Abstract
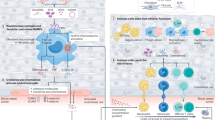
The observation that heart failure with reduced ejection fraction is associated with elevated circulating levels of pro-inflammatory cytokines opened a new area of research that has revealed a potentially important role for the immune system in the pathogenesis of heart failure. However, until the publication in 2019 of the CANTOS trial findings on heart failure outcomes, all attempts to target inflammation in the heart failure setting in phase III clinical trials resulted in neutral effects or worsening of clinical outcomes. This lack of positive results in turn prompted questions on whether inflammation is a cause or consequence of heart failure. This Review summarizes the latest developments in our understanding of the role of the innate and adaptive immune systems in the pathogenesis of heart failure, and highlights the results of phase III clinical trials of therapies targeting inflammatory processes in the heart failure setting, such as anti-inflammatory and immunomodulatory strategies. The most recent of these studies, the CANTOS trial, raises the exciting possibility that, in the foreseeable future, we might be able to identify those patients with heart failure who have a cardio-inflammatory phenotype and will thus benefit from therapies targeting inflammation.
Key points
-
Patients with heart failure with reduced ejection fraction have elevated circulating levels of pro-inflammatory cytokines compared with healthy individuals.
-
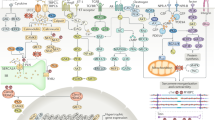
The innate and adaptive immune systems are activated in heart failure and comprise non-cellular and cellular components, including macrophages, mast cells, B cells and T cells.
-
The repertoire of immunological responses differs in acute and chronic myocardial inflammation.
-
Initial phase III clinical trials targeting inflammation in the setting of heart failure had neutral results.
-
In the CANTOS trial, targeted anti-cytokine therapy with a monoclonal antibody against IL-1β resulted in improved heart failure outcomes in patients with myocardial infarction with or without established heart failure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
12 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41569-021-00534-3
References
Levine, B., Kalman, J., Mayer, L., Fillit, H. M. & Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 223, 236–241 (1990).
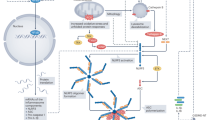
Mann, D. L. Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ. Res. 116, 1254–1268 (2015).
Frantz, S. et al. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur. J. Heart Fail. 20, 445–459 (2018).
Dick, S. A. & Epelman, S. Chronic heart failure and inflammation: what do we really know? Circ. Res. 119, 159–176 (2016).
Van Linthout, S. & Tschope, C. Inflammation – cause or consequence of heart failure or both? Curr. Heart Fail. Rep. 14, 251–265 (2017).
Everett, B. M. et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 139, 1289–1299 (2019).
Paulus, W. J. & Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
Abernethy, A. et al. Pro-inflammatory biomarkers in stable versus acutely decompensated heart failure with preserved ejection fraction. J. Am. Heart Assoc. 7, e007385 (2018).
Goonewardena, S. N. et al. Monocyte subsets and inflammatory cytokines in acute decompensated heart failure. J. Card. Fail. 22, 358–365 (2016).
Mann, D. L. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ. Res. 108, 1133–1145 (2011).
Mackey, D. & McFall, A. J. MAMPs and MIMPs: proposed classifications for inducers of innate immunity. Mol. Microbiol. 61, 1365–1371 (2006).
Toldo, S. & Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 15, 203–214 (2018).
Mann, D. L., Topkara, V. K., Evans, S. & Barger, P. M. Innate immunity in the adult mammalian heart: for whom the cell tolls. Trans. Am. Clin. Climatol. Assoc. 121, 34–50 (2010).
Prabhu, S. D. & Frangogiannis, N. G. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ. Res. 119, 91–112 (2016).
Swirski, F. K. & Nahrendorf, M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 18, 733–744 (2018).
Damas, J. K. et al. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc. Res. 47, 778–787 (2000).
Shahini, N. et al. The alternative complement pathway is dysregulated in patients with chronic heart failure. Sci. Rep. 7, 42532 (2017).
Bajpai, G. et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat. Med. 24, 1234–1245 (2018).
Noutsias, M., Pauschinger, M., Schultheiss, H. & Kühl, U. Phenotypic characterization of infiltrates in dilated cardiomyopathy - diagnostic significance of T-lymphocytes and macrophages in inflammatory cardiomyopathy. Med. Sci. Monit. 8, CR478–CR487 (2002).
Van Praet, L., Jacques, P., Van den Bosch, F. & Elewaut, D. The transition of acute to chronic bowel inflammation in spondyloarthritis. Nat. Rev. Rheumatol. 8, 288–295 (2012).
Torre-Amione, G. et al. Tumor necrosis factor-α and tumor necrosis factor receptors in the failing human heart. Circulation 93, 704–711 (1996).
Bartekova, M., Radosinska, J., Jelemensky, M. & Dhalla, N. S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 23, 733–758 (2018).
Parrillo, J. E. et al. A prospective randomized controlled trial of prednisone for dilated cardiomyopathy. N. Engl. J. Med. 321, 1061–1068 (1989).
Mann, D. L. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ. Res. 91, 988–998 (2002).
Bellinger, D. L. & Lorton, D. Autonomic regulation of cellular immune function. Auton. Neurosci. 182, 15–41 (2014).
Martelli, D., Yao, S. T., McKinley, M. J. & McAllen, R. M. Reflex control of inflammation by sympathetic nerves, not the vagus. J. Physiol. 592, 1677–1686 (2014).
Gullestad, L. et al. Effect of high- versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. J. Am. Coll. Cardiol. 34, 2061–2067 (1999).
Torre-Amione, G. et al. Decreased expression of tumor necrosis factor-α in failing human myocardium after mechanical circulatory support: a potential mechanism for cardiac recovery. Circulation 100, 1189–1193 (1999).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Toldo, S. et al. Interleukin-18 mediates Interleukin-1-induced cardiac dysfunction. Am. J. Physiol. Heart Circ. Physiol. 306, H1025–H1031 (2014).
Gulick, T. S., Chung, M. K., Pieper, S. J., Lange, L. G. & Schreiner, G. F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte β-adrenergic responsiveness. Proc. Natl Acad. Sci. USA 86, 6753–6757 (1989).
Yokoyama, T. et al. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J. Clin. Invest. 92, 2303–2312 (1993).
Yu, X., Kennedy, R. H. & Liu, S. J. JAK2/STAT3, not ERK1/2, mediates interleukin-6-induced activation of inducible nitric-oxide synthase and decrease in contractility of adult ventricular myocytes. J. Biol. Chem. 278, 16304–16309 (2003).
Bozkurt, B. et al. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote progressive left ventricular dysfunction and remodeling in rats. Circulation 97, 1382–1391 (1998).
Sivasubramanian, N. et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation 104, 826–831 (2001).
Hodgson, D. M. et al. Cellular remodeling in heart failure disrupts K(ATP) channel-dependent stress tolerance. EMBO J. 22, 1732–1742 (2003).
Zhang, W. et al. The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions. Circulation 124, 2106–2116 (2011).
Mezzaroma, E. et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc. Natl Acad. Sci. USA 108, 19725–19730 (2011).
Jarrah, A. A. & Tarzami, S. T. The duality of chemokines in heart failure. Expert. Rev. Clin. Immunol. 11, 523–536 (2015).
Aukrust, P., Damas, J. K., Gullestad, L. & Froland, S. S. Chemokines in myocardial failure – pathogenic importance and potential therapeutic targets. Clin. Exp. Immunol. 124, 343–345 (2001).
Aukrust, P. et al. Elevated circulating levels of C-C chemokines in patients with congestive heart failure. Circulation 97, 1136–1143 (1998).
Youker, K. A. et al. High proportion of patients with end-stage heart failure regardless of aetiology demonstrates anti-cardiac antibody deposition in failing myocardium: humoral activation, a potential contributor of disease progression. Eur. Heart J. 35, 1061–1068 (2014).
Aukrust, P. et al. Complement activation in patients with congestive heart failure: effect of high-dose intravenous immunoglobulin treatment. Circulation 104, 1494–1500 (2001).
Zhang, W. et al. Necrotic myocardial cells release damage-associated molecular patterns that provoke fibroblast activation in vitro and trigger myocardial inflammation and fibrosis in vivo. J. Am. Heart Assoc. 4, e001993 (2015).
Tzeng, H. P. et al. Negative inotropic effects of high-mobility group box 1 protein in isolated contracting cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol 294, H1490–H1496 (2008).
Hartupee, J. et al. Diagnostic accuracy of damage-associated molecular patterns (DAMPs) in patients with heart failure with a reduced ejection fraction. J. Clin. Transl. Sci. 1, 208–209 (2017).
Epelman, S. et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during Inflammation. Immunity 40, 91–104 (2014).
Lavine, K. J. et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. USA 111, 16029–16034 (2014).
Lavine, K. J. et al. The macrophage in cardiac homeostasis and disease: JACC Macrophage in CVD Series (Part 4). J. Am. Coll. Cardiol. 72, 2213–2230 (2018).
Dick, S. A., Zaman, R. & Epelman, S. Using high-dimensional approaches to probe monocytes and macrophages in cardiovascular disease. Front. Immunol. 10, 2146 (2019).
Molawi, K. et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 211, 2151–2158 (2014).
Sager, H. B. et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ. Res. 119, 853–864 (2016).
Bajpai, G. et al. Tissue resident CCR2− and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ. Res. 124, 263–278 (2019).
Li, W. et al. Heart-resident CCR2+ macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling. JCI Insight 1, e87315 (2016).
Nahrendorf, M. Myeloid cell contributions to cardiovascular health and disease. Nat. Med. 24, 711–720 (2018).
Ismahil, M. A. et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ. Res. 114, 266–282 (2014).
Dick, S. A. et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 20, 29–39 (2019).
Tang, W. H. W., Li, D. Y. & Hazen, S. L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol. 16, 137–154 (2019).
Oikonomou, E. K. & Antoniades, C. The role of adipose tissue in cardiovascular health and disease. Nat. Rev. Cardiol. 16, 83–99 (2019).
Liao, X. et al. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc. Natl Acad. Sci. USA 115, E4661–E4669 (2018).
Patel, B. et al. CCR2+ monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic. Transl. Sci. 3, 230–244 (2018).
Xia, Y. et al. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem. Cell Biol. 131, 471–481 (2009).
Nevers, T. et al. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ. Heart Fail. 8, 776–787 (2015).
Patel, B., Ismahil, M. A., Hamid, T., Bansal, S. S. & Prabhu, S. D. Mononuclear phagocytes are dispensable for cardiac remodeling in established pressure-overload heart failure. PLOS ONE 12, e0170781 (2017).
Weisheit, C. et al. Ly6C(low) and not Ly6C(high) macrophages accumulate first in the heart in a model of murine pressure-overload. PLOS ONE 9, e112710 (2014).
Lynch, T. L.t. et al. Cardiac inflammation in genetic dilated cardiomyopathy caused by MYBPC3 mutation. J. Mol. Cell Cardiol. 102, 83–93 (2017).
Tang, Q. & Bluestone, J. A. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9, 239–244 (2008).
Abbate, A. et al. Widespread myocardial inflammation and infarct-related artery patency. Circulation 110, 46–50 (2004).
Yndestad, A. et al. Enhanced expression of inflammatory cytokines and activation markers in T-cells from patients with chronic heart failure. Cardiovasc. Res. 60, 141–146 (2003).
Bansal, S. S. et al. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ. Heart Fail. 10, e003688 (2017).
Bansal, S. S. et al. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation 139, 206–221 (2019).
Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532 (2008).
Andersson, B., Aberg, J., Lindelow, B., Tang, M. S. & Wikstrand, J. Dose-related effects of metoprolol on heart rate and pharmacokinetics in heart failure. J. Card. Fail. 7, 311–317 (2001).
Kallikourdis, M. et al. T cell costimulation blockade blunts pressure overload-induced heart failure. Nat. Commun. 8, 14680 (2017).
Laroumanie, F. et al. CD4+ T cells promote the transition from hypertrophy to heart failure during chronic pressure overload. Circulation 129, 2111–2124 (2014).
Nevers, T. et al. Th1 effector T cells selectively orchestrate cardiac fibrosis in nonischemic heart failure. J. Exp. Med. 214, 3311–3329 (2017).
Ngwenyama, N. et al. CXCR3 regulates CD4+ T cell cardiotropism in pressure overload-induced cardiac dysfunction. JCI Insight 4, e125527 (2019).
Kantor, A. B. & Herzenberg, L. A. Origin of murine B cell lineages. Annu. Rev. Immunol. 11, 501–538 (1993).
LeBien, T. W. & Tedder, T. F. B lymphocytes: how they develop and function. Blood 112, 1570–1580 (2008).
Adamo, L. et al. Modulation of subsets of cardiac B lymphocytes improves cardiac function after acute injury. JCI Insight 3, e120137 (2018).
Bonner, F., Borg, N., Burghoff, S. & Schrader, J. Resident cardiac immune cells and expression of the ectonucleotidase enzymes CD39 and CD73 after ischemic injury. PLOS ONE 7, e34730 (2012).
Yan, X. et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell Cardiol. 62, 24–35 (2013).
Zouggari, Y. et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat. Med. 19, 1273–1280 (2013).
Horckmans, M. et al. Pericardial adipose tissue regulates granulopoiesis, fibrosis and cardiac function after myocardial infarction. Circulation 137, 948–960 (2017).
Cordero-Reyes, A. M. et al. Full expression of cardiomyopathy is partly dependent on B-cells: a pathway that involves cytokine activation, immunoglobulin deposition, and activation of apoptosis. J. Am. Heart Assoc. 5, e002484 (2016).
Zhang, M. et al. The role of natural IgM in myocardial ischemia-reperfusion injury. J. Mol. Cell Cardiol. 41, 62–67 (2006).
Krijnen, P. A. et al. IgM colocalises with complement and C reactive protein in infarcted human myocardium. J. Clin. Pathol. 58, 382–388 (2005).
Kaya, Z., Leib, C. & Katus, H. A. Autoantibodies in heart failure and cardiac dysfunction. Circ. Res. 110, 145–158 (2012).
Lazzerini, P. E., Capecchi, P. L., Laghi-Pasini, F. & Boutjdir, M. Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias. Nat. Rev. Cardiol. 14, 521–535 (2017).
Jahns, R. et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J. Clin. Invest. 113, 1419–1429 (2004).
Tschope, C. et al. Targeting CD20+ B-lymphocytes in inflammatory dilated cardiomyopathy with rituximab improves clinical course: a case series. Eur. Heart J. Case Rep. 3, ytz131 (2019).
Vazquez, M. I., Catalan-Dibene, J. & Zlotnik, A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 74, 318–326 (2015).
Mukai, K., Tsai, M., Saito, H. & Galli, S. J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 282, 121–150 (2018).
Frangogiannis, N. G., Burns, A. R., Michael, L. H. & Entman, M. L. Histochemical and morphological characteristics of canine cardiac mast cells. Histochem. J. 31, 221–229 (1999).
Ingason, A. B., Mechmet, F., Atacho, D. A. M., Steingrímsson, E. & Petersen, P. H. Distribution of mast cells within the mouse heart and its dependency on Mitf. Mol. Immunol. 105, 9–15 (2019).
Stewart, J. A. Jr. et al. Cardiac mast cell- and chymase-mediated matrix metalloproteinase activity and left ventricular remodeling in mitral regurgitation in the dog. J. Mol. Cell Cardiol. 35, 311–319 (2003).
Olivetti, G. et al. Long-term pressure-induced cardiac hypertrophy: capillary and mast cell proliferation. Am. J. Physiol. 257, H1766–H1772 (1989).
Panizo, A. et al. Are mast cells involved in hypertensive heart disease? J. Hypertens. 13, 1201–1208 (1995).
Levick, S. P. et al. Cardiac mast cells mediate left ventricular fibrosis in the hypertensive rat heart. Hypertension 53, 1041–1047 (2009).
Engels, W., Reiters, P. H., Daemen, M. J., Smits, J. F. & van der Vusse, G. J. Transmural changes in mast cell density in rat heart after infarct induction in vivo. J. Pathol. 177, 423–429 (1995).
Levick, S. P. et al. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc. Res. 89, 12–19 (2011).
Janicki, J. S., Brower, G. L. & Levick, S. P. The emerging prominence of the cardiac mast cell as a potent mediator of adverse myocardial remodeling. Methods Mol. Biol. 1220, 121–139 (2015).
Brower, G. L. & Janicki, J. S. Pharmacologic inhibition of mast cell degranulation prevents left ventricular remodeling induced by chronic volume overload in rats. J. Card. Fail. 11, 548–556 (2005).
Hara, M. et al. Evidence for a role of mast cells in the evolution to congestive heart failure. J. Exp. Med. 195, 375–381 (2002).
Ayach, B. B. et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc. Natl Acad. Sci. USA 103, 2304–2309 (2006).
Reber, L. L., Marichal, T. & Galli, S. J. New models for analyzing mast cell functions in vivo. Trends Immunol. 33, 613–625 (2012).
Sperr, W. R. et al. The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood 84, 3876–3884 (1994).
Patella, V. et al. Human heart mast cells. Isolation, purification, ultrastructure, and immunologic characterization. J. Immunol. 154, 2855–2865 (1995).
Akgul, A. et al. Role of mast cells and their mediators in failing myocardium under mechanical ventricular support. J. Heart Lung Transpl. 23, 709–715 (2004).
Miyazaki, M., Takai, S., Jin, D. & Muramatsu, M. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol. Ther. 112, 668–676 (2006).
Abel, A. M., Yang, C., Thakar, M. S. & Malarkannan, S. Natural killer cells: development, maturation, and clinical utilization. Front. Immunol. 9, 1869 (2018).
Vivier, E. et al. Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 (2011).
Malhotra, A. & Shanker, A. NK cells: immune cross-talk and therapeutic implications. Immunotherapy 3, 1143–1166 (2011).
Ong, S., Rose, N. R. & Čiháková, D. Natural killer cells in inflammatory heart disease. Clin. Immunol. 175, 26–33 (2017).
Fairweather, D., Kaya, Z., Shellam, G. R., Lawson, C. M. & Rose, N. R. From infection to autoimmunity. J. Autoimmun. 16, 175–186 (2001).
Ong, S. et al. Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration. Am. J. Pathol. 185, 847–861 (2015).
McNerney, M. E. et al. Role of natural killer cell subsets in cardiac allograft rejection. Am. J. Transpl. 6, 505–513 (2006).
Zhang, Z. X. et al. Natural killer cells play a critical role in cardiac allograft vasculopathy in an interleukin-6–dependent manner. Transplantation 98, 1029–1039 (2014).
Vredevoe, D. L. et al. Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure. Am. J. Cardiol. 93, 1007–1011 (2004).
Anderson, J., Carlquist, J. & Hammond, E. Deficient natural killer cell activity in patients with idiopathic dilated cardiomyopathy. Lancet 320, 1124–1127 (1982).
McTiernan, C. F. et al. Circulating T-cell subsets, monocytes, and natural killer cells in peripartum cardiomyopathy: results from the multicenter IPAC study. J. Card. Fail. 24, 33–42 (2018).
Hilgendorf, I. et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ. Res. 114, 1611–1622 (2014).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 (2011).
Courties, G. et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J. Am. Coll. Cardiol. 63, 1556–1566 (2014).
Wang, J. et al. Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction. Int. J. Nanomed. 13, 6441–6451 (2018).
Getts, D. R. et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl Med. 6, 219ra217 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03072199 (2019).
Kang, Y. S., Cha, J. J., Hyun, Y. Y. & Cha, D. R. Novel C-C chemokine receptor 2 antagonists in metabolic disease: a review of recent developments. Expert Opin. Investig. Drugs 20, 745–756 (2011).
Lim, S. Y., Yuzhalin, A. E., Gordon-Weeks, A. N. & Muschel, R. J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 7, 28697–28710 (2016).
Andrews, S. P. & Cox, R. J. Small molecule CXCR3 antagonists. J. Med. Chem. 59, 2894–2917 (2016).
Kuhn, C. & Weiner, H. L. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy 8, 889–906 (2016).
Konig, M., Rharbaoui, F., Aigner, S., Dalken, B. & Schuttrumpf, J. Tregalizumab - a monoclonal antibody to target regulatory T cells. Front. Immunol. 7, 11 (2016).
Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 1, 10–29 (2016).
Mann, D. L. et al. Targeted anti-cytokine therapy in patients with chronic heart failure: results of the randomized etancercept worldwide evaluation (RENEWAL). Circulation 109, 1594–1602 (2004).
Chung, E. S., Packer, M., Lo, K. H., Fasanmade, A. A. & Willerson, J. T. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the Anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation 107, 3133–3140 (2003).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Svensson, E. C. M. A. et al. ET2-driven clonal hematopoiesis predicts enhanced response to canakinumab in the CANTOS trial: an exploratory analysis [abstract]. Circulation 138 (Suppl. 1), A15111 (2018).
Kjekshus, J. et al. Rosuvastatin in older patients with systolic heart failure. N. Engl. J. Med. 357, 2248–2261 (2007).
Tavazzi, L. et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372, 1231–1239 (2008).
Tavazzi, L. et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372, 1223–1230 (2008).
Hare, J. M. et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J. Am. Coll. Cardiol. 51, 2301–2309 (2008).
Gullestad, L. et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation 103, 220–225 (2001).
Moreira, D. M., Vieira, J. L. & Gottschall, C. A. The effects of methotrexate therapy on the physical capacity of patients with ischemic heart failure: a randomized double-blind, placebo-controlled trial (METIS trial). J. Card. Fail. 15, 828–834 (2009).
Torre-Amione, G. et al. Results of a non-specific immunomodulation therapy in chronic heart failure (ACCLAIM trial): a placebo-controlled randomised trial. Lancet 371, 228–236 (2008).
Jahns, R., Schlipp, A., Boivin, V. & Lohse, M. J. Targeting receptor antibodies in immune cardiomyopathy. Semin. Thromb. Hemost. 36, 212–218 (2010).
Mann, D. L. Autoimmunity, immunoglobulin adsorption and dilated cardiomyopathy: has the time come for randomized clinical trials? J. Am. Coll. Cardiol. 38, 184–186 (2001).
Munch, G. et al. Administration of the cyclic peptide COR-1 in humans (phase I study): ex vivo measurements of anti-beta1-adrenergic receptor antibody neutralization and of immune parameters. Eur. J. Heart Fail. 14, 1230–1239 (2012).
Tardif, J.-C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Szulwach, K. E. et al. Single-cell genetic analysis using automated microfluidics to resolve somatic mosaicism. PLOS ONE 10, e0135007 (2015).
Taylor, D. A. et al. Identification of bone marrow cell subpopulations associated with improved functional outcomes in patients with chronic left ventricular dysfunction: an embedded cohort evaluation of the FOCUS-CCTRN trial. Cell Transpl. 25, 1675–1687 (2016).
Gullestad, L. et al. Inflammatory cytokines in heart failure: mediators and markers. Cardiology 122, 23–35 (2012).
Sliwa, K., Skudicky, D., Candy, G., Wisenbaugh, T. & Sareli, P. Randomized investigation of effects of pentoxifylline on left ventricular performance in idiopathic dilated cardiomyopathy. Lancet 351, 1091–1093 (1998).
Skudicky, D., Bergemann, A., Sliwa, K., Candy, G. & Sareli, P. Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensin-converting enzyme inhibitors and carvedilol: results of a randomized study. Circulation 103, 1083–1088 (2001).
Sliwa, K. et al. Effects of pentoxifylline on cytokine profiles and left ventricular performance in patients with decompensated congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am. J. Cardiol. 90, 1118–1122 (2002).
Sliwa, K. et al. Therapy of ischemic cardiomyopathy with the immunomodulating agent pentoxifylline: results of a randomized study. Circulation 109, 750–755 (2004).
McNamara, D. M. et al. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation 103, 2254–2259 (2001).
Gullestad, L. et al. Effect of thalidomide on cardiac remodeling in chronic heart failure: results of a double-blind, placebo-controlled study. Circulation 112, 3408–3414 (2005).
Deftereos, S. et al. Anti-inflammatory treatment with colchcine in stable heart failure: a prospective, randomized study. JACC Heart Fail. 2, 131–137 (2014).
Givertz, M. M. et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the EXACT-HF study. Circulation 131, 1763–1771 (2015).
Acknowledgements
We apologize in advance to colleagues whose work was not directly cited in this Review because of the imposed space limitations. The authors receive support research funds from the NIH (R01 HL58081, HL-73017, HL089543, NIH T32 HL007081 and K08 HL1945108).
Author information
Authors and Affiliations
Contributions
L.A., S.D.P., C.R.R. and D.L.M. researched data for article and wrote the manuscript. All authors provided substantial contribution to discussion of content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
L.A. and D.L.M. are co-founders of iCordis, a start-up company focused on developing B cell modulating therapies for the prevention and treatment of heart failure. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks P. Alcaide, C. Tschöpe and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adamo, L., Rocha-Resende, C., Prabhu, S.D. et al. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol 17, 269–285 (2020). https://doi.org/10.1038/s41569-019-0315-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-019-0315-x
This article is cited by
-
Type 1 diabetes, its complications, and non-ischemic cardiomyopathy: a mendelian randomization study of European ancestry
Cardiovascular Diabetology (2024)
-
Pharmacological properties of Polygonatum and its active ingredients for the prevention and treatment of cardiovascular diseases
Chinese Medicine (2024)
-
Reduced thoracic skeletal muscle size is associated with adverse outcomes in diabetes patients with heart failure and reduced ejection fraction: quantitative analysis of sarcopenia by using cardiac MRI
Cardiovascular Diabetology (2024)
-
Microbial-derived imidazole propionate links the heart failure-associated microbiome alterations to disease severity
Genome Medicine (2024)
-
Exploration of neuron heterogeneity in human heart failure with dilated cardiomyopathy through single-cell RNA sequencing analysis
BMC Cardiovascular Disorders (2024)