Abstract
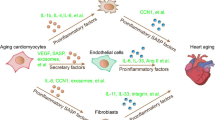
Cardiac ageing manifests as a decline in function leading to heart failure. At the cellular level, ageing entails decreased replicative capacity and dysregulation of cellular processes in myocardial and nonmyocyte cells. Various extrinsic parameters, such as lifestyle and environment, integrate important signalling pathways, such as those involving inflammation and oxidative stress, with intrinsic molecular mechanisms underlying resistance versus progression to cellular senescence. Mitigation of cardiac functional decline in an ageing organism requires the activation of enhanced maintenance and reparative capacity, thereby overcoming inherent endogenous limitations to retaining a youthful phenotype. Deciphering the molecular mechanisms underlying dysregulation of cellular function and renewal reveals potential interventional targets to attenuate degenerative processes at the cellular and systemic levels to improve quality of life for our ageing population. In this Review, we discuss the roles of extrinsic and intrinsic factors in cardiac ageing. Animal models of cardiac ageing are summarized, followed by an overview of the current and possible future treatments to mitigate the deleterious effects of cardiac ageing.
Key points
-
Ageing is a primary risk factor for cardiovascular disease and mortality.
-
The capacity of the adult human heart to maintain function and preserve cellular homeostasis declines with age.
-
Extrinsic factors of environment, behaviour, and lifestyle can promote or blunt cellular and molecular cardiac ageing.
-
Intrinsic processes that promote cellular ageing, such as inflammation and oxidative stress, exacerbate telomere shortening, chromatin remodelling, and epigenetic drift.
-
Cardiovascular ageing is inextricably tied to genetic predisposition and the complex interaction of hereditary influences.
-
Promising advances to antagonize myocardial ageing connect external factors with intrinsic molecular mechanisms, enabling interventional strategies on both behavioural and cellular levels.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135, e146–e603 (2017).
Roth, G. A. et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 70, 1–25 (2017).
Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
Fontana, L., Kennedy, B. K., Longo, V. D., Seals, D. & Melov, S. Medical research: treat ageing. Nature 511, 405–407 (2014).
Paneni, F., Diaz Canestro, C., Libby, P., Luscher, T. F. & Camici, G. G. The aging cardiovascular system: understanding it at the cellular and clinical levels. J. Am. Coll. Cardiol. 69, 1952–1967 (2017).
Bernhard, D. & Laufer, G. The aging cardiomyocyte: a mini-review. Gerontology 54, 24–31 (2008).
Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Lopez-Otin, C., Galluzzi, L., Freije, J. M. P., Madeo, F. & Kroemer, G. Metabolic control of longevity. Cell 166, 802–821 (2016).
Franceschi, C. & Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 69 (Suppl. 1), S4–S9 (2014).
Sack, M. N., Fyhrquist, F. Y., Saijonmaa, O. J., Fuster, V. & Kovacic, J. C. Basic biology of oxidative stress and the cardiovascular system: part 1 of a 3-part series. J. Am. Coll. Cardiol. 70, 196–211 (2017).
Linton, P. J., Gurney, M., Sengstock, D., Mentzer, R. M. Jr & Gottlieb, R. A. This old heart: cardiac aging and autophagy. J. Mol. Cell. Cardiol. 83, 44–54 (2015).
Ren, J. et al. Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling: role of Sirt1-mediated autophagy regulation. Aging Cell 16, 976–987 (2017).
Woodall, B. P. & Gustafsson, A. B. Autophagy-A key pathway for cardiac health and longevity. Acta Physiol. 00, e13074 (2018).
Finkel, T. The metabolic regulation of aging. Nat. Med. 21, 1416–1423 (2015).
Costantino, S., Paneni, F. & Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 594, 2061–2073 (2016).
Eschenhagen, T. et al. Cardiomyocyte regeneration: a consensus statement. Circulation 136, 680–686 (2017).
Beltrami, A. P. et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 (2003).
Anversa, P., Leri, A. & Kajstura, J. Cardiac regeneration. J. Am. Coll. Cardiol. 47, 1769–1776 (2006).
Ellison, G. M. et al. Adult c-kitpos cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842 (2013).
Nadal-Ginard, B., Ellison, G. M. & Torella, D. The cardiac stem cell compartment is indispensable for myocardial cell homeostasis, repair and regeneration in the adult. Stem Cell Res. 13, 615–630 (2014).
Torella, D., Ellison, G. M. & Nadal-Ginard, B. Adult c-kitpos cardiac stem cells fulfill Koch’s postulates as causal agents for cardiac regeneration. Circ. Res. 114, e24–e26 (2014).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Senyo, S. E. et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436 (2013).
Mollova, M. et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl Acad. Sci. USA 110, 1446–1451 (2013).
Ali, S. R. et al. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc. Natl Acad. Sci. USA 111, 8850–8855 (2014).
Bergmann, O. et al. Dynamics of cell generation and turnover in the human heart. Cell 161, 1566–1575 (2015).
Tzahor, E. & Poss, K. D. Cardiac regeneration strategies: staying young at heart. Science 356, 1035–1039 (2017).
Quaife-Ryan, G. A. et al. Multicellular transcriptional analysis of mammalian heart regeneration. Circulation 136, 1123–1139 (2017).
Yuan, X. & Braun, T. Multimodal regulation of cardiac myocyte proliferation. Circ. Res. 121, 293–309 (2017).
Hesse, M., Welz, A. & Fleischmann, B. K. Heart regeneration and the cardiomyocyte cell cycle. Pflugers Arch. 470, 241–248 (2018).
Keith, M. C. & Bolli, R. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ. Res. 116, 1216–1230 (2015).
Tang, J. N. et al. Concise Review: Is cardiac cell therapy dead? embarrassing trial outcomes and new directions for the future. Stem Cells Transl Med. 7, 354–359 (2018).
Broughton, K. M. et al. Mechanisms of cardiac repair and regeneration. Circ. Res. 122, 1151–1163 (2018).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011).
Alkass, K. et al. No evidence for cardiomyocyte number expansion in preadolescent mice. Cell 163, 1026–1036 (2015).
Aix, E., Gutierrez-Gutierrez, O., Sanchez-Ferrer, C., Aguado, T. & Flores, I. Postnatal telomere dysfunction induces cardiomyocyte cell-cycle arrest through p21 activation. J. Cell Biol. 213, 571–583 (2016).
Naqvi, N. et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell 157, 795–807 (2014).
Soonpaa, M. H. et al. Cardiomyocyte cell-cycle activity during preadolescence. Cell 163, 781–782 (2015).
Rubin, N., Harrison, M. R., Krainock, M., Kim, R. & Lien, C. L. Recent advancements in understanding endogenous heart regeneration-insights from adult zebrafish and neonatal mice. Semin. Cell Dev. Biol. 58, 34–40 (2016).
Goldspink, D. F., Burniston, J. G. & Tan, L. B. Cardiomyocyte death and the ageing and failing heart. Exp. Physiol. 88, 447–458 (2003).
Lazar, E., Sadek, H. A. & Bergmann, O. Cardiomyocyte renewal in the human heart: insights from the fall-out. Eur. Heart J. 38, 2333–2342 (2017).
Anversa, P. et al. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ. Res. 67, 871–885 (1990).
Anversa, P., Leri, A., Kajstura, J. & Nadal-Ginard, B. Myocyte growth and cardiac repair. J. Mol. Cell. Cardiol. 34, 91–105 (2002).
Chimenti, C. et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ. Res. 93, 604–613 (2003).
Wang, W. E. et al. Dedifferentiation, proliferation, and redifferentiation of adult mammalian cardiomyocytes after ischemic injury. Circulation 136, 834–848 (2017).
Foglia, M. J. & Poss, K. D. Building and re-building the heart by cardiomyocyte proliferation. Development 143, 729–740 (2016).
Limana, F. et al. bcl-2 overexpression promotes myocyte proliferation. Proc. Natl Acad. Sci. USA 99, 6257–6262 (2002).
Torella, D. et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ. Res. 94, 514–524 (2004).
Pasumarthi, K. B., Nakajima, H., Nakajima, H. O., Soonpaa, M. H. & Field, L. J. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res. 96, 110–118 (2005).
Gude, N. et al. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ. Res. 99, 381–388 (2006).
Muraski, J. A. et al. Pim-1 regulates cardiomyocyte survival downstream of Akt. Nat. Med. 13, 1467–1475 (2007).
Oh, H. et al. Telomerase reverse transcriptase promotes cardiac muscle cell proliferation, hypertrophy, and survival. Proc. Natl Acad. Sci. USA 98, 10308–10313 (2001).
Pinto, A. R. et al. Revisiting cardiac cellular composition. Circ. Res. 118, 400–409 (2016).
Skelly, D. A. et al. Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Rep. 22, 600–610 (2018).
Zlatanova, I., Pinto, C. & Silvestre, J. S. Immune modulation of cardiac repair and regeneration: the art of mending broken hearts. Front. Cardiovasc. Med. 3, 40 (2016).
Mahmoud, A. I. et al. Nerves regulate cardiomyocyte proliferation and heart regeneration. Dev. Cell 34, 387–399 (2015).
Ubil, E. et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature 514, 585–590 (2014).
He, L. et al. Preexisting endothelial cells mediate cardiac neovascularization after injury. J. Clin. Invest. 127, 2968–2981 (2017).
Avolio, E. & Madeddu, P. Discovering cardiac pericyte biology: from physiopathological mechanisms to potential therapeutic applications in ischemic heart disease. Vascul. Pharmacol. 86, 53–63 (2016).
Guimaraes-Camboa, N. et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 20, 345–359 e345 (2017).
Beltrami, A. P. & Madeddu, P. Pericytes and cardiac stem cells: Common features and peculiarities. Pharmacol. Res. 127, 101–109 (2018).
Kovacic, J. C., Moreno, P., Nabel, E. G., Hachinski, V. & Fuster, V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation 123, 1900–1910 (2011).
Kovacic, J. C., Moreno, P., Hachinski, V., Nabel, E. G. & Fuster, V. Cellular senescence, vascular disease, and aging: part 1 of a 2-part review. Circulation 123, 1650–1660 (2011).
Chen, W. & Frangogiannis, N. G. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail. Rev. 15, 415–422 (2010).
Sessions, A. O. & Engler, A. J. Mechanical regulation of cardiac aging in model systems. Circ. Res. 118, 1553–1562 (2016).
Frangogiannis, N. G. The extracellular matrix in myocardial injury, repair, and remodeling. J. Clin. Invest. 127, 1600–1612 (2017).
Trial, J., Entman, M. L. & Cieslik, K. A. Mesenchymal stem cell-derived inflammatory fibroblasts mediate interstitial fibrosis in the aging heart. J. Mol. Cell. Cardiol. 91, 28–34 (2016).
Gude, N. A. & Sussman, M. A. Chasing c-Kit through the heart: taking a broader view. Pharmacol. Res. 127, 110–115 (2018).
Gude, N. A. et al. Cardiac c-Kit Biology Revealed by Inducible Transgenesis. Circ. Res. (2018).
Maroli, G. & Braun, T. The complex biology of KIT+ cells in the heart. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-018-0037-5 (2018).
Bolli, R. et al. Rationale and design of the CONCERT-HF trial (Combination of mesenchymal and c-kit+ cardiac stem cells as regenerative therapy for heart failure). Circ. Res. 122, 1703–1715 (2018).
Cyranoski, D. ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 557, 619–620 (2018).
Natsumeda, M. et al. A combination of allogeneic stem cells promotes cardiac regeneration. J. Am. Coll. Cardiol. 70, 2504–2515 (2017).
Kulandavelu, S. et al. Pim1 kinase overexpression enhances ckit+ cardiac stem cell cardiac repair following myocardial infarction in swine. J. Am. Coll. Cardiol. 68, 2454–2464 (2016).
Urbanek, K. et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc. Natl Acad. Sci. USA 102, 8692–8697 (2005).
Rota, M., Goichberg, P., Anversa, P. & Leri, A. Aging effects on cardiac progenitor cell physiology. Compr. Physiol. 5, 1775–1814 (2015).
Matsumoto, C. et al. Short telomeres induce p53 and autophagy and modulate age-associated changes in cardiac progenitor cell fate. Stem Cells 33, 868–880 (2018).
Mohsin, S. et al. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J. Am. Coll. Cardiol. 60, 1278–1287 (2012).
Mohsin, S. et al. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ. Res. 113, 1169–1179 (2013).
Samse, K. et al. Functional effect of Pim1 depends upon intracellular localization in human cardiac progenitor cells. J. Biol. Chem. 290, 13935–13947 (2015).
Samse, K., Hariharan, N. & Sussman, M. A. Personalizing cardiac regenerative therapy: At the heart of Pim1 kinase. Pharmacol. Res. 103, 13–16 (2016).
Kajstura, J. et al. Telomere shortening is an in vivo marker of myocyte replication and aging. Am. J. Pathol. 156, 813–819 (2000).
Papp, Z., Czuriga, D., Balogh, L., Balogh, A. & Borbely, A. How cardiomyocytes make the heart old. Curr. Pharm. Biotechnol. 13, 2515–2521 (2012).
Sheydina, A., Riordon, D. R. & Boheler, K. R. Molecular mechanisms of cardiomyocyte aging. Clin. Sci. 121, 315–329 (2011).
Jeyapalan, J. C. & Sedivy, J. M. Cellular senescence and organismal aging. Mech. Ageing Dev. 129, 467–474 (2008).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Campisi, J. Cellular senescence: putting the paradoxes in perspective. Curr. Opin. Genet. Dev. 21, 107–112 (2011).
Hall, B. M. et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging 8, 1294–1315 (2016).
Arai, Y. et al. Inflammation, but not telomere length, predicts successful ageing at extreme old age: a longitudinal study of semi-supercentenarians. EBioMedicine 2, 1549–1558 (2015).
Siddiqi, S. & Sussman, M. A. Cardiac hegemony of senescence. Curr. Transl Geriatr. Exp. Gerontol. Rep. 2, 247–254 (2013).
Childs, B. G., Durik, M., Baker, D. J. & van Deursen, J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015).
McHugh, D. & Gil, J. Senescence and aging: causes, consequences, and therapeutic avenues. J. Cell Biol. 217, 65–77 (2018).
Minamino, T. et al. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 105, 1541–1544 (2002).
Matthews, C. et al. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circ. Res. 99, 156–164 (2006).
Wang, M. & Shah, A. M. Age-associated pro-inflammatory remodeling and functional phenotype in the heart and large arteries. J. Mol. Cell. Cardiol. 83, 101–111 (2015).
Curtis, A. B., Karki, R., Hattoum, A. & Sharma, U. C. Arrhythmias in patients ≥80 years of age: pathophysiology, management, and outcomes. J. Am. Coll. Cardiol. 71, 2041–2057 (2018).
Biernacka, A. & Frangogiannis, N. G. Aging and cardiac fibrosis. Aging Dis. 2, 158–173 (2011).
Cesselli, D. et al. Effects of age and heart failure on human cardiac stem cell function. Am. J. Pathol. 179, 349–366 (2011).
Hariharan, N. & Sussman, M. A. Cardiac aging – getting to the stem of the problem. J. Mol. Cell. Cardiol. 83, 32–36 (2015).
Allsopp, R. C., Morin, G. B., DePinho, R., Harley, C. B. & Weissman, I. L. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood 102, 517–520 (2003).
Nollet, E. et al. Accelerated cellular senescence as underlying mechanism for functionally impaired bone marrow-derived progenitor cells in ischemic heart disease. Atherosclerosis 260, 138–146 (2017).
Tang, Y., Liu, M. L., Zang, T. & Zhang, C. L. Direct reprogramming rather than iPSC-based reprogramming maintains aging hallmarks in human motor neurons. Front. Mol. Neurosci. 10, 359 (2017).
Cheng, Z., Peng, H. L., Zhang, R., Fu, X. M. & Zhang, G. S. Rejuvenation of cardiac tissue developed from reprogrammed aged somatic cells. Rejuven. Res. 20, 389–400 (2017).
Ocampo, A., Reddy, P. & Izpisua Belmonte, J. C. Anti-aging strategies based on cellular reprogramming. Trends Mol. Med. 22, 725–738 (2016).
Kim, H. et al. Cardiovascular effects of long-term exposure to air pollution: a population-based study with 900 845 person-years of follow-up. J. Am. Heart Assoc. 6, e007170 (2017).
Sinharay, R. et al. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: a randomised, crossover study. Lancet 391, 339–349 (2017).
Lin, L. Y., Chuang, H. C., Liu, I. J., Chen, H. W. & Chuang, K. J. Reducing indoor air pollution by air conditioning is associated with improvements in cardiovascular health among the general population. Sci. Total Environ. 463–464, 176–181 (2013).
Chuang, H. C. et al. Long-term indoor air conditioner filtration and cardiovascular health: A randomized crossover intervention study. Environ. Int. 106, 91–96 (2017).
Ward-Caviness, C. K. et al. Associations between residential proximity to traffic and vascular disease in a cardiac catheterization cohort. Arterioscler Thromb. Vasc. Biol. 38, 275–282 (2017).
Hoxha, M. et al. Association between leukocyte telomere shortening and exposure to traffic pollution: a cross-sectional study on traffic officers and indoor office workers. Environ. Health 8, 41 (2009).
Lin, N. et al. Accumulative effects of indoor air pollution exposure on leukocyte telomere length among non-smokers. Environ. Pollut. 227, 1–7 (2017).
Martens, D. S. & Nawrot, T. S. Air pollution stress and the aging phenotype: the telomere connection. Curr. Environ. Health Rep. 3, 258–269 (2016).
Corella, D., Coltell, O., Macian, F. & Ordovas, J. M. Advances in understanding the molecular basis of the mediterranean diet effect. Annu. Rev. Food Sci. Technol. 9, 227–249 (2018).
Garcia-Calzon, S. et al. Mediterranean diet and telomere length in high cardiovascular risk subjects from the PREDIMED-NAVARRA study. Clin. Nutr. 35, 1399–1405 (2016).
Fito, M. & Konstantinidou, V. Nutritional genomics and the mediterranean diet’s effects on human cardiovascular health. Nutrients 8, 218 (2016).
Arpon, A. et al. Impact of consuming extra-virgin olive oil or nuts within a mediterranean diet on dna methylation in peripheral white blood cells within the PREDIMED-Navarra randomized controlled trial: a role for dietary lipids. Nutrients 10, E15 (2017).
Gill, S., Le, H. D., Melkani, G. C. & Panda, S. Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347, 1265–1269 (2015).
Melkani, G. C. & Panda, S. Time-restricted feeding for prevention and treatment of cardiometabolic disorders. J. Physiol. 595, 3691–3700 (2017).
Ahmet, I., Wan, R., Mattson, M. P., Lakatta, E. G. & Talan, M. Cardioprotection by intermittent fasting in rats. Circulation 112, 3115–3121 (2005).
Katare, R. G., Kakinuma, Y., Arikawa, M., Yamasaki, F. & Sato, T. Chronic intermittent fasting improves the survival following large myocardial ischemia by activation of BDNF/VEGF/PI3K signaling pathway. J. Mol. Cell. Cardiol. 46, 405–412 (2009).
Wan, R. et al. Cardioprotective effect of intermittent fasting is associated with an elevation of adiponectin levels in rats. J. Nutr. Biochem. 21, 413–417 (2010).
Chausse, B., Vieira-Lara, M. A., Sanchez, A. B., Medeiros, M. H. & Kowaltowski, A. J. Intermittent fasting results in tissue-specific changes in bioenergetics and redox state. PLoS ONE 10, e0120413 (2015).
Godar, R. J. et al. Repetitive stimulation of autophagy-lysosome machinery by intermittent fasting preconditions the myocardium to ischemia-reperfusion injury. Autophagy 11, 1537–1560 (2015).
Maegawa, S. et al. Caloric restriction delays age-related methylation drift. Nat. Commun. 8, 539 (2017).
Horne, B. D., Muhlestein, J. B. & Anderson, J. L. Health effects of intermittent fasting: hormesis or harm? A systematic review. Am. J. Clin. Nutr. 102, 464–470 (2015).
Zuo, L. et al. Comparison of high-protein, intermittent fasting low-calorie diet and heart healthy diet for vascular health of the obese. Front. Physiol. 7, 350 (2016).
St-Onge, M. P. et al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation 135, e96–e121 (2017).
Tinsley, G. M. & Horne, B. D. Intermittent fasting and cardiovascular disease: current evidence and unresolved questions. Future Cardiol. 14, 47–54 (2018).
Melkani, G. C. et al. TRiC/CCT chaperonins are essential for maintaining myofibril organization, cardiac physiological rhythm, and lifespan. FEBS Lett. 591, 3447–3458 (2017).
Barger, J. L. et al. Identification of tissue-specific transcriptional markers of caloric restriction in the mouse and their use to evaluate caloric restriction mimetics. Aging Cell 16, 750–760 (2017).
Booth, F. W., Roberts, C. K., Thyfault, J. P., Ruegsegger, G. N. & Toedebusch, R. G. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol. Rev. 97, 1351–1402 (2017).
Garatachea, N. et al. Exercise attenuates the major hallmarks of aging. Rejuven. Res. 18, 57–89 (2015).
Howden, E. J. et al. Reversing the cardiac effects of sedentary aging in middle age — a randomized controlled trial: implications for heart failure prevention. Circulation 137, 1549–1560 (2018).
Werner, C. et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J. Am. Coll. Cardiol. 52, 470–482 (2008).
Werner, C. et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation 120, 2438–2447 (2009).
Ludlow, A. T. & Roth, S. M. Physical activity and telomere biology: exploring the link with aging-related disease prevention. J. Aging Res. 2011, 790378 (2011).
Ludlow, A. T. et al. Chronic exercise modifies age-related telomere dynamics in a tissue-specific fashion. J. Gerontol. A Biol. Sci. Med. Sci. 67, 911–926 (2012).
Ludlow, A. T., Ludlow, L. W. & Roth, S. M. Do telomeres adapt to physiological stress? Exploring the effect of exercise on telomere length and telomere-related proteins. Biomed. Res. Int. 2013, 601368 (2013).
Loprinzi, P. D. Leisure-time screen-based sedentary behavior and leukocyte telomere length: implications for a new leisure-time screen-based sedentary behavior mechanism. Mayo Clin. Proc. 90, 786–790 (2015).
Loprinzi, P. D. & Sng, E. Mode-specific physical activity and leukocyte telomere length among US adults: implications of running on cellular aging. Prev. Med. 85, 17–19 (2016).
Tucker, L. A. Physical activity and telomere length in US men and women: an NHANES investigation. Prev. Med. 100, 145–151 (2017).
Loprinzi, P. D. & Loenneke, J. P. Leukocyte telomere length and mortality among US adults: effect modification by physical activity behaviour. J. Sports Sci. 36, 213–219 (2018).
Edwards, M. K. & Loprinzi, P. D. Sedentary behavior, physical activity and cardiorespiratory fitness on leukocyte telomere length. Health Promot. Perspect. 7, 22–27 (2017).
Ludlow, A. T., Gratidao, L., Ludlow, L. W., Spangenburg, E. E. & Roth, S. M. Acute exercise activates p38 MAPK and increases the expression of telomere-protective genes in cardiac muscle. Exp. Physiol. 102, 397–410 (2017).
Saban, K. L., Mathews, H. L., DeVon, H. A. & Janusek, L. W. Epigenetics and social context: implications for disparity in cardiovascular disease. Aging Dis. 5, 346–355 (2014).
Kivimaki, M. et al. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet 380, 1491–1497 (2012).
Steptoe, A. & Kivimaki, M. Stress and cardiovascular disease: an update on current knowledge. Annu. Rev. Publ. Health 34, 337–354 (2013).
Steptoe, A. & Kivimaki, M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 9, 360–370 (2012).
Powell, N. D. et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl Acad. Sci. USA 110, 16574–16579 (2013).
Wirtz, P. H. & von Kanel, R. Psychological stress, inflammation, and coronary heart disease. Curr. Cardiol. Rep. 19, 111 (2017).
Chen, Z. et al. Brain-heart interaction: cardiac complications after stroke. Circ. Res. 121, 451–468 (2017).
Arri, S. S., Ryan, M., Redwood, S. R. & Marber, M. S. Mental stress-induced myocardial ischaemia. Heart 102, 472–480 (2016).
Dawson, D. K. Acute stress-induced (takotsubo) cardiomyopathy. Heart 104, 96–102 (2017).
Scally, C. et al. Persistent long-term structural, functional, and metabolic changes after stress-induced (Takotsubo) cardiomyopathy. Circulation 137, 1039–1048 (2018).
Chaix, R. et al. Epigenetic clock analysis in long-term meditators. Psychoneuroendocrinology 85, 210–214 (2017).
Benson, H., Beary, J. F. & Carol, M. P. The relaxation response. Psychiatry 37, 37–46 (1974).
Benson, H., Rosner, B. A., Marzetta, B. R. & Klemchuk, H. M. Decreased blood-pressure in pharmacologically treated hypertensive patients who regularly elicited the relaxation response. Lancet 1, 289–291 (1974).
Bhasin, M. K. et al. Relaxation response induces temporal transcriptome changes in energy metabolism, insulin secretion and inflammatory pathways. PLoS ONE 8, e62817 (2013).
Krishna, B. H., Keerthi, G. S., Kumar, C. K. & Reddy, N. M. Association of leukocyte telomere length with oxidative stress in yoga practitioners. J. Clin. Diagn. Res. 9, CC01–CC03 (2015).
Piao, L. et al. Chronic psychological stress accelerates vascular senescence and impairs ischemia-induced neovascularization: the role of dipeptidyl peptidase-4/glucagon-like peptide-1-adiponectin axis. J. Am. Heart Assoc. 6, e006421 (2017).
Alibhai, F. J. et al. Disrupting the key circadian regulator CLOCK leads to age-dependent cardiovascular disease. J. Mol. Cell. Cardiol. 105, 24–37 (2017).
Yang, Y. C. et al. Social relationships and physiological determinants of longevity across the human life span. Proc. Natl Acad. Sci. USA 113, 578–583 (2016).
Blackburn, E. H., Epel, E. S. & Lin, J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198 (2015).
Anderson, R., Richardson, G. D. & Passos, J. F. Mechanisms driving the ageing heart. Exp. Gerontol. https://doi.org/10.1016/j.exger.2017.10.015 (2017).
Booth, S. A. & Charchar, F. J. Cardiac telomere length in heart development, function, and disease. Physiol. Genom. 49, 368–384 (2017).
Yeh, J. K. & Wang, C. Y. Telomeres and telomerase in cardiovascular diseases. Genes 7, E58 (2016).
de Magalhaes, J. P. & Passos, J. F. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 170, 2–9 (2018).
Aix, E., Gallinat, A. & Flores, I. Telomeres and telomerase in heart regeneration. Differentiation 100, 26–30 (2018).
Chang, A. C. Y. & Blau, H. M. Short telomeres — a hallmark of heritable cardiomyopathies. Differentiation 100, 31–36 (2018).
Cimato, T. R. Biological age and circulating progenitor cell levels as predictors heart disease events. Circ. Res. 120, 1053–1054 (2017).
Hammadah, M. et al. Telomere shortening, regenerative capacity, and cardiovascular outcomes. Circ. Res. 120, 1130–1138 (2017).
Bhattacharyya, J., Mihara, K., Bhattacharjee, D. & Mukherjee, M. Telomere length as a potential biomarker of coronary artery disease. Indian J. Med. Res. 145, 730–737 (2017).
Haycock, P. C. et al. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 349, g4227 (2014).
Gebreab, S. Y. et al. Less than ideal cardiovascular health is associated with shorter leukocyte telomere length: the National Health and Nutrition Examination Surveys, 1999–2002. J. Am. Heart Assoc. 6, e004105 (2017).
Rehkopf, D. H. et al. Leukocyte telomere length in relation to 17 biomarkers of cardiovascular disease risk: a cross-sectional study of US adults. PLoS Med. 13, e1002188 (2016).
Peng, H. et al. Impact of biological aging on arterial aging in American Indians: findings from the Strong Heart Family Study. Aging 8, 1583–1592 (2016).
Passarino, G., De Rango, F. & Montesanto, A. Human longevity: genetics or lifestyle? It takes two to tango. Immun. Ageing 13, 12 (2016).
Bellizzi, D. et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85, 258–263 (2005).
Pawlikowska, L. et al. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell 8, 460–472 (2009).
Ziv, E. & Hu, D. Genetic variation in insulin/IGF-1 signaling pathways and longevity. Ageing Res. Rev. 10, 201–204 (2011).
Morris, B. J., Willcox, D. C., Donlon, T. A. & Willcox, B. J. FOXO3: a major gene for human longevity — a mini-review. Gerontology 61, 515–525 (2015).
Codd, V. et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 45, 422–427 (2013).
Ding, H. et al. Association between previously identified loci affecting telomere length and coronary heart disease (CHD) in Han Chinese population. Clin. Interv. Aging 9, 857–861 (2014).
Smith, J. G. & Newton-Cheh, C. Genome-wide association studies of late-onset cardiovascular disease. J. Mol. Cell. Cardiol. 83, 131–141 (2015).
Navarro, C. L., Cau, P. & Levy, N. Molecular bases of progeroid syndromes. Hum. Mol. Genet. 15, R151–R161 (2006).
Brayson, D. & Shanahan, C. M. Current insights into LMNA cardiomyopathies: Existing models and missing LINCs. Nucleus 8, 17–33 (2017).
Chen, L. et al. WRN, the protein deficient in Werner syndrome, plays a critical structural role in optimizing DNA repair. Aging Cell 2, 191–199 (2003).
Rossi, M. L., Ghosh, A. K. & Bohr, V. A. Roles of Werner syndrome protein in protection of genome integrity. DNA Repair 9, 331–344 (2010).
Broer, L. & van Duijn, C. M. GWAS and meta-analysis in aging/longevity. Adv. Exp. Med. Biol. 847, 107–125 (2015).
Broer, L. et al. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J. Gerontol. A Biol. Sci. Med. Sci. 70, 110–118 (2015).
Fortney, K. et al. Genome-wide scan informed by age-related disease identifies loci for exceptional human longevity. PLoS Genet. 11, e1005728 (2015).
Chen, Z., Yang, S. H., Xu, H. & Li, J. J. ABO blood group system and the coronary artery disease: an updated systematic review and meta-analysis. Sci. Rep. 6, 23250 (2016).
Deelen, J. et al. Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age. Hum. Mol. Genet. 23, 4420–4432 (2014).
Zeng, Y. et al. Novel loci and pathways significantly associated with longevity. Sci. Rep. 6, 21243 (2016).
Joshi, P. K. et al. Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nat. Commun. 8, 910 (2017).
Benson, M. D. et al. The genetic architecture of the cardiovascular risk proteome. Circulation 137, 1158–1172 (2018).
Mailman, M. D. et al. The NCBI dbGaP database of genotypes and phenotypes. Nat. Genet. 39, 1181–1186 (2007).
Berger, S. L., Kouzarides, T., Shiekhattar, R. & Shilatifard, A. An operational definition of epigenetics. Genes Dev. 23, 781–783 (2009).
Benayoun, B. A., Pollina, E. A. & Brunet, A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 16, 593–610 (2015).
Sen, P., Shah, P. P., Nativio, R. & Berger, S. L. Epigenetic mechanisms of longevity and aging. Cell 166, 822–839 (2016).
Booth, L. N. & Brunet, A. The aging epigenome. Mol. Cell 62, 728–744 (2016).
Sierra, M. I., Fernandez, A. F. & Fraga, M. F. Epigenetics of aging. Curr. Genom. 16, 435–440 (2015).
Cruickshanks, H. A. et al. Senescent cells harbour features of the cancer epigenome. Nat. Cell Biol. 15, 1495–1506 (2013).
Zhong, J., Agha, G. & Baccarelli, A. A. The role of DNA methylation in cardiovascular risk and disease: methodological aspects, study design, and data analysis for epidemiological studies. Circ. Res. 118, 119–131 (2016).
Sharma, P. et al. Genome wide DNA methylation profiling for epigenetic alteration in coronary artery disease patients. Gene 541, 31–40 (2014).
Duan, L., Hu, J., Xiong, X., Liu, Y. & Wang, J. The role of DNA methylation in coronary artery disease. Gene 646, 91–97 (2018).
Azad, M. A. K. et al. Hyperhomocysteinemia and cardiovascular disease in animal model. Amino Acids 50, 3–9 (2018).
Heyn, H., Moran, S. & Esteller, M. Aberrant DNA methylation profiles in the premature aging disorders Hutchinson-Gilford Progeria and Werner syndrome. Epigenetics 8, 28–33 (2013).
Issa, J. P. Aging and epigenetic drift: a vicious cycle. J. Clin. Invest. 124, 24–29 (2014).
Gilsbach, R. et al. Distinct epigenetic programs regulate cardiac myocyte development and disease in the human heart in vivo. Nat. Commun. 9, 391 (2018).
Weber, C. M. & Henikoff, S. Histone variants: dynamic punctuation in transcription. Genes Dev. 28, 672–682 (2014).
O’Sullivan, R. J., Kubicek, S., Schreiber, S. L. & Karlseder, J. Reduced histone biosynthesis and chromatin changes arising from a damage signal at telomeres. Nat. Struct. Mol. Biol. 17, 1218–1225 (2010).
Piazzesi, A. et al. Replication-independent histone variant H3.3 controls animal lifespan through the regulation of pro-longevity transcriptional programs. Cell Rep. 17, 987–996 (2016).
Duarte, L. F. et al. Histone H3.3 and its proteolytically processed form drive a cellular senescence programme. Nat. Commun. 5, 5210 (2014).
Bano, D., Piazzesi, A., Salomoni, P. & Nicotera, P. The histone variant H3.3 claims its place in the crowded scene of epigenetics. Aging 9, 602–614 (2017).
Tvardovskiy, A., Schwammle, V., Kempf, S. J., Rogowska-Wrzesinska, A. & Jensen, O. N. Accumulation of histone variant H3.3 with age is associated with profound changes in the histone methylation landscape. Nucleic Acids Res. 45, 9272–9289 (2017).
Goldman, J. A. et al. Resolving heart regeneration by replacement histone profiling. Dev. Cell 40, 392–404.e5 (2017).
Saade, E., Pirozhkova, I., Aimbetov, R., Lipinski, M. & Ogryzko, V. Molecular turnover, the H3.3 dilemma and organismal aging (hypothesis). Aging Cell 14, 322–333 (2015).
Valdivia, M. M., Hamdouch, K., Ortiz, M. & Astola, A. CENPA a genomic marker for centromere activity and human diseases. Curr. Genom. 10, 326–335 (2009).
Lee, S. H., Itkin-Ansari, P. & Levine, F. CENP-A, a protein required for chromosome segregation in mitosis, declines with age in islet but not exocrine cells. Aging 2, 785–790 (2010).
McGregor, M., Hariharan, N., Joyo, A. Y., Margolis, R. L. & Sussman, M. A. CENP-A is essential for cardiac progenitor cell proliferation. Cell Cycle 13, 739–748 (2014).
Feser, J. et al. Elevated histone expression promotes life span extension. Mol. Cell 39, 724–735 (2010).
Wang, Y., Yuan, Q. & Xie, L. Histone modifications in aging the underlying mechanisms and implications. Curr. Stem Cell Res. Ther. 13, 125–135 (2018).
Peleg, S., Feller, C., Ladurner, A. G. & Imhof, A. The metabolic impact on histone acetylation and transcription in ageing. Trends Biochem. Sci. 41, 700–711 (2016).
Dang, W. et al. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature 459, 802–807 (2009).
Ferguson, B. S. & McKinsey, T. A. Non-sirtuin histone deacetylases in the control of cardiac aging. J. Mol. Cell. Cardiol. 83, 14–20 (2015).
McCauley, B. S. & Dang, W. Histone methylation and aging: lessons learned from model systems. Biochim. Biophys. Acta 1839, 1454–1462 (2014).
Matsushima, S. & Sadoshima, J. The role of sirtuins in cardiac disease. Am. J. Physiol. Heart Circ. Physiol. 309, H1375–H1389 (2015).
Cencioni, C. et al. Sirtuin function in aging heart and vessels. J. Mol. Cell. Cardiol. 83, 55–61 (2015).
Winnik, S., Auwerx, J., Sinclair, D. A. & Matter, C. M. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur. Heart J. 36, 3404–3412 (2015).
Tang, X. et al. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation 136, 2051–2067 (2017).
Alcendor, R. R. et al. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ. Res. 100, 1512–1521 (2007).
Canto, C. & Auwerx, J. Caloric restriction, SIRT1 and longevity. Trends Endocrinol. Metab. 20, 325–331 (2009).
Hariharan, N. et al. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ. Res. 107, 1470–1482 (2010).
Hsu, C. P. et al. Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122, 2170–2182 (2010).
D’Onofrio, N., Servillo, L. & Balestrieri, M. L. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid. Redox Signal 28, 711–732 (2018).
Spadari, R. C. et al. Role of β-adrenergic receptors and sirtuin signaling in the heart during aging, heart failure, and adaptation to stress. Cell. Mol. Neurobiol. 38, 109–120 (2018).
Wang, L. et al. Cardiomyocyte specific deletion of Sirt1 gene sensitizes myocardium to ischemia and reperfusion injury. Cardiovasc. Res. 114, 805–821 (2018).
Gomes, P., Outeiro, T. F. & Cavadas, C. Emerging role of Sirtuin 2 in the regulation of mammalian metabolism. Trends Pharmacol. Sci. 36, 756–768 (2015).
Elkhwanky, M. S. & Hakkola, J. Extranuclear sirtuins and metabolic stress. Antioxid. Redox Signal 28, 662–676 (2018).
Tang, X., Chen, X. F., Chen, H. Z. & Liu, D. P. Mitochondrial Sirtuins in cardiometabolic diseases. Clin. Sci. 131, 2063–2078 (2017).
Wood, J. G. et al. Sirt4 is a mitochondrial regulator of metabolism and lifespan in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 115, 1564–1569 (2018).
Vitiello, M. et al. Multiple pathways of SIRT6 at the crossroads in the control of longevity, cancer, and cardiovascular diseases. Ageing Res. Rev. 35, 301–311 (2017).
Sundaresan, N. R. et al. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat. Med. 18, 1643–1650 (2012).
Vakhrusheva, O. et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ. Res. 102, 703–710 (2008).
Ryu, D. et al. A SIRT7-dependent acetylation switch of GABPbeta1 controls mitochondrial function. Cell Metab. 20, 856–869 (2014).
Araki, S. et al. Sirt7 contributes to myocardial tissue repair by maintaining transforming growth factor-β signaling pathway. Circulation 132, 1081–1093 (2015).
Andersen, J. S. et al. Nucleolar proteome dynamics. Nature 433, 77–83 (2005).
Siddiqi, S. et al. Myocardial induction of nucleostemin in response to postnatal growth and pathological challenge. Circ. Res. 103, 89–97 (2008).
Avitabile, D. et al. Nucleolar stress is an early response to myocardial damage involving nucleolar proteins nucleostemin and nucleophosmin. Proc. Natl Acad. Sci. USA 108, 6145–6150 (2011).
Hariharan, N. et al. Nucleostemin rejuvenates cardiac progenitor cells and antagonizes myocardial aging. J. Am. Coll. Cardiol. 65, 133–147 (2015).
Lee, N. et al. Comparative interactomes of SIRT6 and SIRT7: implication of functional links to aging. Proteomics 14, 1610–1622 (2014).
Donlon, T. A. et al. Analysis of polymorphisms in 59 potential candidate genes for association with human longevity. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.org/10.1093/gerona/glx247 (2017).
Santulli, G. et al. Models for preclinical studies in aging-related disorders: one is not for all. Transl Med. UniSa 13, 4–12 (2015).
Koks, S. et al. Mouse models of ageing and their relevance to disease. Mech. Ageing Dev. 160, 41–53 (2016).
Tsang, H. G. et al. Large animal models of cardiovascular disease. Cell Biochem. Funct. 34, 113–132 (2016).
Blackburn, E. H. & Gall, J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J. Mol. Biol. 120, 33–53 (1978).
Blackburn, E. H. et al. Recognition and elongation of telomeres by telomerase. Genome 31, 553–560 (1989).
Allsopp, R. C. et al. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl Acad. Sci. USA 89, 10114–10118 (1992).
Feng, J. et al. The RNA component of human telomerase. Science 269, 1236–1241 (1995).
Blasco, M. A., Funk, W., Villeponteau, B. & Greider, C. W. Functional characterization and developmental regulation of mouse telomerase RNA. Science 269, 1267–1270 (1995).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25–34 (1997).
Lee, H. W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Hande, M. P., Samper, E., Lansdorp, P. & Blasco, M. A. Telomere length dynamics and chromosomal instability in cells derived from telomerase null mice. J. Cell Biol. 144, 589–601 (1999).
Rudolph, K. L. et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell 96, 701–712 (1999).
Goytisolo, F. A. & Blasco, M. A. Many ways to telomere dysfunction: in vivo studies using mouse models. Oncogene 21, 584–591 (2002).
Cheong, C., Hong, K. U. & Lee, H. W. Mouse models for telomere and telomerase biology. Exp. Mol. Med. 35, 141–153 (2003).
Chiang, Y. J. et al. Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo. Mol. Cell. Biol. 24, 7024–7031 (2004).
Leri, A. et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 22, 131–139 (2003).
Perez-Rivero, G. et al. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation 114, 309–317 (2006).
DiMario, J. X., Uzman, A. & Strohman, R. C. Fiber regeneration is not persistent in dystrophic (MDX) mouse skeletal muscle. Dev. Biol. 148, 314–321 (1991).
Straub, V., Rafael, J. A., Chamberlain, J. S. & Campbell, K. P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 139, 375–385 (1997).
Sacco, A. et al. Short telomeres and stem cell exhaustion model Duchenne muscular dystrophy in mdx/mTR mice. Cell 143, 1059–1071 (2010).
Mourkioti, F. et al. Role of telomere dysfunction in cardiac failure in Duchenne muscular dystrophy. Nat. Cell Biol. 15, 895–904 (2013).
Chang, A. C. et al. Telomere shortening and metabolic compromise underlie dystrophic cardiomyopathy. Proc. Natl Acad. Sci. USA 113, 13120–13125 (2016).
Theodoris, C. V. et al. Long telomeres protect against age-dependent cardiac disease caused by NOTCH1 haploinsufficiency. J. Clin. Invest. 127, 1683–1688 (2017).
Pekovic, V. & Hutchison, C. J. Adult stem cell maintenance and tissue regeneration in the ageing context: the role for A-type lamins as intrinsic modulators of ageing in adult stem cells and their niches. J. Anat. 213, 5–25 (2008).
Pacheco, L. M. et al. Progerin expression disrupts critical adult stem cell functions involved in tissue repair. Aging 6, 1049–1063 (2014).
Mounkes, L. C., Kozlov, S., Hernandez, L., Sullivan, T. & Stewart, C. L. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423, 298–301 (2003).
Arimura, T. et al. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 14, 155–169 (2005).
Mounkes, L. C., Kozlov, S. V., Rottman, J. N. & Stewart, C. L. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum. Mol. Genet. 14, 2167–2180 (2005).
Wang, Y., Herron, A. J. & Worman, H. J. Pathology and nuclear abnormalities in hearts of transgenic mice expressing M371K lamin A encoded by an LMNA mutation causing Emery-Dreifuss muscular dystrophy. Hum. Mol. Genet. 15, 2479–2489 (2006).
Lu, D. et al. LMNA E82K mutation activates FAS and mitochondrial pathways of apoptosis in heart tissue specific transgenic mice. PLoS ONE 5, e15167 (2010).
Osorio, F. G. et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci. Transl Med. 3, 106ra107 (2011).
Bertrand, A. T. et al. DelK32-lamin A/C has abnormal location and induces incomplete tissue maturation and severe metabolic defects leading to premature death. Hum. Mol. Genet. 21, 1037–1048 (2012).
Zhang, H., Kieckhaefer, J. E. & Cao, K. Mouse models of laminopathies. Aging Cell 12, 2–10 (2013).
Sullivan, T. et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J. Cell Biol. 147, 913–920 (1999).
Ramos, F. J. et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl Med. 4, 144ra103 (2012).
Gurau, F. et al. Anti-senescence compounds: a potential nutraceutical approach to healthy aging. Ageing Res. Rev. 46, 14–31 (2018).
Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 22, 78–83 (2016).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Baar, M. P. et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 169, 132–147 (2017).
Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236 (2011).
Baker, D. J. et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 530, 184–189 (2016).
Scudellari, M. To stay young, kill zombie cells. Nature 550, 448–450 (2017).
Childs, B. G. et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 16, 718–735 (2017).
Kirkland, J. L. & Tchkonia, T. Cellular senescence: a translational perspective. EBioMedicine 21, 21–28 (2017).
Jeon, O. H. et al. Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat. Med. 23, 775–781 (2017).
Steenman, M. & Lande, G. Cardiac aging and heart disease in humans. Biophys. Rev. 9, 131–137 (2017).
Bhatia-Dey, N., Kanherkar, R. R., Stair, S. E., Makarev, E. O. & Csoka, A. B. Cellular senescence as the causal nexus of aging. Front. Genet. 7, 13 (2016).
Aunan, J. R., Cho, W. C. & Soreide, K. The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. 8, 628–642 (2017).
Seals, D. R., Brunt, V. E. & Rossman, M. J. Strategies for optimal cardiovascular aging. Am. J. Physiol. Heart Circ. Physiol. https://doi.org/10.1152/ajpheart.00734.2017 (2018).
Aiello, A. et al. Nutrigerontology: a key for achieving successful ageing and longevity. Immun. Ageing 13, 17 (2016).
Eisenberg, T. et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22, 1428–1438 (2016).
Zhang, H. et al. Spermine and spermidine reversed age-related cardiac deterioration in rats. Oncotarget 8, 64793–64808 (2017).
Bernardes de Jesus, B. et al. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 10, 604–621 (2011).
Harley, C. B., Liu, W., Flom, P. L. & Raffaele, J. M. A natural product telomerase activator as part of a health maintenance program: metabolic and cardiovascular response. Rejuven. Res. 16, 386–395 (2013).
Salvador, L. et al. A natural product telomerase activator lengthens telomeres in humans: a randomized, double blind, and placebo controlled study. Rejuven. Res. 19, 478–484 (2016).
Martin-Montalvo, A. et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192 (2013).
Barzilai, N., Crandall, J. P., Kritchevsky, S. B. & Espeland, M. A. Metformin as a tool to target aging. Cell Metab. 23, 1060–1065 (2016).
Hershberger, K. A., Martin, A. S. & Hirschey, M. D. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat. Rev. Nephrol. 13, 213–225 (2017).
Mitchell, S. J. et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 6, 836–843 (2014).
Trevino-Saldana, N. & Garcia-Rivas, G. Regulation of sirtuin-mediated protein deacetylation by cardioprotective phytochemicals. Oxid. Med. Cell Longev. 2017, 1750306 (2017).
Jiang, S. et al. Curcumin as a potential protective compound against cardiac diseases. Pharmacol. Res. 119, 373–383 (2017).
Saeidinia, A. et al. Curcumin in heart failure: a choice for complementary therapy? Pharmacol. Res. 131, 112–119 (2018).
Tripathi, V., Chhabria, S., Jadhav, V., Bhartiya, D. & Tripathi, A. Stem cells and progenitors in human peripheral blood get activated by extremely active resveratrol (XAR). Stem Cell Rev. 14, 213–222 (2017).
Fujitsuka, N. et al. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol. Psychiatry 21, 1613–1623 (2016).
Yang, Z. & Ming, X. F. mTOR signalling: the molecular interface connecting metabolic stress, aging and cardiovascular diseases. Obes. Rev. 13 (Suppl. 2), 58–68 (2012).
Weichhart, T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology 64, 127–134 (2018).
Volkers, M. et al. Mechanistic target of rapamycin complex 2 protects the heart from ischemic damage. Circulation 128, 2132–2144 (2013).
Sciarretta, S., Forte, M., Frati, G. & Sadoshima, J. New insights into the role of mTOR signaling in the cardiovascular system. Circ. Res. 122, 489–505 (2018).
Volkers, M. et al. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol. Med. 6, 57–65 (2014).
Mannick, J. B. et al. mTOR inhibition improves immune function in the elderly. Sci. Transl Med. 6, 268ra179 (2014).
Stephens, A. D. et al. Chromatin histone modifications and rigidity affect nuclear morphology independent of lamins. Mol. Biol. Cell 29, 220–233 (2018).
Gilham, D. et al. RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atherosclerosis 247, 48–57 (2016).
Shin, D. G. & Bayarsaihan, D. A. Novel epi-drug therapy based on the suppression of BET family epigenetic readers. Yale J. Biol. Med. 90, 63–71 (2017).
Costantino, S. et al. Epigenetics and cardiovascular regenerative medicine in the elderly. Int. J. Cardiol. 250, 207–214 (2018).
Long, C. et al. Correction of diverse muscular dystrophy mutations in human engineered heart muscle by single-site genome editing. Sci. Adv. 4, eaap9004 (2018).
Zhang, Y., Long, C., Bassel-Duby, R. & Olson, E. N. Myoediting: toward prevention of muscular dystrophy by therapeutic genome editing. Physiol. Rev. 98, 1205–1240 (2018).
Lewis, F. C., Kumar, S. D. & Ellison-Hughes, G. M. Non-invasive strategies for stimulating endogenous repair and regenerative mechanisms in the damaged heart. Pharmacol. Res. 127, 33–40 (2018).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Conboy, I. M. & Rando, T. A. Heterochronic parabiosis for the study of the effects of aging on stem cells and their niches. Cell Cycle 11, 2260–2267 (2012).
Rando, T. A. & Finkel, T. Cardiac aging and rejuvenation—a sense of humors? N. Engl. J. Med. 369, 575–576 (2013).
Loffredo, F. S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Du, G. Q. et al. Targeted myocardial delivery of GDF11 gene rejuvenates the aged mouse heart and enhances myocardial regeneration after ischemia-reperfusion injury. Bas. Res. Cardiol. 112, 7 (2017).
Zimmers, T. A. et al. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Bas. Res. Cardiol. 112, 48 (2017).
Smith, S. C. et al. GDF11 does not rescue aging-related pathological hypertrophy. Circ. Res. 117, 926–932 (2015).
Harper, S. C. et al. Is growth differentiation factor 11 a realistic therapeutic for aging-dependent muscle defects? Circ. Res. 118, 1143–1150 (2016).
Hong, K. U. & Bolli, R. Cardiac stem cell therapy for cardiac repair. Curr. Treat. Opt. Cardiovasc. Med. 16, 324 (2014).
Bolli, R. et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 (2011).
Chugh, A. R. et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126, S54–S64 (2012).
Ren, R., Ocampo, A., Liu, G. H. & Izpisua Belmonte, J. C. Regulation of Stem Cell Aging by Metabolism and Epigenetics. Cell Metab. 26, 460–474 (2017).
Fischer, K. M. et al. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 120, 2077–2087 (2009).
Marotta, P. et al. Combining cell and gene therapy to advance cardiac regeneration. Expert Opin. Biol. Ther. 18, 409–423 (2018).
Hu, X. et al. A large-scale investigation of hypoxia-preconditioned allogeneic mesenchymal stem cells for myocardial repair in nonhuman primates: paracrine activity without remuscularization. Circ. Res. 118, 970–983 (2016).
Zhang, Z. et al. Autophagy mediates the beneficial effect of hypoxic preconditioning on bone marrow mesenchymal stem cells for the therapy of myocardial infarction. Stem Cell Res. Ther. 8, 89 (2017).
Quijada, P. et al. Cardiac stem cell hybrids enhance myocardial repair. Circ. Res. 117, 695–706 (2015).
Finan, A. & Richard, S. Stimulating endogenous cardiac repair. Front. Cell Dev. Biol. 3, 57 (2015).
Jung, J. H., Fu, X. & Yang, P. C. Exosomes generated from ipsc-derivatives: new direction for stem cell therapy in human heart diseases. Circ. Res. 120, 407–417 (2017).
Yang, P. C. Induced pluripotent stem cell (iPSC)-derived exosomes for precision medicine in heart failure. Circ. Res. 122, 661–663 (2018).
Huhne, R., Thalheim, T. & Suhnel, J. AgeFactDB — the JenAge Ageing Factor Database — towards data integration in ageing research. Nucleic Acids Res. 42, D892–D896 (2014).
Zahn, J. M. et al. AGEMAP: a gene expression database for aging in mice. PLOS Genet. 3, e201 (2007).
Kwon, Y., Natori, Y. & Tanokura, M. New approach to generating insights for aging research based on literature mining and knowledge integration. PLoS ONE 12, e0183534 (2017).
Nakayama, H., Nishida, K. & Otsu, K. Macromolecular degradation systems and cardiovascular aging. Circ. Res. 118, 1577–1592 (2016).
Xia, S. et al. An update on inflamm-aging: mechanisms, prevention, and treatment. J. Immunol. Res. 2016, 8426874 (2016).
Shi, R., Guberman, M. & Kirshenbaum, L. A. Mitochondrial quality control: The role of mitophagy in aging. Trends Cardiovasc. Med. 28, 246–260 (2018).
Sun, N., Youle, R. J. & Finkel, T. The mitochondrial basis of aging. Mol. Cell 61, 654–666 (2016).
Karuppagounder, V. et al. The senescence accelerated mouse prone 8 (SAMP8): A novel murine model for cardiac aging. Ageing Res. Rev. 35, 291–296 (2017).
Din, S. et al. Metabolic dysfunction consistent with premature aging results from deletion of Pim kinases. Circ. Res. 115, 376–387 (2014).
Kubben, N. et al. Post-natal myogenic and adipogenic developmental: defects and metabolic impairment upon loss of A-type lamins. Nucleus 2, 195–207 (2011).
Pendas, A. M. et al. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31, 94–99 (2002).
Chang, S. et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat. Genet. 36, 877–882 (2004).
Wijshake, T. et al. Reduced life- and healthspan in mice carrying a mono-allelic BubR1 MVA mutation. PLoS Genet. 8, e1003138 (2012).
Matsumoto, T. et al. Aging-associated vascular phenotype in mutant mice with low levels of BubR1. Stroke 38, 1050–1056 (2007).
Chouchani, E. T. et al. Complex I deficiency due to selective loss of Ndufs4 in the mouse heart results in severe hypertrophic cardiomyopathy. PLoS ONE 9, e94157 (2014).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Nurminen, A., Farnum, G. A. & Kaguni, L. S. Pathogenicity in POLG syndromes: DNA polymerase gamma pathogenicity prediction server and database. BBA Clin. 7, 147–156 (2017).
Acehan, D. et al. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J. Biol. Chem. 286, 899–908 (2011).
Soustek, M. S. et al. Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency. Hum. Gene Ther. 22, 865–871 (2011).
Graham, B. H. et al. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat. Genet. 16, 226–234 (1997).
Strauss, K. A. et al. Severity of cardiomyopathy associated with adenine nucleotide translocator-1 deficiency correlates with mtDNA haplogroup. Proc. Natl Acad. Sci. USA 110, 3453–3458 (2013).
Pfeffer, J. M., Pfeffer, M. A., Fishbein, M. C. & Frohlich, E. D. Cardiac function and morphology with aging in the spontaneously hypertensive rat. Am. J. Physiol. 237, H461–H468 (1979).
Chan, V. et al. Cardiovascular changes during maturation and ageing in male and female spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 57, 469–478 (2011).
Rosa, C. M. et al. Diabetes mellitus activates fetal gene program and intensifies cardiac remodeling and oxidative stress in aged spontaneously hypertensive rats. Cardiovasc. Diabetol 12, 152 (2013).
de Castro, N. M., Yaqoob, P., de la Fuente, M., Baeza, I. & Claus, S. P. Premature impairment of methylation pathway and cardiac metabolic dysfunction in fa/fa obese Zucker rats. J. Proteome Res. 12, 1935–1945 (2013).
Niemann, B. et al. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J. Am. Coll. Cardiol. 57, 577–585 (2011).
Christoffersen, C. et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology 144, 3483–3490 (2003).
Barouch, L. A. et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ. Res. 98, 119–124 (2006).
Aasum, E., Hafstad, A. D., Severson, D. L. & Larsen, T. S. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes 52, 434–441 (2003).
Borgarelli, M. & Buchanan, J. W. Historical review, epidemiology and natural history of degenerative mitral valve disease. J. Vet. Cardiol. 14, 93–101 (2012).
Petzoldt, M. et al. Reliability of transcardiopulmonary thermodilution cardiac output measurement in experimental aortic valve insufficiency. PLoS ONE 12, e0186481 (2017).
Gerrity, R. G., Natarajan, R., Nadler, J. L. & Kimsey, T. Diabetes-induced accelerated atherosclerosis in swine. Diabetes 50, 1654–1665 (2001).
Hamamdzic, D. & Wilensky, R. L. Porcine models of accelerated coronary atherosclerosis: role of diabetes mellitus and hypercholesterolemia. J. Diabetes Res. 2013, 761415 (2013).
Bo-Htay, C., Palee, S., Apaijai, N., Chattipakorn, S. C. & Chattipakorn, N. Effects of d-galactose-induced ageing on the heart and its potential interventions. J. Cell. Mol. Med. 22, 1392–1410 (2018).
Laye, M. J., Thyfault, J. P., Stump, C. S. & Booth, F. W. Inactivity induces increases in abdominal fat. J. Appl. Physiol. (1985) 102, 1341–1347 (2007).
Hughson, R. L., Helm, A. & Durante, M. Heart in space: effect of the extraterrestrial environment on the cardiovascular system. Nat. Rev. Cardiol. 15, 167–180 (2018).
Fuentes, T. I. et al. Simulated microgravity exerts an age-dependent effect on the differentiation of cardiovascular progenitors isolated from the human heart. PLoS ONE 10, e0132378 (2015).
Di Giulio, C. Do we age faster in absence of gravity? Front. Physiol. 4, 134 (2013).
Caiani, E. G. et al. Objective evaluation of changes in left ventricular and atrial volumes during parabolic flight using real-time three-dimensional echocardiography. J. Appl. Physiol. (1985) 101, 460–468 (2006).
Caiani, E. G., Massabuau, P., Weinert, L., Vaida, P. & Lang, R. M. Effects of 5 days of head-down bed rest, with and without short-arm centrifugation as countermeasure, on cardiac function in males (BR-AG1 study). J. Appl. Physiol. (1985) 117, 624–632 (2014).
Demontis, G. C. et al. Human pathophysiological adaptations to the space environment. Front. Physiol. 8, 547 (2017).
Crestani, C. C. Emotional stress and cardiovascular complications in animal models: a review of the influence of stress type. Front. Physiol. 7, 251 (2016).
Acknowledgements
N.A.G. is supported by NIH grants R37HL091102, R01HL117163, R01HL105759, and U54CA132384. K.M.B. is supported by NIH grant F32HL136196. M.A.S. is supported by NIH grants R01HL067245, R37HL091102, R01HL105759, R01HL113647, R01HL117163, P01HL085577, and R01HL122525, as well as by an award from the Fondation Leducq.
Author information
Authors and Affiliations
Contributions
N.A.G. and M.A.S. researched data for the article, discussed its content, wrote the manuscript, and reviewed and edited it before submission. K.M.B. and F.F. contributed to creation of the display items before submission.
Corresponding author
Ethics declarations
Competing interests
K.M.B. has a significant interest in CardioCreate, and M.A.S. is a founding member of CardioCreate. N.A.G. and F.F. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Atlas of Gene Expression in Mouse Aging Project (AGEMAP): https://omictools.com/atlas-of-gene-expression-in-mouse-aging-project-tool
Database of Genotypes and Phenotypes (dbGaP): https://www.ncbi.nlm.nih.gov/gap
Digital Ageing Atlas (DAA): http://ageing-map.org/
Human Ageing Genomic Resources (HAGR): http://genomics.senescence.info/
JenAge Ageing Factor Database (AgeFactDB): http://agefactdb.jenage.de/
Rights and permissions
About this article
Cite this article
Gude, N.A., Broughton, K.M., Firouzi, F. et al. Cardiac ageing: extrinsic and intrinsic factors in cellular renewal and senescence. Nat Rev Cardiol 15, 523–542 (2018). https://doi.org/10.1038/s41569-018-0061-5
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-018-0061-5
This article is cited by
-
Cardiac cell senescence: molecular mechanisms, key proteins and therapeutic targets
Cell Death Discovery (2024)
-
Phosphoglycerate dehydrogenase activates PKM2 to phosphorylate histone H3T11 and attenuate cellular senescence
Nature Communications (2023)
-
Hallmarks of cardiovascular ageing
Nature Reviews Cardiology (2023)
-
Environmental and genetic predictors of human cardiovascular ageing
Nature Communications (2023)
-
Effect of aqueous extract of Indigofera tinctoria (Linn) on aging-induced inflammation and its associated left ventricular hypertrophy and fibrosis in the rat
3 Biotech (2023)