Abstract
Ambulatory monitoring is increasingly important for cardiovascular care but is often limited by the unpredictability of cardiovascular events, the intermittent nature of ambulatory monitors and the variable clinical significance of recorded data in patients. Technological advances in computing have led to the introduction of novel physiological biosignals that can increase the frequency at which abnormalities in cardiovascular parameters can be detected, making expert-level, automated diagnosis a reality. However, use of these biosignals for diagnosis also raises numerous concerns related to accuracy and actionability within clinical guidelines, in addition to medico-legal and ethical issues. Analytical methods such as machine learning can potentially increase the accuracy and improve the actionability of device-based diagnoses. Coupled with interoperability of data to widen access to all stakeholders, seamless connectivity (an internet of things) and maintenance of anonymity, this approach could ultimately facilitate near-real-time diagnosis and therapy. These tools are increasingly recognized by regulatory agencies and professional medical societies, but several technical and ethical issues remain. In this Review, we describe the current state of cardiovascular monitoring along the continuum from biosignal acquisition to the identification of novel biosensors and the development of analytical techniques and ultimately to regulatory and ethical issues. Furthermore, we outline new paradigms for cardiovascular monitoring.
Key points
-
Advances in the use of cardiovascular monitoring technologies, such as the development of novel portable sensors and machine learning algorithms that can provide near-real-time diagnosis, have the potential to provide personalized care.
-
Wearable sensor technologies can detect numerous biosignals, such as cardiac output, blood-pressure levels and heart rhythm, and can integrate multiple modalities.
-
The use of novel biosignals for diagnosis raises concerns regarding accuracy and actionability within clinical guidelines, in addition to medical, legal and ethical issues.
-
Machine learning-based interpretation of biosensor data can facilitate rapid evaluation of the haemodynamic consequences of heart failure or arrhythmias, but is limited by the presence of noise and training data that might not be representative of the real-world clinical setting.
-
The use of data derived from cardiovascular monitoring devices is associated with numerous challenges, such as data security, accessibility and ownership, in addition to other ethical and regulatory concerns.
Similar content being viewed by others
Introduction
Patients with cardiovascular conditions can have variable clinical presentations ranging from no symptoms to haemodynamic collapse, from hypertensive urgency to hypotension and from silent coronary ischaemia to acute coronary syndrome, as well as decompensated heart failure (HF), stroke or sudden death. This diversity in clinical presentation of cardiovascular disorders poses a major challenge for disease monitoring. Although clinicians use a variety of implanted, ambulatory and consumer wearable technologies for disease monitoring, the devices that are best suited to individual patients are difficult to establish. Indeed, optimal monitoring strategies have yet to be developed for some applications.
HF can worsen progressively over days or weeks, but current telemedicine systems might not be sufficient to detect acute exacerbations in HF or to prevent rehospitalization1,2. Conversely, arrhythmias can often occur suddenly or intermittently and might require immediate intervention3,4. Ambulatory rhythm-monitoring devices that allow only sporadic interpretation of data might be appropriate for benign events but not for life-threatening arrhythmias. This misalignment between clinical need and current monitoring technologies is also illustrated by the lack of robust strategies for the detection of impending coronary syndromes, hypertensive emergencies, hypotensive events or stroke in high-risk patients with atrial fibrillation (AF).
Advances in cardiovascular monitoring technologies, such as the use of ubiquitous mobile devices and the development of novel portable sensors with seamless wireless connectivity and machine learning algorithms that can provide specialist-level diagnosis in near real time, have the potential for a more personalized care. Devices have been developed to assess haemodynamics, which can detect potential signs of worsening HF2. Furthermore, continuous electrocardiogram (ECG) recordings have been used to redefine phenotypes for AF4 and ventricular arrhythmias3, and can predict success of antiarrhythmic therapy5. Wearable activity trackers and smartwatches can measure physiological indices such as heart rate, breathing patterns and cardiometabolic activity6, and can even detect AF7. Furthermore, smartphone applications have been successful in shortening the time to first response for sudden cardiac arrest8. This confluence of novel technologies has also attracted much public interest and the promise to expand applications for cardiovascular monitoring.
In this Review, we describe the latest advances in cardiovascular monitoring technology, focusing first on biosignal acquisition and analytical techniques that enable accurate diagnosis, triage and management (Fig. 1). We discuss monitoring in the context of likely future directions in cardiovascular care and identify numerous technical and clinical obstacles, issues regarding data security and privacy, and ethical dilemmas and regulatory challenges that must be overcome before integrated and scalable cardiovascular monitoring tools can be developed.
Numerous innovations in biosignal acquisition, diagnosis and medical triage, and data access enable the curation of data as a dynamic resource that can ultimately be used to alter management guidelines and provide novel pathophysiological insights into cardiovascular diseases. However, the acquisition, processing and use of these innovative technologies is associated with various challenges. EBM, evidence-based medicine.
Biosignal acquisition
Biosignals, physiological signals that can be continuously measured and monitored to provide information on electrical, chemical and mechanical activity, are the foundations of assessment of health and disease, and have been used to develop personalized physiological ‘portraits’ of individuals. Numerous current and emerging wearable technologies can measure multiple physiological biosignals such as pulse, cardiac output, blood-pressure levels, heart rhythm, respiratory rate, electrolyte levels, sympathetic nerve activity, galvanic skin resistance and thoracic and lower-extremity oedema (Fig. 2). Some devices can acquire multiple biosignals simultaneously, which can provide inputs to powerful integrated monitors and diagnostic systems. In developing scalable monitoring technology, the short-term goal is to implement guideline-driven care, whereas a longer-term goal is to expand the scope of care by tracking physiological variables continuously in each individual.
Examples of emerging wearable technologies include mobile phones, body sensors (TempTraq, Blue Spark Technologies, USA), glasses (OrCam MyEye, OrCam Technologies, Israel), necklaces (toSense, USA), earrings (Joule, Ear-O-Smart, BioSensive Technologies, USA), headbands (SmartSleep, Philips, USA, and EPOC, Emotiv, USA), rings (Motiv Ring, Motiv, USA), bracelets (Bangle Activity Tracker, Kate Spade New York, USA), skin patches (BioStampRC, MC10, USA, VitalPatch, VitalConnect, USA, and BodyGuardian Heart, Preventice Solutions, USA), clothing fabric (Nanowear, USA, Hexoskin Smart Shirt, Hexoskin, Canada, and SmartSleep Snoring Relief Band, Philips, USA), belts (Smart Belt Pro, WELT Corp., South Korea, and LumiDiet, Double H, South Korea), socks (Sensoria Socks 2.0, Sensoria, USA, and Siren Diabetic Socks, Siren, USA), shoes (Nike Adapt, Nike, USA) and shoe insoles (Energysole, MEGAComfort, USA).
Table 1 summarizes the use of wearable sensor technologies to detect biosignals. Some sensor technologies can now integrate multiple modalities, such as chest patches that monitor heart rate, heart rhythm, respiration rate and skin temperature7,9. Sensors are being developed to measure myocardial contractility and cardiac output (ballistocardiography), cardiac acoustic data (phonocardiography) and other indices10. We describe various biosensors in the following sections, with reference to their target biosignals and potential clinical applications.
Implanted intracardiac monitors
To date, more than three million people living in the USA have cardiac implantable electronic devices (CIEDs) such as pacemakers, defibrillators or left ventricular assist devices11. Many more patients have other non-CIEDs such as cochlear implants and nerve stimulators. CIEDs are the gold standard for cardiac rhythm detection, providing sensitive and specific measurements with little noise continuously over long time frames of several years. CIEDs are also highly effective prototypes for real-time automatic diagnosis and therapy. Indications for CIED use include pacing for bradyarrhythmias, and tachypacing and defibrillation for tachyarrhythmias. Additionally, most CIEDs also record intracardiac electrograms as a surrogate for ECGs. CIEDs that are prescribed for one indication might provide monitoring that confers clinical benefits for a separate indication, such as the monitoring of atrial arrhythmias by atrial leads in pacemakers or defibrillators, or the monitoring of atrial arrhythmias by far-field atrial electrograms from ventricular leads in some pacemakers or implantable cardioverter–defibrillators (ICDs)12.
CIEDs are well suited to monitor symptoms of HF. In patients with an ICD or a pacemaker, CIEDs can provide indices of heart rate variability and pulmonary impedance, which can track HF and prove an alert for possible decompensation13. Diminished heart rate variability (<100 ms) has been shown to indicate increased sympathetic and decreased vagal modulation, and is associated with increased risk of death, worsening HF and malignant ventricular arrhythmias14. A decline in electrical impedance of the thorax is indicative of pulmonary congestion15. Another promising biosignal for the detection of HF is pulmonary artery pressure. COMPASS-HF16 was the first randomized trial to investigate the efficacy of intracardiac pressure monitoring for HF management with the use of a right ventricular sensor (Chronicle, Medtronic) that measures estimated pulmonary artery diastolic pressure as a surrogate for pulmonary artery pressure. Notably, continuous haemodynamic monitoring did not significantly reduce the incidence of HF-related events compared with optimal medical management. The subsequent CHAMPION study17 showed that monitoring pulmonary artery pressure using the CardioMEMS system (Abbott) significantly lowered the rate of repeated HF hospitalization and was associated with reduced costs compared with standard care. A 2019 meta-analysis involving mostly patients with HF with reduced ejection fraction found that pressure monitoring, but not impedance monitoring, was associated with a lower rate of hospital admission for HF18. Other forms of HF monitors in development integrate pulmonary artery pressure monitoring with vital sign monitoring (Cordella Heart Failure System, Endotronix), left atrial pressure monitoring and various wearable devices19.
Additional CIED-based biosensors for cardiovascular monitoring are likely to emerge in the next 2–3 years. An implanted device that provides neurostimulation of the phrenic nerve has been shown to be effective in reducing episodes of central sleep apnoea20. Such novel CIEDs could, in principle, detect physiological markers that correlate with symptoms of AF or HF that frequently accompany sleep apnoea. Numerous leadless, extravascular devices currently under investigation can defibrillate21 or pace the heart22. Future innovations might eliminate the need to extract the device for battery replacement by using external recharging systems or designs that can transduce energy from respiratory or cardiac motion23.
ECG monitoring
The body surface ECG is a widely used biosignal in medically prescribed monitors and consumer devices (Fig. 2). Ambulatory ECG monitors typically consist of three or more chest electrodes connected to an external recorder or a fully contained patch monitor, and can record continuously for 1–14 days. Some devices have fewer leads, such as the Spider Flash (Datacard Group), which consist of two leads and can record for up to 6 min before and 3 min after detecting an event, and the CardioSTAT (Icentia), a single-lead ECG monitor that can provide continuous recordings. Data from such ECG monitors are uploaded to a central server either wirelessly or by direct device ‘interrogation’, interpreted using semiautomated algorithms and manually confirmed to generate reports and alerts. Some devices can provide near-real-time management options. The mobile cardiac outpatient telemetry (MCOT) system is an ambulatory ECG monitoring system that can transmit signals over a cellular network without activation by the patient and might increase diagnostic yield compared with other systems24,25.
A major application for ECG sensors is to optimize the detection of AF26. AF is, in many ways, an ideal target for biosensors. Numerous ECG sensors focus on detecting rapid and irregularly irregular QRS complexes in AF, but other metrics of rapid and irregular atrial rate and irregular beat-to-beat waveforms might increase diagnostic specificity27. AF can also cause beat-to-beat changes in perfusion and haemodynamics that might allow detection from non-electrical biosignals.
Another major indication for ECG monitors is the detection of ST-segment shifts indicative of coronary ischaemia, which requires relatively noise-free ECGs and sophisticated detection algorithms. Machine learning technologies have been incorporated into wearable devices for the detection of ST-segment elevation with an accuracy of up to 97.4% (ref.28). In principle, coronary ischaemia monitoring could also use optical, electrochemical, mechanical or microRNA-based biosensors, but these applications have not yet been widely adopted. Limitations of ECG-based ambulatory monitoring include noise (particularly during physical activity), the typically limited monitoring duration of 1–2 weeks (which might be insufficient to detect infrequent events) and delays in generating reports and instigating appropriate actions29.
Insertable or implantable loop recorders are minimally invasive devices that can provide long-term ECG monitoring for months or years and include the Reveal LINQ system (Medtronic), the Confirm Rx insertable cardiac monitor (Abbott) and the BioMonitor (Biotronik). The devices are inserted subcutaneously over the sternum or under the clavicle to mimic V leads and to optimize ECG recordings. Data are uploaded during device checks on a 3–6-monthly basis. The advantages of implantable loop recorders include the capacity for long-term monitoring and consistent ECG wave morphologies owing to a fixed spatial orientation. Paradoxically, such devices are suboptimal for the diagnosis of arrhythmias of short durations (tens of seconds to minutes) and for classifying the type of atrial arrhythmia30. These limitations might be overcome with improvements in signal processing algorithms31. Furthermore, most implantable loop recorders cannot establish the haemodynamic significance of detected arrhythmias, although the Reveal LINQ system does include an accelerometer that measures patient activity. A modified Reveal LINQ device was used to capture ECG data, temperature, heart rate and other parameters in American black bears and detected low activity and extreme bradycardia during hibernation32. Lastly, delays in the reporting of urgent events measured by implanted devices might be worsened by longer recording durations between device checks, although some platforms (Reveal LINQ and Confirm Rx) allow home monitoring with programmable alerts.
Finally, numerous wearable ECG devices are available to the public. The Apple Watch (Apple) and KardiaMobile (Alivecor) are approved by the FDA for rhythm monitoring and have clinical-level accuracy for the detection of arrhythmias such as AF33. None of these devices provides continuous monitoring, although daily and nightly use for months might ultimately provide near-continuous recordings. However, at present, these devices require activation by the patient to record the ECG, and smartwatch pulse checks (via photoplethysmography (PPG)) occur only intermittently. Therefore, these monitors can miss paroxysmal arrhythmia events that are too short in duration or too catastrophic in nature to be captured by the patient and cannot measure arrhythmia burden. As wearable devices become increasingly flexible, stretchable and weightless, they can be comfortably worn continuously to provide uninterrupted ECG data34.
At present, unclassifiable tracings are common among all ECG monitoring devices, which is likely to improve with technological advances35. Some systems have increased signal fidelity, such as the KardiaMobile six-lead device (Alivecor) or the CAM device (BardyDx), which might reduce noise and improve P-wave discernment27. Patients are increasingly opting for FDA-approved consumer devices, which increases the urgency to extend guidelines to adopt such technologies when appropriate36.
Photoplethysmography
PPG is an optical technique used to detect fluctuations in reflected light that can provide data on the cycle-by-cycle changes in cardiac haemodynamics37. PPG uses a light source, such as a light-emitting diode, to illuminate the face, fingertips or other accessible parts of the body. Early fitness trackers used this technology to estimate heart rate, but PPG-measured heart rate is associated with a low positive predictive value38, particularly if patients are ambulatory39 or exercising40. The WATCH-AF trial41 was a prospective, case–control study that compared the diagnostic accuracy of a smartwatch-based algorithm using PPG signals with ECG data measured by cardiologists. The PPG algorithm had very high specificity and diagnostic accuracy, but was limited by a high dropout rate owing to insufficient signal quality. Although few comparison studies have been performed, PPG-based analysis of heart rate and rhythm might be less accurate than ECG-based assessment42.
An emerging area for PPG-based sensors is for the monitoring of blood-pressure levels. PPG-based blood-pressure assessment requires the mapping of pulsatile peripheral waveforms to aortic pressure and uses algorithms that incorporate machine learning technologies43,44. However, the sensitivity and specificity of such a sensor in measuring blood-pressure levels in the general population have not yet been defined, and measurement variability might affect their accuracy45. PPG data can also be measured without body surface contact46. Video cameras can detect subtle fluctuations in facial perfusion with normal heartbeats to identify arrhythmias, including AF47. Once technical, workflow and regulatory challenges are overcome, this contactless approach could be used for health screening in a physician’s office, in a nursing home or in public spaces. However, this approach also highlights societal and ethical issues related to patient privacy and confidentiality, and the physician’s responsibility to inform and treat patients48. The infrastructure needed to inform a passer-by of an abnormality detected by contactless sensing technology is not yet available, and whether this protocol is appropriate given that consent for testing was not obtained and which stakeholders would be responsible for informing the individual and then ensuring adequate therapy and follow-up are unclear.
Nevertheless, major advances in PPG sensor technology could facilitate the acquisition of haemodynamic data and assessment of their clinical significance in multiple domains, including HF, coronary ischaemia and arrhythmia monitoring. Importantly, these devices could also be used to augment traditional home sphygmomanometer devices for haemodynamic monitoring.
Innovative biosensors for HF detection
Numerous biosensors are being developed that can monitor HF progression. Intrathoracic impedance can be used to detect pulmonary congestion in patients with HF. Daily self-measurement of lung impedance using non-invasive devices has been described. In patients with HF, use of the Edema Guard Monitor (CardioSet Medical) combined with a symptom diary was associated with increases in self-behaviour score for 30 days after hospital discharge49. In an analysis of more than 500,000 individuals in the UK Biobank, a machine learning model revealed that leg bioimpedance was inversely associated with HF incidence50. Numerous innovative and non-invasive tools can be used to detect leg impedance, such as sock-based sensors51. Furthermore, microphone-based devices have been used to transform cardiac acoustic vibrations to biomedical signals in quantitative versions of the phonocardiogram52. Such devices can track respiratory rate, heart and lung sounds, and body motion or position, and might be superior to physical examination for predicting worsening HF53.
Biosensors for other cardiovascular indications are in development. An external device has been described that can monitor impending thrombosis in intra-arterial mechanical pumps with the use of an accelerometer for real-time analysis of pump vibrations to detect thrombosis and possibly prevent thromboembolic events54. Ballistocardiography, a non-invasive measure of body motion generated by the ejection of blood in each cardiac cycle10, has been incorporated into devices such as weighing scales to measure heart rate55, whereas a digital artificial intelligence (AI)-powered stethoscope that integrates both ECG and phonocardiogram data was approved in 2020 by the FDA to assess patients for the presence of AF and heart murmurs56. The most promising systems might combine multimodality biosignals rather than using a single biosignal.
Challenges of novel monitoring platforms
Several challenges must be overcome before novel monitoring strategies can be adopted for clinical use in the ambulatory setting, which introduces noise from motion, electromagnetic interference and various patient activities, which are more controlled in the clinic. Biosensor design must match hardware specifications to biosignal characteristics for each clinical indication. Furthermore, device design must take into account the trade-off between duration and quantity of collected data, required battery power and device size, and durability in real-world use. Importantly, devices tested under one set of clinical conditions are not applicable for use for other clinical conditions, a particularly relevant point to remember given the growth of poorly regulated consumer medical devices.
Subtle changes in biosignals might also confound analysis, such that testing and validation might need to be repeated de novo for each device being investigated. Of note, many widely used consumer devices have only modest accuracy even for the ‘simple’ biosignals of heart rate or energy expenditure40. Whether accuracy is reduced owing to differences in study cohorts between initial device validation and real-world users57, biological differences in biosignals owing to varying activity levels or other factors is unknown39,58,59. Biosignals that are calibrated in healthy volunteers might differ in accuracy when detecting disease. For example, tachycardia or irregularly irregular AF might introduce noise or variabilities in QRS morphology compared with sinus rhythm and can influence ECG algorithms59. Similarly, variability in pulse waveforms might influence PPG algorithms. Accordingly, algorithms developed with machine learning technology are best applied when the training and test populations are analogous. When these populations differ, learned features might become inaccurate, compounded in machine learning by limited methods to interpret its decisions (justifying why machine learning has sometimes been described as a ‘black box’)60. Testing and validation for each specific clinical application are, therefore, critical in device development.
Machine learning for biosignal analysis
The large quantity of data generated by ambulatory monitoring devices necessitates accurate and automated diagnosis and an infrastructure to enable quick clinical actions. The time-honoured method of human review and annotation of clinical data is also time-consuming, expensive and not scalable. Novel, scalable approaches to data interpretation and actionability might allow the potential of novel ambulatory monitoring to be realized. By reducing the time needed for data interpretation, ambulatory monitoring can detect acute events, such as worsening HF, incipient coronary syndrome or impending sudden cardiac arrest, and provide timely feedback for less urgent events.
Traditional analytical models for ambulatory monitoring rely on a limited number of biosignals and apply intuitive rules, such as those related to rate or regularity of heart rhythm, to flag a normal or abnormal result (Fig. 3a). Such forms of AI systems are known as ‘expert systems’60. Although these traditional models might introduce inaccuracy in data interpretation, slight inaccuracies might be acceptable in traditional health-care paradigms in which data flagged by the device are verified by clinicians. However, this approach might not be safe for wearable consumer devices with little or no clinician input.
a | Traditional systems for the analysis of ambulatory monitoring data rely on a limited number of biosignals and apply signal processing algorithms related to the rate or regularity of heart rhythm to flag a normal or abnormal result. The provider is then alerted to the result for management purposes. In a parallel pathway involving cardiac implanted electronic devices (CIEDs; dashed line), data analysed by the CIED can be used to deliver therapy by altering pacing or delivering implantable cardioverter–defibrillator therapy. b | A potential future model for monitoring might incorporate multiple inputs including biosignals (such as electrograms, haemodynamics and activity levels), patient input and clinical data, which are analysed by a machine learning algorithm. Deep neural networks, a type of machine learning technology, facilitate the classification of multiple diverse inputs even if traditional rules would be difficult to devise. In this scenario, deep neural networks receive inputs (denoted X0, X1, X2, X3 and Xn) and use hidden nodes (denoted h0, h1, h2 and hn) to classify them into actionable outputs (denoted y0 and y1). This model can be tailored to the patient and the type of sensor available. Given that many ambulatory devices are likely to be patient-driven, data will be directly sent to the patient. Additional infrastructure is needed to inform health-care providers of actionable diagnoses138. AF, atrial fibrillation; EMR, electronic medical record.
Machine learning is a rapidly developing branch of AI that has shown early promise for use in cardiovascular medicine61 through the extraction of clinically relevant patterns from complex data, such as detecting myocardial ischaemia from cardiac CT images62 and interpreting arrhythmias from wearable ECG monitors33. Machine learning can also facilitate novel strategies for communication between patients and the health-care team (Fig. 3b). Machine learning-based classification of biosensor data from multiple sensors can automatically evaluate the haemodynamic consequences of HF, arrhythmias or coronary syndromes, and can enable rapid triage without the need to develop, test and separately implement complex rules. Conversely, machine learning algorithms are not perfect and are limited by the presence of noise and training data that might not adequately represent the real-world clinical setting. In a study to detect AF, a third of ECGs could not be interpreted by a consumer device but could be classified by experts35. Furthermore, in a proof-of-concept study involving the use of smartwatch-based PPG sensor data analysed by a deep neural network, AF was diagnosed accurately in recumbent patients (C statistic 0.97) but not in ambulatory patients (C statistic 0.72)39.
Integration of multiple data streams
Advanced monitoring systems that integrate data from multiple streams can better mimic the diagnostic performance of a clinician than current devices that monitor a single data stream. A system that identifies an impending event is likely to be more accurate if an event detected from the ECG is combined with evaluation of potential haemodynamic compromise (such as from a PPG signal) than use of either signal alone. The integration of multiple physiological data streams is a complex task for which simple rules might not readily exist. Machine learning might provide such decision-making potential because of its proven capacity to classify complex data.
Figure 3b illustrates a typical machine learning architecture comprising an artificial neural network with multiple inputs. This type of architecture can capture multimodal biosignals such as ECG, pulse oximetry and electronic medical record (EMR) data (denoted Xn in Fig. 3b) and classify them by adjudicated outcome (denoted y0 or y1), which might represent response or non-response to therapy, or the presence or absence of a haemodynamically significant event. Layers in the model (denoted h0–hn) distil input biosignals into archetypes of data that are relevant to the outcomes, constructed iteratively in the hidden ‘deeper’ layers during algorithm training. These hidden layers are integrated at lower levels to reduce the extent (or dimensionality) of data and identify patterns that best match with the critical event60,61. Although decisions made by such machine learning models are not always readily interpretable, studies have shown that these models make mistakes similar to those made by humans33 and can learn ‘expert’ decision-making processes even if not trained in these processes, raising confidence that machine learning decisions are medically intuitive63.
Machine learning algorithms for diagnosis
Several machine learning-based monitoring systems have been assessed for their efficacy in guiding clinical management. The LINK-HF multicentre study2 investigated the accuracy of a smartphone-based and cloud-based machine learning algorithm that analysed data from a wearable patch for predicting the risk of rehospitalization (via measurement of physiological parameters such as ECG, heart rate, respiratory rate, body temperature, activity level and body position) in 100 patients with HF. This system predicted the risk of imminent HF hospitalization with up to 88% sensitivity and 85% specificity, which is similar to that of implanted devices. A follow-up study to determine whether this approach can prospectively prevent rehospitalizations for HF is ongoing. The 2012 MUSIC study64 was a multicentre, non-randomized trial to validate a multiparameter algorithm in an external multisensor monitoring system to predict impending acute HF decompensation in 543 patients with HF with reduced ejection fraction. Algorithm performance met the prespecified end point with 63% sensitivity and 92% specificity for the detection of HF events.
Numerous monitoring devices that use machine learning technology have been developed to detect ventricular arrhythmias and impending sudden cardiac arrest. The design of the 100Plus Emergency watch (formerly the iBeat Heart Watch) involves a closed-loop system that uses machine learning algorithms to monitor signals detected from a dedicated watch, which then automatically contacts emergency services if the wearer does not respond to a notification within 10 s (ref.65). Machine learning technology (‘deep learning’)60 has also been shown to improve the performance of shock advice algorithms in an automated external defibrillator66 to predict the onset of ventricular arrhythmias with the use of an artificial neural network67 and to predict the onset of sudden cardiac arrest within 72 h by incorporating heart rate variability parameters with vital sign data68. A system that can warn patients of an impending life-threatening cardiac event, even if only by several minutes, will greatly increase the availability and efficacy of a bystander or emergency medical response67.
Pathophysiological insights
The application of machine learning to continuous biosensor data is beginning to provide insights into the pathophysiological mechanisms underlying numerous cardiovascular conditions, such as the identification of novel disease phenotypes that might respond differentially to therapy. Novel immune phenotypes for pulmonary arterial hypertension were identified by unsupervised machine learning analysis of a proteomic panel including 48 cytokines and chemokines from whole-blood samples69. The investigators identified four clusters independent of WHO-defined pulmonary arterial hypertension subtypes, which showed distinct immune profiles and predicted a 5-year transplant-free survival of 47.6% in the highest-risk cluster and 82.4% in the lowest-risk cluster. A machine learning-based cluster analysis of echocardiogram data from patients in the TOPCAT trial revealed three novel phenotypes of HF and preserved ejection fraction with distinct clinical characteristics and long-term outcomes70. In a study involving 44,886 patients with HF with reduced ejection fraction from the Swedish HF Registry, the use of machine learning to analyse demographic, clinical and laboratory data resulted in a random forest-based model that predicted 1-year survival with a C statistic of 0.83 (ref.71). Cluster analysis led to the identification of four distinct phenotypes of HF with reduced ejection fraction that differed in terms of outcomes and response to therapeutics, highlighting the role of such novel analytical strategies in increasing the effectiveness of current therapies.
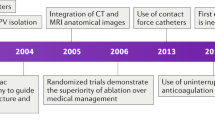
Machine learning data have also provided mechanistic insights into the pathophysiology of AF. Patients with persistent or paroxysmal AF show rates of response to antiarrhythmic medications of 40–60% and to cardiac ablation of 50–70%72. Data from continuous ECGs show that current clinical classifications poorly reflect the true temporal persistence of AF4. Additional studies could identify AF patterns or other physiological phenotypes in patients with ‘less advanced’ persistent AF in whom pulmonary vein isolation alone might be effective, or conversely those with ‘more advanced’ paroxysmal AF in whom pulmonary vein isolation might be less effective. Patients could thus be stratified for treatment according to newly recognized patterns of AF (that is, staccato versus legato)73 or by incorporating haemodynamic or clinical data. A 2019 proof-of-concept study showed that machine learning trained on daily AF burden from continuous CIED tracings revealed signatures with incremental prognostic value for the risk of stroke beyond the CHA2DS2–VASc score74. Patients with HF and arrhythmias could thus show differing prognosis depending on arrhythmia burden75. Therefore, although in the near future digital health platforms are unlikely to provide ‘precision medicine’ at the granular level of individualizing therapy according to genotype, such platforms might still provide the opportunity for personalized care on the basis of deep patient phenotyping to provide novel disease insights.
Regulatory framework and data ownership
The FDA published a discussion paper in April 2019 describing the development, testing and regulatory oversight for machine learning approaches between the stages of premarketing and postmarketing performance76. In general, a desirable system should accurately identify and separate data indicative of urgent or non-urgent clinical states. In the absence of such a system, all biosensor data that meet prescribed cut-off points, such as extreme bradycardia or tachycardia, are flagged and the health-care provider is alerted. This FDA guidance allows device manufacturers to invest in the development of models with a lower-risk pathway to implementation and is intended to increase clinician–patient interactions and promote wellness. However, a drawback of applying traditional regulatory processes to rapidly evolving devices is that machine learning algorithms are typically ‘frozen’, with no further changes permitted, when a ‘software as a medical device’ (SaMD) application is submitted (defined as software that is intended to be used for medical purposes that performs these tasks without being part of a hardware medical device)76. This process limits the opportunity to approve self-learning algorithms, which would ultimately differ from the submitted version, and this limitation is amplified by the inevitable time between receiving trial data and approving the data for use in patients. One potential solution could be to submit several versions of a device for approval, including a base case for the most validated primary labelling indication, plus alternatives with preliminary data for secondary labelling indications. Another approach is to approve a ‘snapshot’ of the SaMD self-learning algorithms associated with a registry, which is similar to postmarketing studies for devices and drugs that require repeated evaluation at predetermined intervals.
Databases for monitoring systems
Development and training of algorithms requires gold-standard data (often termed a ‘ground truth’), yet such data can be difficult to obtain in patients, which complicates the regulatory and clinical pathway. Biosignals are typically complex, non-linear, high-dimensional (comprising many variables) and dynamic. High-quality labelled datasets are scarce both for novel biosignals such as ballistocardiograms and for well-established biosignals such as thoracic impedance, energy expenditure or ECGs measured from atypical locations. Although new datasets can be created for such signals, the accuracy of the sets must be validated de novo. Bias is introduced whenever humans interact with data, which should be considered when scalable systems are being designed. One ideal solution would be the development of curated databases with specific biosignal data streams that are labelled by adjudicated outcomes and tailored to each use77. Although standardized databases such as Physionet have been useful for testing algorithms for research78, these databases are small and might not include data from novel biosensors. The plethora of commercially available health monitoring devices has facilitated the generation of large proprietary datasets, yet these databases are not always transparent or available for validation33. Therefore, the regulatory pathway might require several clinical tests with prototypes in each class of device or algorithm, and multiple well-curated datasets. Device manufacturers should demonstrate that emerging devices can be operated by untrained users to acquire recordings that will perform well with their systems, including analysis of human factors that can bias the results and analyses specific to their algorithms. Therefore, although standardization of novel biosensors across manufacturers is ultimately desirable, this goal might need to be deferred until technologies become more mature.
Patient-centred data access
Regulatory agencies in the USA, including the FDA, and patient advocacy groups have unanimously taken the position that patients must be empowered in their relationship with health-care providers and have access to their data79. Meaningful use criteria for EMRs require data sharing through patient access portals, yet such data might be difficult for patients to interpret (Table 2). Historically, medical device data have been kept in databases owned and maintained by industry and accessible by health-care providers, yet with more limited accessibility for patients. Consumer devices have shifted this landscape, empowering individuals to access their data from device companies, who then directly provide automated reports without having to notify a caregiver (Fig. 1).
This model introduced several potential challenges. Whether meaningful use criteria for EMRs apply to consumer device-based data is unclear. Moreover, whether a health-care organization can have timely and unfettered access to data ‘ordered’ then paid for by a consumer and then stored in devices that are also paid for by the consumer is unclear (Table 3).
One important additional point is that these devices have already been developed with use of data that arguably belong to the consumer. In 2016, the Alphabet-owned AI company DeepMind Technologies partnered with health-care authorities in the UK to access health data without the need for patients’ permission80. This model introduces potential risks of a ‘services for data’ social media business model in which personal data are commoditized for sale to or by third-party companies. Alternatively, if medical devices and data are owned and paid for by consumers, an opportunity exists for market forces or legislation to return control to data owners. Device manufacturers or third parties could conceivably compete in providing patient-friendly data visualization tools, to which medical providers could also pay for access. This scenario has its own challenges and is likely to be a point of contention in coming years.
Data security
A complicated responsibility exists for data that are shared between users (patients, health-care providers and algorithm developers), data owners (health-care organizations, individuals and industry) and industry. Health-care organizations are liable for unauthorized access to EMRs, yet numerous privacy concerns exist for non-health-related mobile data. Consumer devices are also likely to encounter cybersecurity risks, which must be addressed proactively.
Data breaches, both unintentional and malicious in nature, have been reported by many companies that are now entering the health-care market, as well as diagnostic companies81 and CIED manufacturers82. The technical shift to consumer-driven technology might provide a catalyst to standardize biosensor and data formats, and in turn increase security. Blockchain technology, which has been successfully used in financial markets and other industries, might have a role in patient-centred monitoring by tagging data ownership, providing traceability and enabling incentive programmes for sharing data83.
Geopolitical regulations are also in development. The General Data Protection Regulation was enacted in the EU in 2016 with the primary goal of giving individuals control over their personal data, and aims to unify the regulations within the region and provide safeguards to protect data, requiring all stakeholders to disclose data collection practices and breaches that occur. This regulation has become a model for privacy laws elsewhere and is similar in structure to the California Consumer Privacy Act. However, it is unclear how general consumer regulations will apply to or potentially influence the US Health Insurance Portability and Accountability Act, which could also be modified given that it covers only a fraction of an individual’s health-related data84.
Real-time cardiovascular care delivery
Devices that integrate high-fidelity biosignal detection with broadband wireless connectivity and cloud processing could, in principle, facilitate real-time care. A similar landscape is rapidly developing in the automotive industry with regard to the design of autonomous driving vehicles that apply multimodal, ultrafast fusion algorithms to multiple data streams that can provide an immediate response. To apply this technology to wearable devices, collected data must interact within a rapidly changing clinical context, which has already occurred for ICD therapy for tachyarrhythmia or pacing technology for bradycardia85. However, this technology is less developed for other domains such as AF management and HF or blood-pressure monitoring and devices that require multimodal data. Several clinical studies of mobile and wearable device platforms are summarized in Table 3.
One early model is the currently available MCOT system for arrhythmia monitoring. The MCOT system includes ECG sensors and a device that automatically transmits data to a central analysis hub for annotation and alerts the health-care provider25. The cycle time for this process ranges from minutes to hours. This approach can increase the diagnostic yield over that of other ambulatory ECG systems25 and has been used during the coronavirus disease 2019 (COVID-19) pandemic to monitor the QT interval in patients receiving hydroxychloroquine or azithromycin while simultaneously minimizing clinician exposure and preserving personal protective equipment resources86. During the COVID-19 pandemic, the Heart Rhythm Society (HRS) recommended the use of digital wearable devices to obtain vital signs and ECG tracings, as well as the use of MCOT after hospital discharge87. Furthermore, the HRS recommended the replacement of in-person clinic visits and CIED checks with telehealth consultations whenever feasible. These approaches are not yet recommended as an ‘emergency response’ system for scenarios such as impending sudden cardiac arrest.
New real-time systems might lay the foundation for real-time data transmission and response that are coordinated with emergency medical services and bystanders88. Early proof-of-concept systems have shown success in rapidly alerting bystanders and emergency medical providers to expedite first response89. In Europe, community volunteers can rapidly deliver automated external defibrillator to people experiencing sudden cardiac arrest90. Possible future directions include the development of a wireless internet of things (in which multiple devices are connected in their own dedicated network) for real-time cardiovascular care delivery. An important consideration is that medical care systems are not required to be fully automatic, unlike self-driving cars. Optimal medical systems might require only conditional autonomy, in that input from medical professionals and patients should be considered, rather than complete autonomy61. Although this need for conditional autonomy reduces some technical challenges, conditional autonomy also introduces limitations such as the need for integration with contemporaneous medical systems and to allow practitioner oversight while retaining speed of response and accuracy.
Cardiovascular monitoring guidelines
A growing number of publications support the use of monitoring devices in cardiovascular diagnostics and decision-making, including those that integrate machine learning technology. This rapid expansion of the evidence base has coincided with increased FDA guidance supporting the use of wearable devices for health care. Table 3 summarizes clinical studies of mobile and wearable device platforms.
Current monitoring strategies
Detection of subclinical AF in patients with cryptogenic stroke
The 2019 AHA/ACC/HRS guidelines for the management of AF recommend ambulatory monitoring to screen patients for AF and, if this is inconclusive, a cardiac monitor should be implanted91. The CRYSTAL-AF trial92 showed that ECG monitoring with an insertable cardiac monitor was superior to conventional follow-up for detecting AF in patients after cryptogenic stroke. The EMBRACE trial93 extended these observations by showing that a high burden of premature atrial beats predicted AF in patients with cryptogenic stroke. The long recording duration of wearable ECG devices makes them desirable for detecting subclinical AF, although whether such information can influence therapeutic decisions to prevent stroke is yet to be shown. Future studies should thus compare the accuracy and cost-effectiveness of wearable devices with those of traditional monitors in patients at risk of stroke and after stroke.
Screening for sudden cardiac arrest
Individuals at risk of sudden cardiac death have a diverse spectrum of phenotypes. The 2017 AHA/ACC/HRS guidelines provided a class I indication for ambulatory monitoring in patients with palpitations, presyncope or syncope to undergo monitoring to detect potential ventricular arrhythmias85. A class IIA recommendation was indicated for patients with suspected long QT syndrome and to determine whether symptoms, including palpitations, presyncope or syncope, are caused by ventricular arrhythmias. Ambulatory ECG monitoring was also recommended for patients starting certain antiarrhythmic medications (including disopyramide, dofetilide, ibutilide, procainamide or sotalol) with or without risk factors for torsades de pointes85. The 2014 ESC guidelines on the diagnosis and management of hypertrophic cardiomyopathy recommended ambulatory ECG monitoring every 6–12 months in patients with hypertrophic cardiomyopathy with left atrial dilation of ≥45 mm or after septal reduction therapies94. The diversity of patient phenotypes in this group introduces challenges and might require non-uniform monitoring intensity between patient populations. The current lack of infrastructure to facilitate actions in response to data from wearable devices might limit their use in detecting life-threatening arrhythmias. However, professional society guidelines have provided recommendations on the use of wearable cardioverter–defibrillators to prevent sudden cardiac death95 and have called for increased transparency in monitoring data from CIEDs and consumer arrhythmia-monitoring devices96.
Arrhythmia screening in patients with syncope
The 2018 ESC guidelines for the diagnosis and management of syncope recommend ambulatory ECG monitoring in patients with recurrent and unexplained syncope97. Depending on the frequency of events and the clinical context, patients can be monitored with the use of implanted devices or external devices that send alerts to health-care providers. Devices that encompass multiple sensor streams, such as activity, pulse oximetry and haemodynamics, to track the temporal relationship between episodes of hypotension, posture and cardiac rhythm might provide pathophysiological insights in different populations and are currently under investigation6.
Monitoring for patients with non-arrhythmic conditions
The 2017 AHA guidelines and the 2017 ESC guidelines recommend ambulatory arrhythmia monitoring for various subgroups of patients with acute coronary syndromes, including those with left ventricular ejection fraction <40%, failed reperfusion and high risk of ventricular arrhythmia, and patients requiring β-blocker therapy adequacy assessment85,98. Similarly, a 2017 expert consensus statement from the International Society for Holter and Noninvasive Electrocardiology and the HRS provided a class I recommendation for ambulatory monitoring in patients with arrhythmic and non-arrhythmic conditions, including non-ischaemic cardiomyopathy99. Although these recommendations were largely instituted for arrhythmia detection, signals for recurrent ischaemia might also be derived from these data.
Fitness and health-tracking devices
In July 2016, the FDA issued guidance for general wellness devices such as activity trackers, smartwatches and other products intended to improve physical fitness, nutrition or other wellness goals99. Subsequently, in September 2019, the FDA issued new draft guidance for clinical support applications that provides diagnostic and treatment recommendations for physicians but not for patients76.
Emerging monitoring strategies
Screening of the general population for AF
In 2018, the US Preventive Services Task Force concluded that insufficient evidence is available to determine whether the benefits of AF screening outweigh the associated risks100. This conclusion was formed on the basis of the potential physical and psychological risks of unnecessary treatment (false positives) in asymptomatic individuals aged ≥65 years. Conversely, the 2016 ESC guidelines recommend screening for AF in individuals older than 65 years in order to consider anticoagulation101 on the basis of findings from the SAFE102 and STROKESTOP103 studies, in which AF screening of asymptomatic individuals aged ≥65 years and ≥75 years, respectively, was shown to be cost-effective. Investigators in the ongoing SCREEN-AF trial104 will randomly assign individuals aged ≥75 years to 2 weeks of ambulatory ECG monitoring with a home blood-pressure monitor that can automatically detect AF or to the standard of care, to assess the primary end point of AF detection.
The Apple Heart study7 enroled 419,297 participants in the USA over 8 months to ascertain whether a PPG-enabled device could detect AF in individuals without a known history of the disease. Inclusion criteria included absence of self-reported AF, atrial flutter or oral anticoagulation use in individuals with a compatible Apple smartphone and smartwatch. Overall, 2,161 participants (0.52%) were notified of irregular rhythms with this technology7. In a subset of 450 enrollees who wore and returned clinical gold-standard ECG patches containing data that could be analysed, AF (≥30 s) was present in 34% of all participants and in 35% of participants aged ≥65 years. The positive predictive value for simultaneous AF on ambulatory ECG patch monitoring was 84% (95% CI 76–92%). The HUAWEI Heart study105, conducted by the MAFA II investigators, assessed the use of a wristband or wristwatch with PPG technology to monitor pulse rhythm in 246,541 individuals. Of these individuals, 262 were notified as having suspected AF, including 227 who had AF confirmed by a gold-standard clinical device. Therefore, this wristwatch provided a positive predictive value of 91.6% (95% CI 91.5–91.8%) in the subset of individuals who also had clinical monitoring105. The proportion of individuals with positive test results in both studies reflects the expected pretest probability of AF in a wide and relatively healthy population, and can inform on the design of future screening trials and the best target populations for such a strategy.
Personalization of oral anticoagulation therapy
The 2019 AHA/ACC/HRS guidelines for the management of AF emphasize that anticoagulation should not be tailored by the detection of AF episodes, the precise onset of AF or the temporal patterns of AF91. Indeed, the IMPACT-AF trial106 showed that pill-in-the-pocket use of non-vitamin K oral anticoagulants on the basis of detected AF did not reduce bleeding or thromboembolic event rates compared with standard therapy in patients with an indication for oral anticoagulation. Furthermore, the REACT.COM study107 showed the feasibility of a targeted strategy of implantable cardiac monitor-guided intermittent administration of non-vitamin K oral anticoagulants on the basis of remote monitoring in low-risk AF populations. However, this strategy might be less effective in other patient populations, and the investigators did not assess treatment adherence among participants108.
In standard clinical practice, oral anticoagulation is indicated as soon as AF is detected in patients with a single CHA2DS2–VASc risk factor91. Emerging monitoring devices might facilitate the definition of a specific device-detected AF threshold that warrants the initiation of anticoagulation therapy. In the TRENDS study109, this AF threshold might be an AF duration as short as 5.5 h. By contrast, a substudy of the ASSERT trial suggested a threshold duration of subclinical AF of ≥24 h (ref.110). Ongoing clinical trials are testing the use of oral anticoagulants for several proposed thresholds of AF duration. The ARTESiA trial111 is currently enrolling patients with AF of ≥6 min, and the NOAH trial112 is enrolling patients with an atrial high rate (≥170 bpm) of duration of ≥6 min. Both trials are enrolling patients with a CIED with an atrial lead and exclude individuals with a single AF episode longer than 24 h. Finally, the LOOP study113 is using the Reveal LINQ system to detect AF of ≥6 min, confirmed by at least two senior cardiologists. The results of these and other trials will help to define the device-detected AF threshold that warrants the initiation of anticoagulation therapy.
Conclusions
Cardiovascular monitoring is poised for dramatic technological advances through developments in novel biosignal definition and biosensor acquisition, automated diagnosis and expert-level triage, secure data transmission and patient-centric disease management. Numerous challenges remain in ensuring that data are owned and fully accessible by patients, but at the same time allowing relevant stakeholders to access data and enable timely disease management. Once data security and the other ethical and regulatory concerns associated with wearable technologies are addressed, this expanded monitoring paradigm has the potential to revolutionize the cardiovascular care of ambulatory patients.
References
Ong, M. K. et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition — heart failure (BEAT-HF) randomized clinical trial. JAMA Intern. Med. 176, 310–318 (2016).
Stehlik, J. et al. Continuous wearable monitoring analytics predict heart failure hospitalization. Circ. Heart Fail. 13, e006513 (2020).
Barbosa, R. S. et al. Defining the pattern of initiation of monomorphic ventricular tachycardia using the beat-to-beat intervals recorded on implantable cardioverter defibrillators from the RAFT study: a computer-based algorithm. J. Electrocardiol. 51, 470–474 (2018).
Charitos, E. I., Pürerfellner, H., Glotzer, T. V. & Ziegler, P. D. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J. Am. Coll. Cardiol. 63, 2840–2848 (2014).
Andrade, J. G. et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 140, 1779–1788 (2019).
Nam, Y., Kong, Y., Reyes, B., Reljin, N. & Chon, K. H. Monitoring of heart and breathing rates using dual cameras on a smartphone. PLoS ONE 11, e0151013 (2016).
Perez, M. V. et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N. Engl. J. Med. 381, 1909–1917 (2019).
Berglund, E. et al. A smartphone application for dispatch of lay responders to out-of-hospital cardiac arrests. Resuscitation 126, 160–165 (2018).
Khandwalla, R. M. et al. Predicting heart failure events with home monitoring: use of a novel, wearable necklace to measure stroke volume, cardiac output and thoracic impedance. J. Am. Coll. Cardiol. 67, 1296 (2016).
Shao, D., Tsow, F., Liu, C., Yang, Y. & Tao, N. Simultaneous monitoring of ballistocardiogram and photoplethysmogram using a camera. IEEE Trans. Biomed. Eng. 64, 1003–1010 (2017).
Kremers, M. S. et al. The national ICD registry report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 10, e59–e65 (2013).
Kurt, M. et al. Avoiding inappropriate therapy of single-lead implantable cardioverter-defibrillator by using atrial-sensing electrodes. J. Cardiovasc. Electrophysiol. 29, 1682–1689 (2018).
Morgan, J. M. et al. Remote management of heart failure using implantable electronic devices. Eur. Heart J. 38, 2352–2360 (2017).
Kleiger, R. E., Stein, P. K. & Bigger, J. T. Jr. Heart rate variability: measurement and clinical utility. Ann. Noninvasive Electrocardiol. 10, 88–101 (2005).
Yu, C. M. et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation 112, 841–848 (2005).
Bourge, R. C. et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure. J. Am. Coll. Cardiol. 51, 1073 (2008).
Desai, A. S. et al. Ambulatory hemodynamic monitoring reduces heart failure hospitalizations in “Real-World” clinical practice. J. Am. Coll. Cardiol. 69, 2357–2365 (2017).
Halawa, A., Enezate, T. & Flaker, G. Device monitoring in heart failure management: outcomes based on a systematic review and meta-analysis. Cardiovasc. Diagn. Ther. 9, 386–393 (2019).
Ritzema, J. et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation 116, 2952–2959 (2007).
Costanzo, M. R. et al. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet 388, 974–982 (2016).
Bardy, G. H. et al. An entirely subcutaneous implantable cardioverter-defibrillator. N. Engl. J. Med. 363, 36–44 (2010).
Reddy, V. Y. et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation 129, 1466–1471 (2014).
Ouyang, H. et al. Symbiotic cardiac pacemaker. Nat. Commun. 10, 1821 (2019).
Joshi, A. K. et al. First experience with a mobile cardiac outpatient telemetry (MCOT) system for the diagnosis and management of cardiac arrhythmia. Am. J. Cardiol. 95, 878–881 (2005).
Rothman, S. A. et al. The diagnosis of cardiac arrhythmias: a prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J. Cardiovasc. Electrophysiol. 18, 241–247 (2007).
Chugh, S. S. et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation 129, 837–847 (2014).
Rho, R., Vossler, M., Blancher, S. & Poole, J. E. Comparison of 2 ambulatory patch ECG monitors: the benefit of the P-wave and signal clarity. Am. Heart J. 203, 109–117 (2018).
Chowdhury, M. E. H. et al. Wearable real-time heart attack detection and warning system to reduce road accidents. Sensors 19, 2780 (2019).
Varon, C. et al. A comparative study of ECG-derived respiration in ambulatory monitoring using the single-lead ECG. Sci. Rep. 10, 5704 (2020).
Mittal, S. et al. Real-world performance of an enhanced atrial fibrillation detection algorithm in an insertable cardiac monitor. Heart Rhythm. 13, 1624–1630 (2016).
Hindricks, G. et al. Performance of a new leadless implantable cardiac monitor in detecting and quantifying atrial fibrillation: results of the XPECT trial. Circ. Arrhythm. Electrophysiol. 3, 141–147 (2010).
Laske, T. G., Iaizzo, P. A. & Garshelis, D. L. Six years in the life of a mother bear - the longest continuous heart rate recordings from a free-ranging mammal. Sci. Rep. 7, 40732 (2017).
Hannun, A. Y. et al. Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat. Med. 25, 65–69 (2019).
Mackanic, D. G. et al. Decoupling of mechanical properties and ionic conductivity in supramolecular lithium ion conductors. Nat. Commun. 10, 5384 (2019).
Bumgarner, J. M. et al. Smartwatch algorithm for automated detection of atrial fibrillation. J. Am. Coll. Cardiol. 71, 2381–2388 (2018).
Turakhia, M. P. et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: the apple heart study. Am. Heart J. 207, 66–75 (2019).
Reisner, A., Shaltis, P. A., McCombie, D. & Asada, H. H. Utility of the photoplethysmogram in circulatory monitoring. Anesthesiology 108, 950–958 (2008).
Koshy, A. N. et al. Smart watches for heart rate assessment in atrial arrhythmias. Int. J. Cardiol. 266, 124–127 (2018).
Tison, G. H. et al. Passive detection of atrial fibrillation using a commercially available smartwatch. JAMA Cardiol. 3, 409–416 (2018).
Shcherbina, A. et al. Accuracy in wrist-worn, sensor-based measurements of heart rate and energy expenditure in a diverse cohort. J. Pers. Med. https://doi.org/10.3390/jpm7020003 (2017).
Dörr, M. et al. The WATCH AF trial: SmartWATCHes for Detection of Atrial Fibrillation. JACC Clin. Electrophysiol. 5, 199–208 (2019).
Pereira, T. et al. Photoplethysmography based atrial fibrillation detection: a review. NPJ Digit. Med. 3, 3 (2020).
Watanabe, N. et al. Development and validation of a novel cuff-less blood pressure monitoring device. JACC Basic. Transl Sci. 2, 631 (2017).
Xiao, H., Qasem, A., Butlin, M. & Avolio, A. Estimation of aortic systolic blood pressure from radial systolic and diastolic blood pressures alone using artificial neural networks. J. Hypertens. 35, 1577–1585 (2017).
Kollias, A. & Stergiou, G. S. Automated measurement of office, home and ambulatory blood pressure in atrial fibrillation. Clin. Exp. Pharmacol. Physiol. 41, 9–15 (2014).
Wang, W., Brinker, A. C. D., Stuijk, S. & Haan, G. D. Algorithmic principles of remote PPG. IEEE Trans. Biomed. Eng. 64, 1479–1491 (2017).
Yan, B. P. et al. High-throughput, contact-free detection of atrial fibrillation from video with deep learning. JAMA Cardiol. 5, 105–107 (2019).
Turakhia, M. P. Diagnosing with a camera from a distance-proceed cautiously and responsibly. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2019.4572 (2019).
Aamodt, I. T. et al. Self-care monitoring of heart failure symptoms and lung impedance at home following hospital discharge: longitudinal study. J. Med. Internet Res. 22, e15445 (2020).
Lindholm, D., Fukaya, E., Leeper, N. J. & Ingelsson, E. Bioimpedance and new-onset heart failure: a longitudinal study of >500 000 individuals from the general population. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.118.008970 (2018).
Zhang, S. & Rajamani, R. Sensors on instrumented socks for detection of lower leg edema-an in vitro study. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 3153–3156 (2015).
Gupta, P. et al. Precision wearable accelerometer contact microphones for longitudinal monitoring of mechano-acoustic cardiopulmonary signals. NPJ Digit. Med. 3, 19 (2020).
Cao, M. et al. Ambulatory monitoring of heart sounds via an implanted device is superior to auscultation for prediction of heart failure events. J. Card. Fail. 26, 151–159 (2020).
Schalit, I. et al. Accelerometer detects pump thrombosis and thromboembolic events in an in vitro HVAD circuit. ASAIO J. 64, 601–609 (2018).
Díaz, D. H., Óscar, C. & Pallas-Areny, R. Heart rate detection from single-foot plantar bioimpedance measurements in a weighing scale. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2010, 6489–6492 (2010).
GlobalData Healthcare. FDA approves Eko cardiology technology advances. Verdict Media https://www.medicaldevice-network.com/comment/fda-eko-cardiology/ (2020).
Modave, F. et al. Mobile device accuracy for step counting across age groups. JMIR Mhealth Uhealth 5, e88 (2017).
Brooke, S. M. et al. Concurrent validity of wearable activity trackers under free-living conditions. J. Strength. Cond. Res. 31, 1097–1106 (2017).
Choi, A. & Shin, H. Photoplethysmography sampling frequency: pilot assessment of how low can we go to analyze pulse rate variability with reliability? Physiol. Meas. 38, 586–600 (2017).
Krittanawong, C. et al. Deep learning for cardiovascular medicine: a practical primer. Eur. Heart J. 40, 2058–2073 (2019).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56 (2019).
Beecy, A. N. et al. A novel deep learning approach for automated diagnosis of acute ischemic infarction on computed tomography. JACC Cardiovasc. Imaging 11, 1723 (2018).
Alhusseini M. et al. Classifying and interpreting disorganized electrical patterns within the fibrillating human heart using machine learning. Circ. Arrhythmia Electrophysiol. 13, e008160 (2020).
Anand, I. S. et al. Design and performance of a multisensor heart failure monitoring algorithm: results from the multisensor monitoring in congestive heart failure (MUSIC) study. J. Card. Fail. 18, 289–295 (2012).
100Plus. 100Plus Emergency Watch. 100Plus https://www.100plus.com/emergency-watch/ (2020).
Nguyen, M. T., Nguyen, B. V. & Kim, K. Deep feature learning for sudden cardiac arrest detection in automated external defibrillators. Sci. Rep. 8, 17196 (2018).
Lee, H., Shin, S.-Y., Seo, M., Nam, G.-B. & Joo, S. Prediction of ventricular tachycardia one hour before occurrence using artificial neural networks. Sci. Rep. 6, 32390 (2016).
Ong, M. E. et al. Prediction of cardiac arrest in critically ill patients presenting to the emergency department using a machine learning score incorporating heart rate variability compared with the modified early warning score. Crit. Care 16, R108 (2012).
Sweatt, A. J. et al. Discovery of distinct immune phenotypes using machine learning in pulmonary arterial hypertension. Circ. Res. 124, 904–919 (2019).
Segar, M. W. et al. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning-based unsupervised cluster analysis. Eur. J. Heart Fail. 22, 148–158 (2020).
Ahmad, T. et al. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.117.008081 (2018).
Calkins, H. et al. Harmonized outcome measures for use in atrial fibrillation patient registries and clinical practice: endorsed by the Heart Rhythm Society Board of Trustees. Heart Rhythm. 16, e3–e16 (2019).
Wineinger, N. E. et al. Identification of paroxysmal atrial fibrillation subtypes in over 13,000 individuals. Heart Rhythm. 16, 26–30 (2019).
Han, L. et al. Atrial fibrillation burden signature and near-term prediction of stroke: a machine learning analysis. Circ. Cardiovasc. Interv. 12, e005595 (2019).
Marrouche, N. F., Kheirkhahan, M. & Brachmann, J. Huff and puff, this CASTLE is made of bricks. Circulation 138, 754–755 (2018).
The US Food and Drug Administration. US FDA artificial intelligence and machine learning discussion paper, https://www.fda.gov/files/medical%20devices/published/US-FDA-Artificial-Intelligence-and-Machine-Learning-Discussion-Paper.pdf (2019).
Narayan, S. M., Wang, P. J. & Daubert, J. P. New concepts in sudden cardiac arrest to address an intractable epidemic: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 70–88 (2019).
Goldberger Ary, L. et al. PhysioBank, PhysioToolkit, and PhysioNet. Circulation 101, e215–e220 (2000).
Kostkova, P. et al. Who owns the data? Open data for healthcare. Front. Public Health 4, 7 (2016).
Ledford, H. Google health-data scandal spooks researchers. Nature https://doi.org/10.1038/d41586-019-03574-5 (2019).
Davis, J. 11.9M quest diagnostics patients impacted by AMCA data breach. HealthITSecurity https://healthitsecurity.com/news/11.9m-quest-diagnostics-patients-impacted-by-amca-data-breach (2019).
King, R. Devicemaker data breach exposes 277K patients’ information. Modern Healthcare https://www.modernhealthcare.com/technology/devicemaker-data-breach-exposes-277k-patients-information (2019).
Krittanawong, C. et al. Integrating blockchain technology with artificial intelligence for cardiovascular medicine. Nat. Rev. Cardiol. 17, 1–3 (2020).
US Department of Health and Human Services. Health information privacy beyond HIPAA: a 2018 environmental scan of major trends and challenges, https://ncvhs.hhs.gov/wp-content/uploads/2018/05/NCVHS-Beyond-HIPAA_Report-Final-02-08-18.pdf (2017).
Al-Khatib, S. M. et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 15, e190–e252 (2018).
Chang, D. et al. Inpatient use of ambulatory telemetry monitors for COVID-19 patients treated with hydroxychloroquine and/or azithromycin. J. Am. Coll. Cardiol. 75, 2992 (2020).
Lakkireddy, D. R. et al. Guidance for cardiac electrophysiology during the COVID-19 pandemic from the Heart Rhythm Society COVID-19 task force; electrophysiology section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm https://doi.org/10.1016/j.hrthm.2020.03.028 (2020).
Brooks, S. C., Simmons, G., Worthington, H., Bobrow, B. J. & Morrison, L. J. The PulsePoint Respond mobile device application to crowdsource basic life support for patients with out-of-hospital cardiac arrest: challenges for optimal implementation. Resuscitation 98, 20–26 (2016).
Ringh, M. et al. The challenges and possibilities of public access defibrillation. J. Intern. Med. 283, 238–256 (2018).
Capucci, A. et al. Community-based automated external defibrillator only resuscitation for out-of-hospital cardiac arrest patients. Am. Heart J. 172, 192–200 (2016).
January Craig, T. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 140, e125–e151 (2019).
Sanna, T. et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 370, 2478–2486 (2014).
Gladstone, D. J. et al. Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 370, 2467–2477 (2014).
Authors/Task Force Members. et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 35, 2733–2779 (2014).
Piccini Jonathan, P. et al. Wearable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. Circulation 133, 1715–1727 (2016).
Slotwiner, D. J. et al. Transparent sharing of digital health data: a call to action. Heart Rhythm. 16, e95–e106 (2019).
Brignole, M. et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 39, 1883–1948 (2018).
Ibanez, B. et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the Management of Acute Myocardial Infarction in Patients Presenting with ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39, 119–177 (2018).
Steinberg, J. S. et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. 14, e55–e96 (2017).
Curry, S. J. Screening for atrial fibrillation with electrocardiography: US Preventive Services Task Force recommendation statement. JAMA 320, 478–484 (2018).
Kirchhof, P. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. heart J. 37, 2893–2962 (2016).
Hobbs, F. D. et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol. Assess. 9, 1–74 (2005).
Svennberg, E. et al. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 131, 2176–2184 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02392754 (2020).
Guo, Y. et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J. Am. Coll. Cardiol. 74, 2365–2375 (2019).
Vinereanu, D. et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet 390, 1737–1746 (2017).
Passman, R. et al. Targeted anticoagulation for atrial fibrillation guided by continuous rhythm assessment with an insertable cardiac monitor: the Rhythm Evaluation for Anticoagulation with Continuous Monitoring (REACT.COM) pilot study. J. Cardiovasc. Electrophysiol. 27, 264–270 (2016).
Turakhia, M. P. & Estes, N. A. M. III Stroke risk stratification in atrial fibrillation: bridging the evidence gaps. J. Cardiovasc. Electrophysiol. 27, 271–273 (2016).
Glotzer Taya, V. et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk. Circ. Arrhythm. Electrophysiol. 2, 474–480 (2009).
Van Gelder, I. C. et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur. Heart J. 38, 1339–1344 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01938248 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02618577 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02036450 (2020).
Vetrovsky, T. et al. Effect of a 6-month pedometer-based walking intervention on functional capacity in patients with chronic heart failure with reduced (HFrEF) and with preserved (HFpEF) ejection fraction: study protocol for two multicenter randomized controlled trials. J. Transl Med. 15, 153 (2017).
Redfield, M. M. et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N. Engl. J. Med. 373, 2314–2324 (2015).
Brasier, N. et al. Detection of atrial fibrillation with a smartphone camera: first prospective, international, two-centre, clinical validation study (DETECT AF PRO). Europace 21, 41–47 (2019).
Gliner, V., Behar, J. & Yaniv, Y. Novel method to efficiently create an mHealth App: implementation of a real-time electrocardiogram R peak detector. JMIR Mhealth Uhealth 6, e118 (2018).
Jaakkola, J. et al. Mobile phone detection of atrial fibrillation with mechanocardiography. Circulation 137, 1524–1527 (2018).
Steinhubl, S. R. et al. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 320, 146–155 (2018).
Lown, M. et al. Screening for atrial fibrillation using economical and accurate technology (from the SAFETY study). Am. J. Cardiol. 122, 1339–1344 (2018).
Goldenthal, I. L. et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: iHEART results. J. Cardiovasc. Electrophysiol. 30, 2220–2228 (2019).
Guo, Y. et al. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF App Trial. Am. J. Med. 130, 1388–1396.e1386 (2017).
Ghanbari, H. et al. Feasibility and usability of a mobile application to assess symptoms and affect in patients with atrial fibrillation: a pilot study. J. Atr. Fibrillation 10, 1672 (2017).
Benezet-Mazuecos, J., García-Talavera, C. S. & Rubio, J. M. Smart devices for a smart detection of atrial fibrillation. J. Thorac. Dis. 10, S3824–S3827 (2018).
Halcox, J. P. J. et al. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 136, 1784–1794 (2017).
Soni, A. et al. Age-and-sex stratified prevalence of atrial fibrillation in rural western India: results of SMART-India, a population-based screening study. Int. J. Cardiol. 280, 84–88 (2019).
Chan, P. H. et al. Head-to-head comparison of the AliveCor heart monitor and Microlife WatchBP office AFIB for atrial fibrillation screening in a primary care setting. Circulation 135, 110–112 (2017).
Kroll, R. R., Boyd, J. G. & Maslove, D. M. Accuracy of a wrist-worn wearable device for monitoring heart rates in hospital inpatients: a prospective observational study. J. Med. Internet Res. 18, e253 (2016).
Lowres, N. et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb. Haemost. 111, 1167–1176 (2014).
de Asmundis, C. et al. Comparison of the patient-activated event recording system vs. traditional 24 h Holter electrocardiography in individuals with paroxysmal palpitations or dizziness. Europace 16, 1231–1235 (2014).
Kearley, K. et al. Triage tests for identifying atrial fibrillation in primary care: a diagnostic accuracy study comparing single-lead ECG and modified BP monitors. BMJ Open 4, e004565 (2014).
Wiesel, J., Arbesfeld, B. & Schechter, D. Comparison of the Microlife blood pressure monitor with the Omron blood pressure monitor for detecting atrial fibrillation. Am. J. Cardiol. 114, 1046–1048 (2014).
Lau, J. K. et al. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int. J. Cardiol. 165, 193–194 (2013).
Kaleschke, G. et al. Prospective, multicentre validation of a simple, patient-operated electrocardiographic system for the detection of arrhythmias and electrocardiographic changes. Europace 11, 1362–1368 (2009).
Doliwa, P. S., Frykman, V. & Rosenqvist, M. Short-term ECG for out of hospital detection of silent atrial fibrillation episodes. Scand. Cardiovasc. J. 43, 163–168 (2009).
Wiesel, J., Fitzig, L., Herschman, Y. & Messineo, F. C. Detection of atrial fibrillation using a modified microlife blood pressure monitor. Am. J. Hypertens. 22, 848–852 (2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02918175 (2020).
Etemadi, M. & Inan, O. T. Wearable ballistocardiogram and seismocardiogram systems for health and performance. J. Appl. Physiol. 124, 452–461 (2018).
Acknowledgements
S.M.N. has received funding from the NIH (R01 HL149134 and R01 HL83359).
Author information
Authors and Affiliations
Contributions
C.K., A.J.R., K.W.J. and S.M.N. researched data for the article. C.K., A.J.R., K.W.J., J.L.H. and S.M.N. wrote the manuscript. C.K. A.J.R., K.W.J., Z.W., J.L.H and S.M.N. reviewed and edited the manuscript before submission, and all authors contributed to the discussion of its content.
Corresponding authors
Ethics declarations
Competing interests
C.K. is on the ACC Solution Set Oversight Committee, on the advisory board of Lancet Digital Health and on the editorial board of EHJ Digital Health and Journal of the American Heart Association. K.W.J. has received a salary and equity from Tempus Labs and equity from Oova. M.P.T. has received research grants from AHA, Apple, Bayer, Bristol Myers Squibb, Bristol Myers Squibb–Pfizer Alliance and Janssen and Medtronic and consulting fees from Abbott, Biotronik, Cardiva Medical, Johnson & Johnson and Medtronic, has equity in AliveCor and is an editor for JAMA Cardiology. S.M.N. has received consulting fees from Abbott, Beyondlimits.ai and TDK Corporation, and has co-ownership of intellectual property owned by the Regents of the University of California and Stanford University. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks N. Varma, D. Miller and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Krittanawong, C., Rogers, A.J., Johnson, K.W. et al. Integration of novel monitoring devices with machine learning technology for scalable cardiovascular management. Nat Rev Cardiol 18, 75–91 (2021). https://doi.org/10.1038/s41569-020-00445-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-020-00445-9
This article is cited by
-
Motion artefact management for soft bioelectronics
Nature Reviews Bioengineering (2024)
-
Machine learning for the development of diagnostic models of decompensated heart failure or exacerbation of chronic obstructive pulmonary disease
Scientific Reports (2023)
-
Artificial intelligence-powered electronic skin
Nature Machine Intelligence (2023)
-
Recent successes in heart failure treatment
Nature Medicine (2023)
-
Use of mobile diagnostics and digital clinical trials in cardiology
Nature Medicine (2023)