failure with preserved ejection fraction a kidney disorder? Curr. Hypertens. Rep. 21, 86 (2019).
van de Wouw, J. et al. Chronic kidney disease as a risk factor for heart failure with preserved ejection fraction: a focus on microcirculatory factors and therapeutic targets. Front. Physiol. 10, 1108 (2019).
Agrawal, A., Naranjo, M., Kanjanahattakij, N., Rangaswami, J. & Gupta, S. Cardiorenal syndrome in heart failure with preserved ejection fraction–an under-recognized clinical entity. Heart Fail. Rev. 24, 421–437 (2019).
Upadhya, B., Amjad, A. & Stacey, R. B. Optimizing the management of obese HFpEF phenotype: can we mind both the heart and the kidney? J. Card. Fail. 26, 108–111 (2020).
Robinson, T. W. & Freedman, B. I. The impact of APOL1 on chronic kidney disease and hypertension. Adv. Chronic Kidney Dis. 26, 131–136 (2019).
Franceschini, N. et al. Association of APOL1 with heart failure with preserved ejection fraction in postmenopausal African American women. JAMA Cardiol. 3, 712–720 (2018).
Jhund, P. S. et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation https://doi.org/10.1161/circulationaha.120.050391 (2020).
Packer, M. et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Eng.l J. Med. 383, 1413–1424 (2020).
Ferreira, J. P. et al. Covariate adjusted reanalysis of the I-Preserve trial. Clin. Res. Cardiol. 109, 1358–1365 (2020).
Borlaug, B. A. et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA 320, 1764–1773 (2018).
Pieske, B. et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur. Heart J. 38, 1119–1127 (2017).
Udelson, J. E. et al. Rationale and design for a multicenter, randomized, double-blind, placebo-controlled, phase 2 study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator praliciguat over 12 weeks in patients with heart failure with preserved ejection fraction (CAPACITY HFpEF). Am. Heart J. 222, 183–190 (2020).
Hahn, V. S. et al. Myocardial transcriptomics reveal distinct gene expression in human heart failure with preserved ejection fraction. Circulation 140 (Suppl. 1), abstr. A16227 (2019).
Khush, K. K. et al. Obese patients have lower B-type and atrial natriuretic peptide levels compared with nonobese. Cong. Heart Fail. 12, 85–90 (2006).
Solomon, S. D. et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N. Engl. J. Med. 381, 1609–1620 (2019).
McMurray, J. J. et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 371, 993–1004 (2014).
Vaduganathan, M. et al. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J. Am. Coll. Cardiol. 75, 245–254 (2019).
Sharma, K. et al. Randomized evaluation of heart failure with preserved ejection fraction patients with acute heart failure and dopamine: the ROPA-DOP trial. JACC Heart Fail. 6, 859–870 (2018).
Van Tassell, B. W. et al. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ. Heart Fail. 11, e005036 (2018).
Reddy, Y. N. V. et al. The β-adrenergic agonist albuterol improves pulmonary vascular reserve in heart failure with preserved ejection fraction. Circ. Res. 124, 306–314 (2019).
Singh, S. et al. Randomized double-blind placebo-controlled trial of perhexiline in heart failure with preserved ejection fraction syndrome. Fut. Cardiol. 10, 693–698 (2014).
Abraham, W. T. et al. Rationale and design of the EMPERIAL-Preserved and EMPERIAL-Reduced trials of empagliflozin in patients with chronic heart failure. Eur. J. Heart Fail. 21, 932–942 (2019).
Physicians’ Academy for Cardiovascular Education. No Improvement in Exercise Ability with SGLT2i in Two HF Trials, One in HFrEF and One in HFpEF Patients https://pace-cme.org/2020/01/06/no-improvement-in-exercise-ability-with-sglt2i-in-two-hf-trials-one-in-hfref-and-one-in-hfpef-patients/ (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03448406 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02941705 (2020).
Kaye, D. M., Nanayakkara, S., Vizi, D., Byrne, M. & Mariani, J. A. Effects of milrinone on rest and exercise hemodynamics in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 67, 2554–2556 (2016).
Nanayakkara, S. et al. Extended-release oral milrinone for the treatment of heart failure with preserved ejection fraction. J. Am. Heart Assoc. 9, e015026 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03541603 (2020).
Swain, E. Mixed results for levosimendan in HFpEF, pulmonary hypertension. Healio.com https://www.healio.com/news/cardiology/20201004/mixed-results-for-levosimendan-in-hfpef-pulmonary-hypertension (2020).
Patel, H. C. et al. Effects of renal denervation on vascular remodelling in patients with heart failure and preserved ejection fraction: a randomised control trial. JRSM Cardiovasc. Dis. 6, 2048004017690988 (2017).
Feldman, T. et al. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): a phase 2, randomized, sham-controlled trial. Circulation 137, 364–375 (2018).
Serova, M. et al. A new algorithm for optimization of rate-adaptive pacing improves exercise tolerance in patients with HFpEF. Pacing Clin. Electrophysiol. 43, 223–233 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02145351 (2020).
Borlaug, B. A. et al. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ. Heart Fail. 10, e003612 (2017).
Tucker, W. J. et al. Mechanisms of the improvement in peak VO2 with exercise training in heart failure with reduced or preserved ejection fraction. Heart Lung Circ. 27, 9–21 (2018).
Pandey, A. et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ. Heart Fail. 8, 33–40 (2015).
Rodriguez Flores, M., Aguilar Salinas, C., Piche, M. E., Auclair, A. & Poirier, P. Effect of bariatric surgery on heart failure. Expert Rev. Cardiovasc. Ther. 15, 567–579 (2017).
Mikhalkova, D. et al. Bariatric surgery-induced cardiac and lipidomic changes in obesity-related heart failure with preserved ejection fraction. Obesity 26, 284–290 (2018).
de Boer, R. A. et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 3, 215–224 (2018).
Chan, M. M. et al. Growth differentiation factor 15 in heart failure with preserved vs. reduced ejection fraction. Eur. J. Heart Fail. 18, 81–88 (2016).
Putko, B. N. et al. Circulating levels of tumor necrosis factor-alpha receptor 2 are increased in heart failure with preserved ejection fraction relative to heart failure with reduced ejection fraction: evidence for a divergence in pathophysiology. PLoS ONE 9, e99495 (2014).
Gohar, A. et al. The prognostic value of highly sensitive cardiac troponin assays for adverse events in men and women with stable heart failure and a preserved vs. reduced ejection fraction. Eur. J. Heart Fail. 19, 1638–1647 (2017).
Sanders-van Wijk, S. et al. Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur. J. Heart Fail. 17, 1006–1014 (2015).
Salah, K. et al. Prognosis and NT-proBNP in heart failure patients with preserved versus reduced ejection fraction. Heart 105, 1182–1189 (2019).
Pandey, A. et al. Factors associated with and prognostic implications of cardiac troponin elevation in decompensated heart failure with preserved ejection fraction: findings from the American Heart Association Get With The Guidelines-Heart Failure program. JAMA Cardiol. 2, 136–145 (2017).
Author information
Authors and Affiliations
Contributions
Both authors researched data for the article, discussed its content, wrote the manuscript, and reviewed and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks B. Borlaug, S. Shah and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishra, S., Kass, D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 18, 400–423 (2021). https://doi.org/10.1038/s41569-020-00480-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-020-00480-6
This article is cited by
-
HFpEF as systemic disease, insight from a diagnostic prediction model reminiscent of systemic inflammation and organ interaction in HFpEF patients
Scientific Reports (2024)
-
Empagliflozin and liraglutide ameliorate HFpEF in mice via augmenting the Erbb4 signaling pathway
Acta Pharmacologica Sinica (2024)
-
Elevated ITGA1 levels in type 2 diabetes: implications for cardiac function impairment
Diabetologia (2024)
-
RETRACTED ARTICLE: Effects of Dapagliflozin on myocardial remodeling, inflammatory factors, and cardiac events in heart failure with preserved ejection fraction
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Enhancing cardiac diagnostics through semantic-driven image synthesis: a hybrid GAN approach
Neural Computing and Applications (2024)