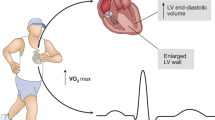
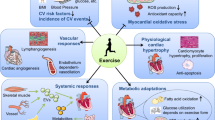
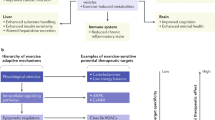
Abstract
Despite strong scientific evidence supporting the benefits of regular exercise for the prevention and management of cardiovascular disease (CVD), physical inactivity is highly prevalent worldwide. In addition to merely changing well-known risk factors for systemic CVD, regular exercise can also improve cardiovascular health through non-traditional mechanisms. Understanding the pathways through which exercise influences different physiological systems is important and might yield new therapeutic strategies to target pathophysiological mechanisms in CVD. This Review includes a critical discussion of how regular exercise can have antiatherogenic effects in the vasculature, improve autonomic balance (thereby reducing the risk of malignant arrhythmias), and induce cardioprotection against ischaemia–reperfusion injury, independent of effects on traditional CVD risk factors. This Review also describes how exercise promotes a healthy anti-inflammatory milieu (largely through the release of muscle-derived myokines), stimulates myocardial regeneration, and ameliorates age-related loss of muscle mass and strength, a frequently overlooked non-traditional CVD risk factor. Finally, we discuss how the benefits of exercise might also occur via promotion of a healthy gut microbiota. We argue, therefore, that a holistic view of all body systems is necessary and useful when analysing the role of exercise in cardiovascular health.
Key points
-
Regular exercise induces antiatherogenic adaptations in vascular function and structure, irrespective of traditional cardiovascular disease (CVD) risk factors.
-
Regular exercise training improves cardiac parasympathetic regulation, thereby conferring protection against malignant arrhythmias, and also provides cardioprotection against ischaemia–reperfusion injury.
-
Muscle-derived myokines are responsible for many of the beneficial effects of exercise, particularly by promoting a healthy anti-inflammatory milieu.
-
Exercise can improve myocardial regeneration capacity, in part through stimulation of circulating angiogenic cells.
-
Loss of muscle strength and mass is a forgotten hallmark of — and, in fact, a risk factor for — CVD that can be largely reversed with resistance (strength) training, including in elderly individuals.
-
Regular exercise can promote a healthy gut microbiota while protecting the permeability and function of the gut barrier.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Raichlen, D. A. et al. Physical activity patterns and biomarkers of cardiovascular disease risk in hunter-gatherers. Am. J. Hum. Biol. https://doi.org/10.1002/ajhb.22919 (2017).
Hallal, P. C. et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 380, 247–257 (2012). In this study, the authors examine in detail physical activity levels worldwide and highlight the worryingly high proportion (80.3% of total) of inactive adolescents (aged 13–15 years and doing less than the WHO-recommended minimum of 60 min daily of MVPA).
Santos-Lozano, A., Lucia, A., Ruilope, L. & Pitsiladis, Y. P. Born to run: our future depends on it. Lancet 390, 635–636 (2017).
Booth, F. W., Roberts, C. K., Thyfault, J. P., Ruegsegger, G. N. & Toedebusch, R. G. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol. Rev. 97, 1351–1402 (2017).
Gurven, M., Jaeggi, A. V., Kaplan, H. & Cummings, D. Physical activity and modernization among Bolivian Amerindians. PLoS ONE 8, e55679 (2013).
World Health Organization. Global Recommendations on Physical Activity for Health (WHO, 2010).This manuscript compiles the WHO Physical Activity for Health recommendations.
Kaplan, H. et al. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet 389, 1730–1739 (2017).
Lindeberg, S., Eliasson, M., Lindahl, B. & Ahren, B. Low serum insulin in traditional Pacific Islanders — the Kitava Study. Metabolism 48, 1216–1219 (1999).
Kohl, H. W. 3rd et al. The pandemic of physical inactivity: global action for public health. Lancet 380, 294–305 (2012).
Katzmarzyk, P. T., Lee, I. M., Martin, C. K. & Blair, S. N. Epidemiology of physical activity and exercise training in the United States. Prog. Cardiovasc. Dis. 60, 3–10 (2017).
Tremblay, M. S. et al. Sedentary Behavior Research Network (SBRN) — Terminology Consensus Project process and outcome. Int. J. Behav. Nutr. Phys. Act. 14, 75 (2017). Herein, the Sedentary Behavior Research Network presents a Terminology Consensus Project to standardize important definitions related to physical inactivity and activity or sedentary time.
Karlsen, T., Aamot, I. L., Haykowsky, M. & Rognmo, O. high intensity interval training for maximizing health outcomes. Prog. Cardiovasc. Dis. 60, 67–77 (2017).
Gillen, J. B. & Gibala, M. J. Is high-intensity interval training a time-efficient exercise strategy to improve health and fitness? Appl. Physiol. Nutr. Metab. 39, 409–412 (2014).
Warburton, D. E. & Bredin, S. S. Reflections on physical activity and health: what should we recommend? Can. J. Cardiol. 32, 495–504 (2016).
Carson, V. et al. Light-intensity physical activity and cardiometabolic biomarkers in US adolescents. PLoS ONE 8, e71417 (2013).
Zhao, G. et al. Leisure-time aerobic physical activity, muscle-strengthening activity and mortality risks among US adults: the NHANES linked mortality study. Br. J. Sports Med. 48, 244–249 (2014).
Ekelund, U. et al. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC). Am. J. Clin. Nutr. 101, 613–621 (2015).
Wen, C. P. et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 378, 1244–1253 (2011).
Lee, D. C. et al. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 64, 472–481 (2014). This study is a good example of the growing evidence that even low physical activity loads, below the WHO minimum recommendations, can also provide major benefits; notably, running 5–10 min per day at slow speeds ( < 6 mph) was associated with a considerably reduced risk of CVD death in US adults.
Lear, S. A. et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: the PURE study. Lancet 390, 2643–2654 (2017). This epidemiological study (n = 168,916 participants) found that higher recreational and non-recreational physical activity levels were associated with a lower risk of death and cardiovascular events, irrespective of country income.
Arem, H. et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose–response relationship. JAMA Intern. Med. 175, 959–967 (2015). This population-based, prospective analysis of US and European cohorts (total n = 661,137 men and women aged 21–98 years) shows that meeting the minimum international recommendations for MVPA is associated with virtually the maximum longevity benefit and that the benefit threshold was quite high at ~3–5 times the recommended minimum, and no excess risk was found at ≥10 times the minimum.
Ding, D. et al. The economic burden of physical inactivity: a global analysis of major non-communicable diseases. Lancet 388, 1311–1324 (2016).
Harber, M. P. et al. Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog. Cardiovasc. Dis. 60, 11–20 (2017).
Kodama, S. et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301, 2024–2035 (2009).
Kulinski, J. P. et al. Association between cardiorespiratory fitness and accelerometer-derived physical activity and sedentary time in the general population. Mayo Clin. Proc. 89, 1063–1071 (2014).
Hamilton, M. T., Hamilton, D. G. & Zderic, T. W. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56, 2655–2667 (2007).
Ekelund, U. et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 388, 1302–1310 (2016).
Fiuza-Luces, C., Garatachea, N., Berger, N. A. & Lucia, A. Exercise is the real polypill. Physiology 28, 330–358 (2013).
Green, D. J., Hopman, M. T., Padilla, J., Laughlin, M. H. & Thijssen, D. H. Vascular adaptation to exercise in humans: role of hemodynamic stimuli. Physiol. Rev. 97, 495–528 (2017).
Sahebkar, A. et al. Statin therapy reduces plasma endothelin-1 concentrations: a meta-analysis of 15 randomized controlled trials. Atherosclerosis 241, 433–442 (2015).
Ashor, A. W. et al. Exercise modalities and endothelial function: a systematic review and dose–response meta-analysis of randomized controlled trials. Sports Med. 45, 279–296 (2015).
Green, D. J., O’Driscoll, G., Joyner, M. J. & Cable, N. T. Exercise and cardiovascular risk reduction: time to update the rationale for exercise? J. Appl. Physiol. (1985) 105, 766–768 (2008).
Erkens, R. et al. Modulation of local and systemic heterocellular communication by mechanical forces: a role of endothelial nitric oxide synthase. Antioxid. Redox Signal. 26, 917–935 (2017).
Hambrecht, R. et al. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N. Engl. J. Med. 342, 454–460 (2000). In this study in patients with coronary endothelial dysfunction, a 4-week exercise intervention improves endothelium-dependent vasodilatation, both in epicardial coronary and resistance vessels.
Hambrecht, R. et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107, 3152–3158 (2003). This study shows that, in patients with stable coronary artery disease, a 4-week exercise training programme improves agonist-mediated endothelium-dependent vasodilatory capacity.
Durand, M. J. & Gutterman, D. D. Exercise and vascular function: how much is too much? Can. J. Physiol. Pharmacol. 92, 551–557 (2014).
Currens, J. H. & White, P. D. Half a century of running. Clinical, physiologic and autopsy findings in the case of Clarence DeMar (“Mr. Marathon”). N. Engl. J. Med. 265, 988–993 (1961).
Haskell, W. L. et al. Coronary artery size and dilating capacity in ultradistance runners. Circulation 87, 1076–1082 (1993).
Nguyen, P. K. et al. Physical activity in older subjects is associated with increased coronary vasodilation: the ADVANCE study. JACC Cardiovasc. Imag. 4, 622–629 (2011).
Zeppilli, P. et al. Echocardiographic size of conductance vessels in athletes and sedentary people. Int. J. Sports Med. 16, 38–44 (1995).
Thijssen, D. H., Cable, N. T. & Green, D. J. Impact of exercise training on arterial wall thickness in humans. Clin. Sci. 122, 311–322 (2012).
Shimada, K. et al. Atherosclerotic plaques induced by marble-burying behavior are stabilized by exercise training in experimental atherosclerosis. Int. J. Cardiol. 151, 284–289 (2011).
Yoshikawa, D. et al. Association of cardiorespiratory fitness with characteristics of coronary plaque: assessment using integrated backscatter intravascular ultrasound and optical coherence tomography. Int. J. Cardiol. 162, 123–128 (2013).
Madssen, E. et al. Coronary atheroma regression and plaque characteristics assessed by grayscale and radiofrequency intravascular ultrasound after aerobic exercise. Am. J. Cardiol. 114, 1504–1511 (2014).
Aengevaeren, V. L. et al. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation 136, 138–148 (2017).
Garatachea, N. et al. Elite athletes live longer than the general population: a meta-analysis. Mayo Clin. Proc. 89, 1195–1200 (2014).
Heaps, C. L., Robles, J. C., Sarin, V., Mattox, M. L. & Parker, J. L. Exercise training-induced adaptations in mediators of sustained endothelium-dependent coronary artery relaxation in a porcine model of ischemic heart disease. Microcirculation 21, 388–400 (2014).
Seals, D. R., Desouza, C. A., Donato, A. J. & Tanaka, H. Habitual exercise and arterial aging. J. Appl. Physiol. (1985) 105, 1323–1332 (2008).
Bhella, P. S. et al. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. J. Am. Coll. Cardiol. 64, 1257–1266 (2014).
Glinge, C., Sattler, S., Jabbari, R. & Tfelt-Hansen, J. Epidemiology and genetics of ventricular fibrillation during acute myocardial infarction. J. Geriatr. Cardiol. 13, 789–797 (2016).
Billman, G. E. et al. Exercise training-induced bradycardia: evidence for enhanced parasympathetic regulation without changes in intrinsic sinoatrial node function. J. Appl. Physiol. (1985) 118, 1344–1355 (2015). This study is an illustrative example of the relevant work performed over the years by G. E. Billman’s group in dog models, showing the anti-arrhythmogenic and salutary effects of moderate endurance exercise training through enhanced cardiac parasympathetic regulation.
Sessa, F. et al. Heart rate variability as predictive factor for sudden cardiac death. Aging 10, 166–177 (2018).
Hillebrand, S. et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace 15, 742–749 (2013).
Pearson, M. J. & Smart, N. A. Exercise therapy and autonomic function in heart failure patients: a systematic review and meta-analysis. Heart Fail. Rev. 23, 91–108 (2018). This meta-analysis shows that endurance exercise training improves parasympathetic tone and reduces sympathetic activity in patients with heart failure, which is in line with the findings of the aforementioned mechanistic studies in canine models by G. E. Billman’s group.
Villafaina, S., Collado-Mateo, D., Fuentes, J. P., Merellano-Navarro, E. & Gusi, N. Physical exercise improves heart rate variability in patients with type 2 diabetes: a systematic review. Curr. Diab. Rep. 17, 110 (2017).
Billman, G. E. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am. J. Physiol. Heart Circ. Physiol. 297, H1171–H1193 (2009).
Joyner, M. J. & Green, D. J. Exercise protects the cardiovascular system: effects beyond traditional risk factors. J. Physiol. 587, 5551–5558 (2009).
Deley, G., Picard, G. & Taylor, J. A. Arterial baroreflex control of cardiac vagal outflow in older individuals can be enhanced by aerobic exercise training. Hypertension 53, 826–832 (2009).
Mueller, P. J. Physical (in)activity-dependent alterations at the rostral ventrolateral medulla: influence on sympathetic nervous system regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R1468–R1474 (2010).
Bonilla, I. M. et al. Endurance exercise training normalizes repolarization and calcium-handling abnormalities, preventing ventricular fibrillation in a model of sudden cardiac death. J. Appl. Physiol. (1985) 113, 1772–1783 (2012). This preclinical study finds that exercise training could prevent ischaemia-induced ventricular fibrillation owing to a combination of beneficial effects on cellular electrophysiology and calcium handling.
Frasier, C. R., Moore, R. L. & Brown, D. A. Exercise-induced cardiac preconditioning: how exercise protects your achy-breaky heart. J. Appl. Physiol. (1985) 111, 905–915 (2011). This detailed review discusses how exercise training evokes sustainable protection against cardiac ischaemia–reperfusion injury through several mechanisms, such as improved oxidant buffering capacity, decreased cellular and/or mitochondrial calcium overload, and preservation of bioenergetics.
Thijssen, D. H. J., Redington, A., George, K. P., Hopman, M. T. E. & Jones, H. Association of exercise preconditioning with immediate cardioprotection: a review. JAMA Cardiol. 3, 169–176 (2017).
Demirel, H. A. et al. Short-term exercise improves myocardial tolerance to in vivo ischemia–reperfusion in the rat. J. Appl. Physiol. (1985) 91, 2205–2212 (2001).
Hamilton, K. L. et al. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am. J. Physiol. Heart Circ. Physiol. 281, H1346–H1352 (2001).
Lalonde, F., Poirier, P., Sylvestre, M. P., Arvisais, D. & Curnier, D. Exercise-induced ischemic preconditioning detected by sequential exercise stress tests: a meta-analysis. Eur. J. Prev. Cardiol. 22, 100–112 (2015).
Miller, L. E. et al. Involvement of the δ-opioid receptor in exercise-induced cardioprotection. Exp. Physiol. 100, 410–421 (2015).
Calvert, J. W. et al. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β3-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ. Res. 108, 1448–1458 (2011). This preclinical study shows that a 4-week exercise programme protects the heart against myocardial ischaemia–reperfusion injury, in part by stimulating β 3 -adrenergic receptors and increasing cardiac storage of NO metabolites.
Bowles, D. K., Farrar, R. P. & Starnes, J. W. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am. J. Physiol. 263, H804–H809 (1992).
Lennon, S. L. et al. Exercise and myocardial tolerance to ischaemia-reperfusion. Acta Physiol. Scand. 182, 161–169 (2004).
Pons, S. et al. Regular treadmill exercise restores cardioprotective signaling pathways in obese mice independently from improvement in associated co-morbidities. J. Mol. Cell. Cardiol. 54, 82–89 (2013).
Dregan, A., Charlton, J., Chowienczyk, P. & Gulliford, M. C. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population-based cohort study. Circulation 130, 837–844 (2014).
Kaptoge, S. et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur. Heart J. 35, 578–589 (2014).
Liu, C. et al. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine 86, 100–109 (2016).
Fuster, J. J., Ouchi, N., Gokce, N. & Walsh, K. Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ. Res. 118, 1786–1807 (2016).
Fedewa, M. V., Hathaway, E. D. & Ward-Ritacco, C. L. Effect of exercise training on C reactive protein: a systematic review and meta-analysis of randomised and non-randomised controlled trials. Br. J. Sports Med. 51, 670–676 (2017).
Hayashino, Y. et al. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metabolism 63, 431–440 (2014).
Niessner, A. et al. Endurance training reduces circulating inflammatory markers in persons at risk of coronary events: impact on plaque stabilization? Atherosclerosis 186, 160–165 (2006).
Chen, Y. L. et al. Correlation between serum interleukin-6 level and type 1 diabetes mellitus: a systematic review and meta-analysis. Cytokine 94, 14–20 (2017).
Zhang, B. et al. Correlative association of interleukin-6 with intima media thickness: a meta-analysis. Int. J. Clin. Exp. Med. 8, 4731–4743 (2015).
Li, H., Liu, W. & Xie, J. Circulating interleukin-6 levels and cardiovascular and all-cause mortality in the elderly population: a meta-analysis. Arch. Gerontol. Geriatr. 73, 257–262 (2017).
Fischer, C. P. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc. Immunol. Rev. 12, 6–33 (2006).
Ostrowski, K., Schjerling, P. & Pedersen, B. K. Physical activity and plasma interleukin-6 in humans — effect of intensity of exercise. Eur. J. Appl. Physiol. 83, 512–515 (2000).
Steensberg, A., Fischer, C. P., Keller, C., Moller, K. & Pedersen, B. K. IL-6 enhances plasma IL-1RA, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 285, E433–E437 (2003). This study is an example of work by B. K. Pedersen’s group representing a paradigm shift regarding the role of IL-6; when released from working muscles, this molecule can have anti-inflammatory rather than pro-inflammatory effects.
Starkie, R., Ostrowski, S. R., Jauffred, S., Febbraio, M. & Pedersen, B. K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-α production in humans. FASEB J. 17, 884–886 (2003).
Pedersen, B. K. & Febbraio, M. A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 88, 1379–1406 (2008).
Lambernd, S. et al. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia 55, 1128–1139 (2012).
Nielsen, A. R. et al. Association between interleukin-15 and obesity: interleukin-15 as a potential regulator of fat mass. J. Clin. Endocrinol. Metab. 93, 4486–4493 (2008).
Kim, H. J. et al. Effect of treadmill exercise on interleukin-15 expression and glucose tolerance in Zucker diabetic fatty rats. Diabetes Metab. J. 37, 358–364 (2013).
Busquets, S., Figueras, M., Almendro, V., Lopez-Soriano, F. J. & Argiles, J. M. Interleukin-15 increases glucose uptake in skeletal muscle. An antidiabetogenic effect of the cytokine. Biochim. Biophys. Acta 1760, 1613–1617 (2006).
Jedrychowski, M. P. et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 22, 734–740 (2015).
Raschke, S. et al. Evidence against a beneficial effect of irisin in humans. PLoS ONE 8, e73680 (2013).
Albrecht, E. et al. Irisin — a myth rather than an exercise-inducible myokine. Sci. Rep. 5, 8889 (2015).
Bostrom, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Emanuele, E. et al. Serum irisin levels, precocious myocardial infarction, and healthy exceptional longevity. Am. J. Med. 127, 888–890 (2014).
Rao, R. R. et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291 (2014).
Izumiya, Y. et al. FGF21 is an Akt-regulated myokine. FEBS Lett. 582, 3805–3810 (2008).
Lee, M. S. et al. Fibroblast growth factor-21 protects human skeletal muscle myotubes from palmitate-induced insulin resistance by inhibiting stress kinase and NF-κB. Metabolism 61, 1142–1151 (2012).
Lee, P. et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 19, 302–309 (2014).
Ouchi, N. et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 283, 32802–32811 (2008).
Jiang, M., Wan, F., Wang, F. & Wu, Q. Irisin relaxes mouse mesenteric arteries through endothelium-dependent and endothelium-independent mechanisms. Biochem. Biophys. Res. Commun. 468, 832–836 (2015).
Lu, J. et al. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-null diabetic mice. Atherosclerosis 243, 438–448 (2015).
Besse-Patin, A. et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int. J. Obes. 38, 707–713 (2014). This study shows that endurance exercise training upregulated the expression of apelin in the muscle of individuals with obesity, and the authors postulate that this molecule is a novel myokine.
Zhong, J. C. et al. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta 1863, 1942–1950 (2017).
Subbotina, E. et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl Acad. Sci. USA 112, 16042–16047 (2015).
Roberts, L. D. et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 19, 96–108 (2014).
Chin, S. O. et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLOS ONE 8, e60119 (2013).
Medina-Inojosa, J. R. et al. Association between adiposity and lean mass with long-term cardiovascular events in patients with coronary artery disease: no paradox. J. Am. Heart. Assoc. 7, e007505 (2018).
Shah, R. V. et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc. Imag. 7, 1221–1235 (2014).
Norheim, F. et al. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am. J. Physiol. Endocrinol. Metab. 301, E1013–E1021 (2011). In this study, the authors show that muscle strength training increases the levels of several secretory muscle proteins in humans, many of which are candidate myokines.
Sanchis-Gomar, F. & Lucia, A. Acute myocardial infarction: ‘telomerasing’ for cardioprotection. Trends Mol. Med. 21, 203–205 (2015).
Haykowsky, M. et al. A meta-analysis of the effects of exercise training on left ventricular remodeling following myocardial infarction: start early and go longer for greatest exercise benefits on remodeling. Trials 12, 92 (2011).
Cai, M. X. et al. Exercise training activates neuregulin 1/ErbB signaling and promotes cardiac repair in a rat myocardial infarction model. Life Sci. 149, 1–9 (2016).
Sanchis-Gomar, F., Perez-Quilis, C. & Lucia, A. Overexpressing FSTL1 for heart repair. Trends Mol. Med. 22, 353–354 (2016).
Bakogiannis, C. et al. Circulating endothelial progenitor cells as biomarkers for prediction of cardiovascular outcomes. Curr. Med. Chem. 19, 2597–2604 (2012).
Adams, V. et al. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler. Thromb. Vasc. Biol. 24, 684–690 (2004).
Artero, E. G. et al. Effects of muscular strength on cardiovascular risk factors and prognosis. J. Cardiopulm. Rehabil. Prev. 32, 351–358 (2012).
Celis-Morales, C. A. et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK Biobank participants. BMJ 361, k1651 (2018).
Hamasaki, H. et al. Association of handgrip strength with hospitalization, cardiovascular events, and mortality in Japanese patients with type 2 diabetes. Sci. Rep. 7, 7041 (2017).
Lopez-Jaramillo, P. et al. Association of handgrip strength to cardiovascular mortality in pre-diabetic and diabetic patients: a subanalysis of the ORIGIN trial. Int. J. Cardiol. 174, 458–461 (2014).
Jurca, R. et al. Association of muscular strength with incidence of metabolic syndrome in men. Med. Sci. Sports Exerc. 37, 1849–1855 (2005).
Harada, K. et al. Impact of skeletal muscle mass on long-term adverse cardiovascular outcomes in patients with chronic kidney disease. Am. J. Cardiol. 119, 1275–1280 (2017). In this study, low skeletal muscle mass is proved to be an independent predictor of major adverse cardiovascular events in patients with chronic kidney disease.
Ko, B. J. et al. Relationship between low relative muscle mass and coronary artery calcification in healthy adults. Arterioscler. Thromb. Vasc. Biol. 36, 1016–1021 (2016).
Spahillari, A. et al. The association of lean and fat mass with all-cause mortality in older adults: the Cardiovascular Health Study. Nutr. Metab. Cardiovasc. Dis. 26, 1039–1047 (2016).
Minn, Y. K. & Suk, S. H. Higher skeletal muscle mass may protect against ischemic stroke in community-dwelling adults without stroke and dementia: The PRESENT project. BMC Geriatr. 17, 45 (2017).
Kamiya, K. et al. Prognostic usefulness of arm and calf circumference in patients ≤ 65 years of age with cardiovascular disease. Am. J. Cardiol. 119, 186–191 (2017).
Bekfani, T. et al. Sarcopenia in patients with heart failure with preserved ejection fraction: impact on muscle strength, exercise capacity and quality of life. Int. J. Cardiol. 222, 41–46 (2016).
Kinugasa, Y. & Yamamoto, K. The challenge of frailty and sarcopenia in heart failure with preserved ejection fraction. Heart 103, 184–189 (2017). In this report, the authors explain the possible mechanisms linking myokines, sarcopenia, and the development of HFpEF.
Paulus, W. J. & Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 62, 263–271 (2013).
Dos Santos, M. R. et al. Sarcopenia and endothelial function in patients with chronic heart failure: results from the Studies Investigating Comorbidities Aggravating Heart Failure (SICA-HF). J. Am. Med. Dir. Assoc. 18, 240–245 (2017).
Omori, Y. et al. L-Carnitine prevents the development of ventricular fibrosis and heart failure with preserved ejection fraction in hypertensive heart disease. J. Hypertens. 30, 1834–1844 (2012).
Tanaka, K. et al. Effects of adiponectin on calcium-handling proteins in heart failure with preserved ejection fraction. Circ. Heart Fail. 7, 976–985 (2014).
Cadore, E. L. & Izquierdo, M. Exercise interventions in polypathological aging patients that coexist with diabetes mellitus: improving functional status and quality of life. Age 37, 64 (2015).
Yamamoto, S., Hotta, K., Ota, E., Mori, R. & Matsunaga, A. Effects of resistance training on muscle strength, exercise capacity, and mobility in middle-aged and elderly patients with coronary artery disease: a meta-analysis. J. Cardiol. 68, 125–134 (2016).
Mehta, S. et al. Resistance training for gait speed and total distance walked during the chronic stage of stroke: a meta-analysis. Top. Stroke Rehabil. 19, 471–478 (2012).
Cornelissen, V. A., Fagard, R. H., Coeckelberghs, E. & Vanhees, L. Impact of resistance training on blood pressure and other cardiovascular risk factors: a meta-analysis of randomized, controlled trials. Hypertension 58, 950–958 (2011).
Serra-Rexach, J. A. et al. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: a randomized controlled trial. J. Am. Geriatr. Soc. 59, 594–602 (2011).
Maeda, S. et al. Resistance exercise training reduces plasma endothelin-1 concentration in healthy young humans. J. Cardiovasc. Pharmacol. 44 (Suppl. 1), S443–S446 (2004).
Jie, Z. et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 8, 845 (2017).
Koeth, R. A. et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585 (2013).
Patel, P. N., Shah, R. Y., Ferguson, J. F. & Reilly, M. P. Human experimental endotoxemia in modeling the pathophysiology, genomics, and therapeutics of innate immunity in complex cardiometabolic diseases. Arterioscler. Thromb. Vasc. Biol. 35, 525–534 (2015).
Lanter, B. B., Sauer, K. & Davies, D. G. Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. MBio 5, e01206–e01214 (2014).
Fu, J. et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ. Res. 117, 817–824 (2015).
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Yang, Y. et al. The association between cardiorespiratory fitness and gut microbiota composition in premenopausal women. Nutrients 9, E792 (2017).
Bressa, C. et al. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS ONE 12, e0171352 (2017). This paper reports novel evidence for an interdependence between some genera of gut bacteria and sedentary behaviour, and further shows that the dose and type of exercise can influence the composition of the gut microbiota.
Petersen, L. M. et al. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome 5, 98 (2017).
Allen, J. M. et al. Exercise alters gut microbiota composition and function in lean and obese humans. Med. Sci. Sports Exerc. 50, 747–757 (2018).
Estaki, M. et al. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 4, 42 (2016).
Kallio, K. A. et al. Endotoxemia, nutrition and cardiometabolic disorders. Acta Diabetol. 52, 395–404 (2015).
Lira, F. S. et al. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis. 9, 82 (2010).
O’Keefe, J. H., Franklin, B. & Lavie, C. J. Exercising for health and longevity versus peak performance: different regimens for different goals. Mayo Clin. Proc. 89, 1171–1175 (2014).
Sanchis-Gomar, F., Perez, L. M., Joyner, M. J., Lollgen, H. & Lucia, A. Endurance exercise and the heart: friend or foe? Sports Med. 46, 459–466 (2016).
Mons, U., Hahmann, H. & Brenner, H. A reverse J-shaped association of leisure time physical activity with prognosis in patients with stable coronary heart disease: evidence from a large cohort with repeated measurements. Heart 100, 1043–1049 (2014).
Williams, P. T. & Thompson, P. D. Increased cardiovascular disease mortality associated with excessive exercise in heart attack survivors. Mayo Clin. Proc. 89, 1187–1194 (2014).
Acknowledgements
Research in the field of exercise and health by A.L. and M.I. is funded by Fondo de Investigaciones Sanitarias (Instituto de Salud Carlos III) and Fondos FEDER (grant #PI15/00558 and PI17/01814). C.F.-L. has a Juan de la Cierva postdoctoral fellowship from the Spanish Ministry of Economy, Industry, and Competitiveness (#JCI-2016-30253). The authors thank K. McCreath for editorial assistance.
Author information
Authors and Affiliations
Contributions
All authors contributed to researching data for this article, discussions of its content, writing the paper, and review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Physical activity
-
Any bodily movement produced by skeletal muscles that requires energy expenditure.
- Moderate-to-vigorous physical activity
-
(MVPA). Any activity with an energy expenditure of ≥3 metabolic equivalents (for example, brisk walking); the WHO minimum recommendations are 150 min of MVPA each week (or 20 min or 10,000 steps on most days of the week) for adults and 60 min of active playing on most days of the week for children and adolescents.
- Physical inactivity
-
Also termed ‘lack of physical activity’; defined as not meeting the WHO-recommended minimum levels of moderate-to-vigorous physical activity (note, physical inactivity is not a synonym for sedentary behaviour).
- Exercise
-
Also termed ‘exercise training’; a subset of physical activity that is planned, structured, and repetitive and has a final or an intermediate objective of improving or maintaining physical fitness. In this Review, the terms ‘exercise’ and ‘exercise training’ are used interchangeably to refer to the cardiovascular adaptations produced by this specific type of physical activity; a single bout of exercise is referred to as ‘acute exercise’.
- Interval exercise
-
Exercise that typically involves short, repeated bouts of intense effort (for example, fast running or intensive bicycling) interspersed with short recovery periods (each lasting a few minutes or less); common variants are high-intensity interval training (HIIT), in which near maximal effort is expended in exercise bouts of 1 min, interspersed with 1-min recovery periods, and sprint interval training (SIT), in which supramaximal effort is expended in exercise bouts of ~30 min, interspersed with recovery periods lasting a few minutes.
- Aerobic exercise
-
Exercise involving dynamic movements and large muscle groups that predominantly rely on aerobic metabolism for fuelling muscle contractions; examples include jogging, running, swimming, and rowing.
- Myokine
-
A cytokine or peptide produced by skeletal muscle cells and subsequently released into the circulation, where it exerts endocrine or paracrine effects in other cells, tissues, or organs.
- Cardiorespiratory fitness
-
The capacity of the circulatory and respiratory systems to supply oxygen to skeletal muscles during sustained physical activity; the primary measure is maximal oxygen consumption (VO2 max) reached during graded exercise testing until exhaustion.
- Metabolic equivalent
-
(MET). A unit for quantifying cardiorespiratory fitness: 1 MET is equivalent to the basal metabolic rate (consumption of 3.5 ml O2/kg/min, on average).
- Sedentary behaviour
-
Any waking activities conducted in a sitting, reclining, or lying posture and characterized by an energy expenditure ≤1.5 metabolic equivalents.
- Sarcopenia
-
Derived from the Greek ‘sarx’ (flesh) and ‘penia’ (loss); sarcopenia is the age-induced loss of muscle mass and function, which typically manifests as reduced gait speed.
- Shear stress
-
The frictional force exerted by blood flow on the endothelium of vessel walls.
- Resistance exercise
-
Movement performed against a specific external force that is regularly increased during training; examples include weightlifting and exercises using resistance machines.
- Muscle strength
-
The ability of a muscle to exert force on physical objects; muscle strength is determined by the mass of the muscle and its ability to recruit motor units.
- Gut microbiota
-
The collective microorganisms (bacteria, archaea, fungi, and viruses) that reside in the gastrointestinal tract.
- Gut microbiome
-
The collective genomes of the gut microbiota.
- Short-chain fatty acids
-
Fatty acids (such as butyrate) that are produced by the gut microbiota during the fermentation of partially digestible and indigestible carbohydrates; the highest levels of short-chain fatty acids are found in the proximal colon, where they are used locally by enterocytes or transported across the gut epithelium into the bloodstream.
- Intestinal permeability
-
The capacity of material to pass from the lumen of the gastrointestinal tract through the cells lining the gut wall into the rest of the body.
- Lipopolysaccharide
-
Also known as endotoxin; an active component of the cell wall of Gram-negative bacteria originating from food intake and/or the microbiota of the oral cavity and gut.
Rights and permissions
About this article
Cite this article
Fiuza-Luces, C., Santos-Lozano, A., Joyner, M. et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol 15, 731–743 (2018). https://doi.org/10.1038/s41569-018-0065-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-018-0065-1
This article is cited by
-
The Effects of Stretching Exercise on Levels of Blood Glucose: A Systematic Review with Meta-Analysis
Sports Medicine - Open (2024)
-
Effect of exercise training on heath, quality of life, exercise capacity in juvenile idiopathic arthritis: a meta-analysis of randomized controlled trials
Pediatric Rheumatology (2024)
-
Exercise mimetics: a novel strategy to combat neuroinflammation and Alzheimer’s disease
Journal of Neuroinflammation (2024)
-
Association of physical activity with socio-economic status and chronic disease in older adults in China: cross-sectional findings from the survey of CLASS 2020 after the outbreak of COVID-19
BMC Public Health (2024)
-
The effect of physical exercise on anticancer immunity
Nature Reviews Immunology (2024)