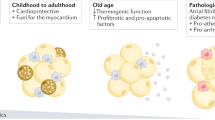
Abstract
Diabesity is a term used to describe the combined adverse health effects of obesity and diabetes mellitus. The worldwide dual epidemic of obesity and type 2 diabetes is an important public health issue. Projections estimate a sixfold increase in the number of adults with obesity in 40 years and an increase in the number of individuals with diabetes to 642 million by 2040. Increased adiposity is the strongest risk factor for developing diabetes. Early detection of the effects of diabesity on the cardiovascular system would enable the optimal implementation of effective therapies that prevent atherosclerosis progression, cardiac remodelling, and the resulting ischaemic heart disease and heart failure. Beyond conventional imaging techniques, such as echocardiography, CT and cardiac magnetic resonance, novel post-processing tools and techniques provide information on the biological processes that underlie metabolic heart disease. In this Review, we summarize the effects of obesity and diabetes on myocardial structure and function and illustrate the use of state-of-the-art multimodality cardiac imaging to elucidate the pathophysiology of myocardial dysfunction, prognosticate long-term clinical outcomes and potentially guide treatment strategies.
Key points
-
Diabesity describes the combined detrimental health effects of obesity and diabetes mellitus.
-
Early detection of the effects of diabesity on the cardiovascular system would enable the optimal implementation of effective therapies to prevent ischaemic heart disease and heart failure.
-
Advanced imaging techniques have helped us to understand how diabesity leads to atherosclerosis and cardiac remodelling and dysfunction.
-
Advanced imaging techniques have improved our understanding of how various therapies can halt or reverse the atherosclerotic and cardiac remodelling processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387, 1377–1396 (2016).
Ward, Z. J. et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 381, 2440–2450 (2019).
Ogurtsova, K. et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 128, 40–50 (2017).
Karwi, Q. G., Uddin, G. M., Ho, K. L. & Lopaschuk, G. D. Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 5, 68 (2018).
Mudaliar, S., Alloju, S. & Henry, R. R. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care 39, 1115–1122 (2016).
Wisneski, J. A. et al. Metabolic fate of extracted glucose in normal human myocardium. J. Clin. Invest. 76, 1819–1827 (1985).
Stanley, W. C., Recchia, F. A. & Lopaschuk, G. D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 85, 1093–1129 (2005).
Wisneski, J. A., Gertz, E. W., Neese, R. A. & Mayr, M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J. Clin. Invest. 79, 359–366 (1987).
Balaban, R. S. & Heineman, F. W. Control of mitochondrial respiration in the heart in vivo. Mol. Cell Biochem. 89, 191–197 (1989).
Montaigne, D. et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 130, 554–564 (2014).
Rider, O. J. et al. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation 125, 1511–1519 (2012).
Scheuermann-Freestone, M. et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation 107, 3040–3046 (2003).
Ng, A. C. et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation 122, 2538–2544 (2010).
Hernando, D. et al. Joint estimation of water/fat images and field inhomogeneity map. Magn. Reson. Med. 59, 571–580 (2008).
Kellman, P. et al. Multiecho dixon fat and water separation method for detecting fibrofatty infiltration in the myocardium. Magn. Reson. Med. 61, 215–221 (2009).
Ng, A. C. T. et al. Impact of epicardial adipose tissue, left ventricular myocardial fat content, and interstitial fibrosis on myocardial contractile function. Circ. Cardiovasc. Imaging 11, e007372 (2018).
Antonopoulos, A. S. & Antoniades, C. Cardiac magnetic resonance imaging of epicardial and intramyocardial adiposity as an early sign of myocardial disease. Circ. Cardiovasc. Imaging 11, e008083 (2018).
Gillinder, L. et al. Quantification of intramyocardial metabolites by proton magnetic resonance spectroscopy. Front. Cardiovasc. Med. 2, 24 (2015).
Peterson, L. R. et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109, 2191–2196 (2004).
Listenberger, L. L., Ory, D. S. & Schaffer, J. E. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 276, 14890–14895 (2001).
Chokshi, A. et al. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation 125, 2844–2853 (2012).
McGavock, J. M. et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 116, 1170–1175 (2007).
Burkhoff, D. et al. Influence of metabolic substrate on rat heart function and metabolism at different coronary flows. Am. J. Physiol. 261 (3 Pt 2), H741–H750 (1991).
Sacks, H. S. & Fain, J. N. Human epicardial adipose tissue: a review. Am. Heart J. 153, 907–917 (2007).
Djaberi, R. et al. Relation of epicardial adipose tissue to coronary atherosclerosis. Am. J. Cardiol. 102, 1602–1607 (2008).
Alexopoulos, N. et al. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 210, 150–154 (2010).
Nelson, R. H., Prasad, A., Lerman, A. & Miles, J. M. Myocardial uptake of circulating triglycerides in nondiabetic patients with heart disease. Diabetes 56, 527–530 (2007).
Malavazos, A. E. et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am. J. Cardiol. 105, 1831–1835 (2010).
Higuchi, Y. et al. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation 109, 1892–1897 (2004).
Sinagra, E., Perricone, G., Romano, C. & Cottone, M. Heart failure and anti tumor necrosis factor-alpha in systemic chronic inflammatory diseases. Eur. J. Intern. Med. 24, 385–392 (2013).
Greulich, S. et al. Secretory products of guinea pig epicardial fat induce insulin resistance and impair primary adult rat cardiomyocyte function. J. Cell Mol. Med. 15, 2399–2410 (2011).
Greulich, S. et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 126, 2324–2334 (2012).
Obokata, M., Reddy, Y. N. V., Pislaru, S. V., Melenovsky, V. & Borlaug, B. A. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 136, 6–19 (2017).
Borlaug, B. A. & Reddy, Y. N. V. The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Heart Fail. 7, 574–585 (2019).
McInnes, I. B. et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. 74, 694–702 (2015).
Mazurek, T. et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 108, 2460–2466 (2003).
Park, H. Y. et al. Potential role of leptin in angiogenesis: leptin induces endothelial cell proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp. Mol. Med. 33, 95–102 (2001).
Dahl, T. B. et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation 115, 972–980 (2007).
Antonopoulos, A. S. et al. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circ. Res. 118, 842–855 (2016).
Zhang, H. et al. Role of TNF-alpha in vascular dysfunction. Clin. Sci. 116, 219–230 (2009).
Salazar, J. et al. Epicardial fat: physiological, pathological, and therapeutic implications. Cardiol. Res. Pract. 2016, 1291537 (2016).
Iacobellis, G., Leonetti, F., Singh, N. & Sharma, M. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 115, 272–273 (2007).
Iacobellis, G., Ribaudo, M. C., Zappaterreno, A., Iannucci, C. V. & Leonetti, F. Relation between epicardial adipose tissue and left ventricular mass. Am. J. Cardiol. 94, 1084–1087 (2004).
Nyman, K. et al. Cardiac steatosis and left ventricular function in men with metabolic syndrome. J. Cardiovasc. Magn. Reson. 15, 103 (2013).
Zhao, L., Harrop, D. L., Ng, A. C. T. & Wang, W. Y. S. Epicardial adipose tissue is associated with left atrial dysfunction in people without obstructive coronary artery disease or atrial fibrillation. Can. J. Cardiol. 34, 1019–1025 (2018).
Ng, A. C., Goo, S. Y., Roche, N., van der Geest, R. J. & Wang, W. Y. Epicardial Adipose tissue volume and left ventricular myocardial function using 3-dimensional speckle tracking echocardiography. Can. J. Cardiol. 32, 1485–1492 (2016).
Kinlay, S., Libby, P. & Ganz, P. Endothelial function and coronary artery disease. Curr. Opin. Lipidol. 12, 383–389 (2001).
Vinik, A. I., Freeman, R. & Erbas, T. Diabetic autonomic neuropathy. Semin. Neurol. 23, 365–372 (2003).
Hanna, P. et al. Cardiac neuroanatomy - Imaging nerves to define functional control. Auton. Neurosci. 207, 48–58 (2017).
Matsunari, I. et al. Iodine-123 metaiodobenzylguanidine imaging and carbon-11 hydroxyephedrine positron emission tomography compared in patients with left ventricular dysfunction. Circ. Cardiovasc. Imaging 3, 595–603 (2010).
Tentolouris, N., Liatis, S. & Katsilambros, N. Sympathetic system activity in obesity and metabolic syndrome. Ann. N. Y. Acad. Sci. 1083, 129–152 (2006).
Egan, B. M. Insulin resistance and the sympathetic nervous system. Curr. Hypertens. Rep. 5, 247–254 (2003).
Falcao-Pires, I. & Leite-Moreira, A. F. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail. Rev. 17, 325–344 (2012).
Pellegrino, T. et al. Impact of obesity and acquisition protocol on 123I-metaiodobenzylguanidine indexes of cardiac sympathetic innervation. Quant. Imaging Med. Surg. 5, 822–828 (2015).
Allman, K. C. et al. Noninvasive assessment of cardiac diabetic neuropathy by carbon-11 hydroxyephedrine and positron emission tomography. J. Am. Coll. Cardiol. 22, 1425–1432 (1993).
Vinik, A. I. & Ziegler, D. Diabetic cardiovascular autonomic neuropathy. Circulation 115, 387–397 (2007).
Nagamachi, S. et al. Prognostic value of cardiac I-123 metaiodobenzylguanidine imaging in patients with non-insulin-dependent diabetes mellitus. J. Nucl. Cardiol. 13, 34–42 (2006).
Hattori, N. et al. Regional abnormality of iodine-123-MIBG in diabetic hearts. J. Nucl. Med. 37, 1985–1990 (1996).
Burke, G. L. et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch. Intern. Med. 168, 928–935 (2008).
Kramer, C. K., Zinman, B. & Retnakaran, R. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Ann. Intern. Med. 159, 758–769 (2013).
Chang, Y. et al. Metabolically-healthy obesity and coronary artery calcification. J. Am. Coll. Cardiol. 63, 2679–2686 (2014).
Echouffo-Tcheugui, J. B. et al. Natural history of obesity sub-phenotypes: dynamic changes over two decades and prognosis in The Framingham Heart Study. J. Clin. Endocrinol. Metab. 104, 738–752 (2019).
Kronmal, R. A. et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 115, 2722–2730 (2007).
Park, G. M. et al. Comparison of coronary computed tomographic angiographic findings in asymptomatic subjects with versus without diabetes mellitus. Am. J. Cardiol. 116, 372–378 (2015).
Malik, S. et al. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the multi-ethnic study of atherosclerosis. Diabetes Care 34, 2285–2290 (2011).
Scholte, A. J. et al. Prevalence of coronary artery disease and plaque morphology assessed by multi-slice computed tomography coronary angiography and calcium scoring in asymptomatic patients with type 2 diabetes. Heart 94, 290–295 (2008).
Nance, J. W. Jr. et al. Incremental prognostic value of different components of coronary atherosclerotic plaque at cardiac CT angiography beyond coronary calcification in patients with acute chest pain. Radiology 264, 679–690 (2012).
Kwan, A. C. et al. Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology 272, 690–699 (2014).
Oikonomou, E. K. et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet 392, 929–939 (2018).
Antonopoulos, A. S. et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 9, eaal2658 (2017).
Yun, C. H. et al. Pericardial and thoracic peri-aortic adipose tissues contribute to systemic inflammation and calcified coronary atherosclerosis independent of body fat composition, anthropometric measures and traditional cardiovascular risks. Eur. J. Radiol. 81, 749–756 (2012).
Marwan, M. & Achenbach, S. Quantification of epicardial fat by computed tomography: why, when and how? J. Cardiovasc. Comput. Tomogr. 7, 3–10 (2013).
Iles, L. et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J. Am. Coll. Cardiol. 52, 1574–1580 (2008).
Sibley, C. T. et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology 265, 724–732 (2012).
Miller, C. A. et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ. Cardiovasc. Imaging 6, 373–383 (2013).
Cavalera, M., Wang, J. & Frangogiannis, N. G. Obesity, metabolic dysfunction, and cardiac fibrosis: pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl. Res. 164, 323–335 (2014).
Wong, C. Y. et al. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 110, 3081–3087 (2004).
Ng, A. C. et al. Association between diffuse myocardial fibrosis by cardiac magnetic resonance contrast-enhanced T1 mapping and subclinical myocardial dysfunction in diabetic patients: a pilot study. Circ. Cardiovasc. Imaging 5, 51–59 (2012).
Wong, T. C. et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur. Heart J. 35, 657–664 (2014).
Ng, A. C. T. et al. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am. J. Cardiol. 104, 1398–1401 (2009).
Ernande, L. et al. Impaired myocardial radial function in asymptomatic patients with type 2 diabetes mellitus: a speckle-tracking imaging study. J. Am. Soc. Echocardiogr. 23, 1266–1272 (2010).
Ernande, L. et al. Diastolic dysfunction in patients with type 2 diabetes mellitus: is it really the first marker of diabetic cardiomyopathy? J. Am. Soc. Echocardiogr. 24, 1268–1275 (2011).
Yingchoncharoen, T., Agarwal, S., Popovic, Z. B. & Marwick, T. H. Normal ranges of left ventricular strain: a meta-analysis. J. Am. Soc. Echocardiogr. 26, 185–191 (2013).
Holland, D. J. et al. Subclinical LV dysfunction and 10-year outcomes in type 2 diabetes mellitus. Heart 101, 1061–1066 (2015).
Turkbey, E. B. et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA). J. Am. Coll. Cardiol. Imaging 3, 266–274 (2010).
Rider, O. J. et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J. Am. Coll. Cardiol. 54, 718–726 (2009).
de Divitiis, O. et al. Obesity and cardiac function. Circulation 64, 477–482 (1981).
Alpert, M. A. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am. J. Med. Sci. 321, 225–236 (2001).
Ng, A. C. T. et al. Impact of diabetes and increasing body mass index category on left ventricular systolic and diastolic function. J. Am. Soc. Echocardiogr. 31, 916–925 (2018).
Gustafson, B., Hammarstedt, A., Andersson, C. X. & Smith, U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 2276–2283 (2007).
Andersson, C. X., Gustafson, B., Hammarstedt, A., Hedjazifar, S. & Smith, U. Inflamed adipose tissue, insulin resistance and vascular injury. Diabetes Metab. Res. Rev. 24, 595–603 (2008).
Horwich, T. B. & Fonarow, G. C. Glucose, obesity, metabolic syndrome, and diabetes: relevance to incidence of heart failure. J. Am. Coll. Cardiol. 55, 283–293 (2010).
Seferovic, P. M. & Paulus, W. J. Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 36, 1718–1727c (2015).
Paulus, W. J. & Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
van, H. L. et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117, 43–51 (2008).
Shah, S. J. et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 39, 3439–3450 (2018).
Yang, J. H. et al. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 22, 432–441 (2020).
Lupon, J. et al. Heart failure with preserved ejection fraction infrequently evolves toward a reduced phenotype in long-term survivors. Circ. Heart Fail. 12, e005652 (2019).
Shah, S. J. et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134, 73–90 (2016).
Savji, N. et al. The association of obesity and cardiometabolic traits with Incident HFpEF and HFrEF. JACC Heart Fail. 6, 701–709 (2018).
Pandey, A. et al. Relationship between physical activity, body mass index, and risk of heart failure. J. Am. Coll. Cardiol. 69, 1129–1142 (2017).
Pandey, A. et al. Body mass index and cardiorespiratory fitness in mid-life and risk of heart failure hospitalization in older age: findings from the Cooper Center longitudinal study. JACC Heart Fail. 5, 367–374 (2017).
Kenchaiah, S. et al. Obesity and the risk of heart failure. N. Engl. J. Med. 347, 305–313 (2002).
Echouffo-Tcheugui, J. B. et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get With The Guidelines-Heart Failure registry. Am. Heart J. 182, 9–20 (2016).
Yap, J. et al. Association of diabetes mellitus on cardiac remodeling, quality of life, and clinical outcomes in heart failure with reduced and preserved ejection fraction. J. Am. Heart Assoc. 8, e013114 (2019).
Kristensen, S. L. et al. Clinical and echocardiographic characteristics and cardiovascular outcomes according to diabetes status in patients with heart failure and preserved ejection fraction: a report from the I-Preserve Trial (Irbesartan in Heart Failure With Preserved Ejection Fraction). Circulation 135, 724–735 (2017).
Shah, K. S. et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J. Am. Coll. Cardiol. 70, 2476–2486 (2017).
Reddy, Y. N. V. et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur. J. Heart Fail. 22, 1009–1018 (2020).
Reddy, Y. N. V. et al. Characterization of the obese phenotype of heart failure with preserved ejection fraction: A RELAX trial ancillary study. Mayo Clin. Proc. 94, 1199–1209 (2019).
Reddy, Y. N. V. et al. Adverse renal response to decongestion in the obese phenotype of heart failure with preserved ejection fraction. J. Card. Fail. 26, 101–107 (2020).
Shah, A. M. et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation 132, 402–414 (2015).
Kannel, W. B., Hjortland, M. & Castelli, W. P. Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 34, 29–34 (1974).
Iribarren, C. et al. Glycemic control and heart failure among adult patients with diabetes. Circulation 103, 2668–2673 (2001).
Pazin-Filho, A. et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 51, 2197–2204 (2008).
Dandamudi, S. et al. The prevalence of diabetic cardiomyopathy: a population-based study in Olmsted County, Minnesota. J. Card. Fail. 20, 304–309 (2014).
Liu, J. H. et al. Incremental prognostic value of global longitudinal strain in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 15, 22 (2016).
Hubert, H. B., Feinleib, M., McNamara, P. M. & Castelli, W. P. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67, 968–977 (1983).
Morkedal, B., Vatten, L. J., Romundstad, P. R., Laugsand, L. E. & Janszky, I. Risk of myocardial infarction and heart failure among metabolically healthy but obese individuals: HUNT (Nord-Trondelag Health Study), Norway. J. Am. Coll. Cardiol. 63, 1071–1078 (2014).
Haufe, S. et al. Left ventricular mass and function with reduced-fat or reduced-carbohydrate hypocaloric diets in overweight and obese subjects. Hypertension 59, 70–75 (2012).
Algahim, M. F. et al. Progressive regression of left ventricular hypertrophy two years after bariatric surgery. Am. J. Med. 123, 549–555 (2010).
Obert, P. et al. Impact of diet and exercise training-induced weight loss on myocardial mechanics in severely obese adolescents. Obesity 21, 2091–2098 (2013).
Lambadiari, V. et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc. Diabetol. 17, 8 (2018).
Cuspidi, C., Rescaldani, M., Tadic, M., Sala, C. & Grassi, G. Effects of bariatric surgery on cardiac structure and function: a systematic review and meta-analysis. Am. J. Hypertens. 27, 146–156 (2014).
Aggarwal, R. et al. The effects of bariatric surgery on cardiac structure and function: a systematic review of cardiac imaging outcomes. Obes. Surg. 26, 1030–1040 (2016).
Kurnicka, K. et al. Improvement of left ventricular diastolic function and left heart morphology in young women with morbid obesity six months after bariatric surgery. Cardiol. J. 25, 97–105 (2018).
Kaier, T. E. et al. Ventricular remodelling post-bariatric surgery: is the type of surgery relevant? A prospective study with 3D speckle tracking. Eur. Heart J. Cardiovasc. Imaging 15, 1256–1262 (2014).
Tuluce, K. et al. Early reverse cardiac remodeling effect of laparoscopic sleeve gastrectomy. Obes. Surg. 27, 364–375 (2017).
Shin, S. H. et al. Beneficial Effects of bariatric surgery on cardiac structure and function in obesity. Obes. Surg. 27, 620–625 (2017).
Leung, M., Xie, M., Durmush, E., Leung, D. Y. & Wong, V. W. Weight loss with sleeve gastrectomy in obese type 2 diabetes mellitus: impact on cardiac function. Obes. Surg. 26, 321–326 (2016).
Reddy, Y. N. V. et al. Hemodynamic effects of weight loss in obesity: a systematic review and meta-analysis. JACC Heart Fail. 7, 678–687 (2019).
Utz, W. et al. Moderate dietary weight loss reduces myocardial steatosis in obese and overweight women. Int. J. Cardiol. 167, 905–909 (2013).
Hsuan, C. F. et al. The effect of surgical weight reduction on left ventricular structure and function in severe obesity. Obesity 18, 1188–1193 (2010).
de las Fuentes, L. et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J. Am. Coll. Cardiol. 54, 2376–2381 (2009).
Olsen, R. H. et al. A randomised trial comparing the effect of exercise training and weight loss on microvascular function in coronary artery disease. Int. J. Cardiol. 185, 229–235 (2015).
Schuster, I. et al. Diastolic dysfunction and intraventricular dyssynchrony are restored by low intensity exercise training in obese men. Obesity 20, 134–140 (2012).
Umpierre, D. et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 305, 1790–1799 (2011).
Swift, D. L. et al. Effects of clinically significant weight loss with exercise training on insulin resistance and cardiometabolic adaptations. Obesity 24, 812–819 (2016).
Hordern, M. D. et al. Effects of exercise intervention on myocardial function in type 2 diabetes. Heart 95, 1343–1349 (2009).
Jonker, J. T. et al. Exercise and type 2 diabetes mellitus: changes in tissue-specific fat distribution and cardiac function. Radiology 269, 434–442 (2013).
Schrauwen-Hinderling, V. B. et al. Cardiac lipid content is unresponsive to a physical activity training intervention in type 2 diabetic patients, despite improved ejection fraction. Cardiovasc. Diabetol. 10, 47 (2011).
Look AHEAD Research Group et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 369, 145–154 (2013).
Connolly, H. M. et al. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 337, 581–588 (1997).
Fitzgerald, L. W. et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol. Pharmacol. 57, 75–81 (2000).
James, W. P. et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N. Engl. J. Med. 363, 905–917 (2010).
Topol, E. J. et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet 376, 517–523 (2010).
Greenway, F. L. et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376, 595–605 (2010).
Apovian, C. M. et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity 21, 935–943 (2013).
Smith, S. R. et al. Combination therapy with naltrexone and bupropion for obesity reduces total and visceral adiposity. Diabetes Obes. Metab. 15, 863–866 (2013).
Hollander, P. et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 36, 4022–4029 (2013).
Allison, D. B. et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity 20, 330–342 (2012).
Gadde, K. M. et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 377, 1341–1352 (2011).
Garvey, W. T. et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 95, 297–308 (2012).
Hsia, D. S. et al. A randomized, double-blind, placebo-controlled, pharmacokinetic and pharmacodynamic study of a fixed-dose combination of phentermine/topiramate in adolescents with obesity. Diabetes Obes. Metab. 22, 480–491 (2020).
Smith, S. R. et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 363, 245–256 (2010).
Fidler, M. C. et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J. Clin. Endocrinol. Metab. 96, 3067–3077 (2011).
O’Neil, P. M. et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity 20, 1426–1436 (2012).
Bohula, E. A. et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N. Engl. J. Med. 379, 1107–1117 (2018).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Tamborlane, W. V. et al. Liraglutide in children and adolescents with type 2 diabetes. N. Engl. J. Med. 381, 1786–1787 (2019).
le Roux, C. W. et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 389, 1399–1409 (2017).
Kumarathurai, P. et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide on systolic function in patients with coronary artery disease and type 2 diabetes: a randomized double-blind placebo-controlled crossover study. Cardiovasc. Diabetol. 15, 105 (2016).
O’Neil, P. M. et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet 392, 637–649 (2018).
Pratley, R. et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet 394, 39–50 (2019).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015).
McMurray, J. J. V. et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 381, 1995–2008 (2019).
Wiviott, S. D. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 380, 347–357 (2019).
Neal, B. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 377, 644–657 (2017).
Hsu, J. C., Wang, C. Y., Su, M. M.-Y., Lin, L. Y. & Yang, W. S. Effect of empagliflozin on cardiac function, adiposity, and diffuse fibrosis in patients with type 2 diabetes mellitus. Sci. Rep. 9, 15348 (2019).
Verma, S. et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation 140, 1693–1702 (2019).
Ferrannini, E. et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 65, 1190–1195 (2016).
Tardif, A. et al. Chronic exposure to beta-hydroxybutyrate impairs insulin action in primary cultures of adult cardiomyocytes. Am. J. Physiol. Endocrinol. Metab. 281, E1205–E1212 (2001).
Hasselbaink, D. M., Glatz, J. F., Luiken, J. J. F. P., Roemen, T. H. M. & Van der Vusse, G. J. Ketone bodies disturb fatty acid handling in isolated cardiomyocytes derived from control and diabetic rats. Biochem. J. 371 (Pt 3), 753–760 (2003).
Janardhan, A., Chen, J. & Crawford, P. A. Altered systemic ketone body metabolism in advanced heart failure. Tex. Heart Inst. J. 38, 533–538 (2011).
Beer, M. et al. Effects of exercise training on myocardial energy metabolism and ventricular function assessed by quantitative phosphorus-31 magnetic resonance spectroscopy and magnetic resonance imaging in dilated cardiomyopathy. J. Am. Coll. Cardiol. 51, 1883–1891 (2008).
Sjostrom, L. et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 357, 741–752 (2007).
Adams, T. D. et al. Long-term mortality after gastric bypass surgery. N. Engl. J. Med. 357, 753–761 (2007).
Jamaly, S., Carlsson, L., Peltonen, M., Jacobson, P. & Karason, K. Surgical obesity treatment and the risk of heart failure. Eur. Heart J. 40, 2131–2138 (2019).
Sundstrom, J. et al. Weight loss and heart failure: a nationwide study of gastric bypass surgery versus intensive lifestyle treatment. Circulation 135, 1577–1585 (2017).
Owan, T. et al. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2-year follow-up in the Utah obesity study. J. Am. Coll. Cardiol. 57, 732–739 (2011).
Shah, R. V. et al. Weight loss and progressive left ventricular remodelling: The Multi-Ethnic Study of Atherosclerosis (MESA). Eur. J. Prev. Cardiol. 22, 1408–1418 (2015).
Hannukainen, J. C. et al. Reversibility of myocardial metabolism and remodelling in morbidly obese patients 6 months after bariatric surgery. Diabetes Obes. Metab. 20, 963–973 (2018).
SCOT-HEART Investigators et al. Coronary CT angiography and 5-year risk of myocardial infarction. N. Engl. J. Med. 379, 924–933 (2018).
Davidson, M. H. et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA 281, 235–242 (1999).
Hollander, P. A. et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care 21, 1288–1294 (1998).
Leblanc, E. S., O’Connor, E., Whitlock, E. P., Patnode, C. D. & Kapka, T. Effectiveness of primary care-relevant treatments for obesity in adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 155, 434–447 (2011).
Astrup, A. et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374, 1606–1616 (2009).
Astrup, A. et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 36, 843–854 (2012).
Wadden, T. A. et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int. J. Obes. 37, 1443–1451 (2013).
Fryar, C. D., Carroll, M. D., & Ogden, C. L. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2015–2016. CDC https://www.cdc.gov/nchs/data/hestat/obesity_adult_15_16/obesity_adult_15_16.htm#Tables (2018).
Fryar, C. D., Carroll, M. D., & Ogden, C. L. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2015–2016. CDC https://www.cdc.gov/nchs/data/hestat/obesity_child_15_16/obesity_child_15_16.htm#Figure (2018).
Beadle, R. & Frenneaux, M. Magnetic resonance spectroscopy in myocardial disease. Expert Rev. Cardiovasc. Ther. 8, 269–277 (2010).
Ng, A. C. et al. Multimodality imaging in diabetic heart disease. Curr. Probl. Cardiol. 36, 9–47 (2011).
Author information
Authors and Affiliations
Contributions
A.C.T.N., B.A.B. and J.J.B. researched data for the article. All the authors provided substantial contribution to the discussion of its content, wrote the manuscript, and reviewed and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
V.D. and J.J.B. received speaker fees from Abbott Vascular and Edwards Lifesciences. B.A.B. receives support from the NIH (R01 HL128526). The Department of Cardiology of the Leiden University Medical Centre receives unrestricted research grants from Biotronik, Boston Scientific, Edwards Lifesciences, GE Healthcare and Medtronic. A.C.T.N. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ng, A.C.T., Delgado, V., Borlaug, B.A. et al. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nat Rev Cardiol 18, 291–304 (2021). https://doi.org/10.1038/s41569-020-00465-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-020-00465-5
This article is cited by
-
The effect of hyperlipidemia and body fat distribution on subclinical left ventricular function in obesity: a cardiovascular magnetic resonance study
Cardiovascular Diabetology (2024)
-
The impact of obesity on different glucose tolerance status with incident cardiovascular disease and mortality events over 15 years of follow-up: a pooled cohort analysis
Diabetology & Metabolic Syndrome (2024)
-
Left ventricular concentric hypertrophy with cardiac magnetic resonance imaging improves risk stratification in patients with Duchenne muscular dystrophy: a prospective cohort study
Pediatric Radiology (2024)
-
Myocardial Metabolic Reprogramming in HFpEF
Journal of Cardiovascular Translational Research (2024)
-
Gut microbiome as therapeutic target for diabesity management: opportunity for nanonutraceuticals and associated challenges
Drug Delivery and Translational Research (2024)