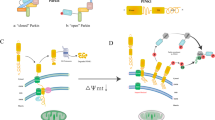
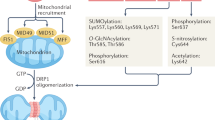
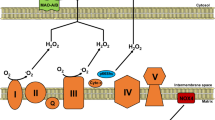
Abstract
Cardiovascular disease is the leading cause of morbidity and mortality worldwide. Advancing age is a major risk factor for developing cardiovascular disease because of the lifelong exposure to cardiovascular risk factors and specific alterations affecting the heart and the vasculature during ageing. Indeed, the ageing heart is characterized by structural and functional changes that are caused by alterations in fundamental cardiomyocyte functions. In particular, the myocardium is heavily dependent on mitochondrial oxidative metabolism and is especially susceptible to mitochondrial dysfunction. Indeed, primary alterations in mitochondrial function, which are subsequently amplified by defective quality control mechanisms, are considered to be major contributing factors to cardiac senescence. In this Review, we discuss the mechanisms linking defective mitochondrial quality control mechanisms (that is, proteostasis, biogenesis, dynamics, and autophagy) to organelle dysfunction in the context of cardiac ageing. We also illustrate relevant molecular pathways that might be exploited for the prevention and treatment of age-related heart dysfunction.
Key points
-
Older adults are especially vulnerable to developing cardiovascular disease owing to long-term exposure to risk factors and intrinsic cardiovascular alterations occurring during ageing.
-
Mitochondrial quality control (MQC) operates through the coordination of various processes (proteostasis, biogenesis, dynamics, and mitophagy) to ensure cell homeostasis.
-
Mitochondrial dysfunction, amplified by failing quality control processes, is believed to be a major mechanism underlying cardiac ageing and cardiovascular disease.
-
Preclinical evidence suggests that modulation of MQC can be harnessed for therapeutic benefit against cardiac ageing and cardiovascular disease.
-
Current unknowns include the optimal window of MQC functioning to achieve cardioprotection, the timing and intensity of interventions, and noninvasively accessible biomarkers of MQC in the heart.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Mozaffarian, D. et al. Heart disease and stroke statistics—2016 update. Circulation 133, e38–e360 (2016).
Chiao, Y. A. & Rabinovitch, P. S. The aging heart. Cold Spring Harb. Perspect. Med. 5, a025148 (2015).
Zhang, Y. et al. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic. Biol. Med. 71, 208–220 (2014).
Baris, O. R. et al. Mosaic deficiency in mitochondrial oxidative metabolism promotes cardiac arrhythmia during aging. Cell Metab. 21, 667–677 (2015).
Lok, N. S. & Lau, C. P. Prevalence of palpitations, cardiac arrhythmias and their associated risk factors in ambulant elderly. Int. J. Cardiol. 54, 231–236 (1996).
Dutta, D., Calvani, R., Bernabei, R., Leeuwenburgh, C. & Marzetti, E. Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ. Res. 110, 1125–1138 (2012).
Marzetti, E. et al. Cellular mechanisms of cardioprotection by calorie restriction: state of the science and future perspectives. Clin. Geriatr. Med. 25, 715–732 (2009).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
North, B. J. & Sinclair, D. A. The intersection between aging and cardiovascular disease. Circ. Res. 110, 1097–1108 (2012).
Theurey, P. & Pizzo, P. The aging mitochondria. Genes (Basel) 9, 22 (2018).
Hernandez-Segura, A., Nehme, J. & Demaria, M. Hallmarks of cellular senescence. Trends Cell Biol. 28, 436–453 (2018).
Garinis, G. A., van der Horst, G. T. J., Vijg, J. & Hoeijmakers, J. H. J. DNA damage and ageing: new-age ideas for an age-old problem. Nat. Cell Biol. 10, 1241–1247 (2008).
Sahin, E. et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470, 359–365 (2011).
Zierer, J. et al. Metabolomics profiling reveals novel markers for leukocyte telomere length. Aging (Albany, NY) 8, 77–94 (2016).
Yen, W.-L. & Klionsky, D. J. How to live long and prosper: autophagy, mitochondria, and aging. Physiology 23, 248–262 (2008).
Marzetti, E. et al. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am. J. Physiol. Heart Circ. Physiol. 305, H459–H476 (2013).
Wohlgemuth, S. E., Calvani, R. & Marzetti, E. The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J. Mol. Cell. Cardiol. 71, 62–70 (2014).
Chung, H. Y. et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res. Rev. 8, 18–30 (2009).
Fougère, B., Boulanger, E., Nourhashémi, F., Guyonnet, S. & Cesari, M. Chronic inflammation: accelerator of biological aging. J. Gerontol. A Biol. Sci. Med. Sci. 72, 1218–1225 (2017).
Lin, C.-C. et al. NADPH oxidase/ROS-dependent VCAM-1 induction on TNF-α-challenged human cardiac fibroblasts enhances monocyte adhesion. Front. Pharmacol. 6, 310 (2015).
Sallam, N. & Laher, I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxid. Med. Cell. Longev. 2016, 7239639 (2016).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Gimbrone, M. A. & García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016).
Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 75, 685–705 (2013).
Muñoz-Espín, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Senft, D. & Ronai, Z. A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 40, 141–148 (2015).
Sano, R. et al. Endoplasmic reticulum protein BI-1 regulates Ca2+-mediated bioenergetics to promote autophagy. Genes Dev. 26, 1041–1054 (2012).
Adam-Vizi, V. & Starkov, A. A. Calcium and mitochondrial reactive oxygen species generation: how to read the facts. J. Alzheimers Dis. 20 (Suppl. 2), S413–S426 (2010).
Andersson, D. C. et al. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 14, 196–207 (2011).
Bánsághi, S. et al. Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J. Biol. Chem. 289, 8170–8181 (2014).
Rhee, S. G. & Kil, I. S. Mitochondrial H2O2 signaling is controlled by the concerted action of peroxiredoxin III and sulfiredoxin: linking mitochondrial function to circadian rhythm. Free Radic. Biol. Med. 100, 73–80 (2016).
Manella, G. & Asher, G. The circadian nature of mitochondrial biology. Front. Endocrinol. (Lausanne) 7, 162 (2016).
Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
Duicu, O. M. et al. Ageing-induced decrease in cardiac mitochondrial function in healthy rats. Can. J. Physiol. Pharmacol. 91, 593–600 (2013).
Kuka, S. et al. Effect of aging on formation of reactive oxygen species by mitochondria of rat heart. Gen. Physiol. Biophys. 32, 415–420 (2014).
Wong, H.-S., Dighe, P. A., Mezera, V., Monternier, P.-A. & Brand, M. D. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 292, 16804–16809 (2017).
Kennedy, S. R., Salk, J. J., Schmitt, M. W. & Loeb, L. A. Ultra-sensitive sequencing reveals an age-related increase in somatic mitochondrial mutations that are inconsistent with oxidative damage. PLoS Genet. 9, e1003794 (2013).
Itsara, L. S. et al. Oxidative stress is not a major contributor to somatic mitochondrial DNA mutations. PLoS Genet. 10, e1003974 (2014).
Trifunovic, A. et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429, 417–423 (2004).
Kujoth, G. C. et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 309, 481–484 (2005).
Dai, D.-F. et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9, 536–544 (2010).
Lewis, K. N., Andziak, B., Yang, T. & Buffenstein, R. The naked mole-rat response to oxidative stress: just deal with it. Antioxid. Redox Signal. 19, 1388–1399 (2013).
Someya, S. et al. Effects of calorie restriction on the lifespan and healthspan of POLG mitochondrial mutator mice. PLoS ONE 12, e0171159 (2017).
Das, K. C. & Muniyappa, H. Age-dependent mitochondrial energy dynamics in the mice heart: role of superoxide dismutase-2. Exp. Gerontol. 48, 947–959 (2013).
Logan, A. et al. In vivo levels of mitochondrial hydrogen peroxide increase with age in mtDNA mutator mice. Aging Cell 13, 765–768 (2014).
Eisenberg, T. et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22, 1428–1438 (2016).
Feng, W. et al. Increased age-related cardiac dysfunction in bradykinin B2 receptor-deficient mice. J. Gerontol. A Biol. Sci. Med. Sci. 71, 178–187 (2016).
Marzetti, E. et al. Shorter telomeres in peripheral blood mononuclear cells from older persons with sarcopenia: results from an exploratory study. Front. Aging Neurosci. 6, 233 (2014).
Tocchi, A., Quarles, E. K., Basisty, N., Gitari, L. & Rabinovitch, P. S. Mitochondrial dysfunction in cardiac aging. Biochim. Biophys. Acta 1847, 1424–1433 (2015).
Sena, L. A. & Chandel, N. S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 48, 158–167 (2012).
Wojtovich, A. P., Nadtochiy, S. M., Brookes, P. S. & Nehrke, K. Ischemic preconditioning: the role of mitochondria and aging. Exp. Gerontol. 47, 1–7 (2012).
Mishra, P. & Chan, D. C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 212, 379–387 (2016).
Wu, H., Wei, H., Sehgal, S. A., Liu, L. & Chen, Q. Mitophagy receptors sense stress signals and couple mitochondrial dynamic machinery for mitochondrial quality control. Free Radic. Biol. Med. 100, 199–209 (2016).
Rossignol, R. et al. Mitochondrial threshold effects. Biochem. J. 370, 751–762 (2003).
Wanrooij, S. et al. In vivo mutagenesis reveals that OriL is essential for mitochondrial DNA replication. EMBO Rep. 13, 1130–1137 (2012).
Elson, J. L., Samuels, D. C., Turnbull, D. M. & Chinnery, P. F. Random intracellular drift explains the clonal expansion of mitochondrial DNA mutations with age. Am. J. Hum. Genet. 68, 802–806 (2001).
Greaves, L. C. et al. Comparison of mitochondrial mutation spectra in ageing human colonic epithelium and disease: absence of evidence for purifying selection in somatic mitochondrial DNA point mutations. PLoS Genet. 8, e1003082 (2012).
Kauppila, T. E. S., Kauppila, J. H. K. & Larsson, N.-G. Mammalian mitochondria and aging: an update. Cell Metab. 25, 57–71 (2017).
Müller-Höcker, J., Droste, M., Kadenbach, B., Pongratz, D. & Hübner, G. Fatal mitochondrial myopathy with cytochrome-c-oxidase deficiency and subunit-restricted reduction of enzyme protein in two siblings: an autopsy-immunocytochemical study. Hum. Pathol. 20, 666–672 (1989).
Cottrell, D. A. et al. Cytochrome c oxidase deficient cells accumulate in the hippocampus and choroid plexus with age. Neurobiol. Aging 22, 265–272 (2001).
Khrapko, K., Kraytsberg, Y., de Grey, A. D. N. J., Vijg, J. & Schon, E. A. Does premature aging of the mtDNA mutator mouse prove that mtDNA mutations are involved in natural aging? Aging Cell 5, 279–282 (2006).
Inoue, K. et al. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat. Genet. 26, 176–181 (2000).
Nakada, K. et al. Inter-mitochondrial complementation: mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat. Med. 7, 934–940 (2001).
Vermulst, M. et al. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat. Genet. 39, 540–543 (2007).
Meissner, C. et al. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: a useful biomarker or more? Exp. Gerontol. 43, 645–652 (2008).
Kraytsberg, Y. et al. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 38, 518–520 (2006).
Khrapko, K. & Vijg, J. Mitochondrial DNA mutations and aging: a case closed? Nat. Genet. 39, 445–446 (2007).
Srivastava, S. The mitochondrial basis of aging and age-related disorders. Genes (Basel) 8, 398 (2017).
Taylor, S. D. et al. Targeted enrichment and high-resolution digital profiling of mitochondrial DNA deletions in human brain. Aging Cell 13, 29–38 (2014).
Taylor, R. W. et al. Mitochondrial DNA mutations in human colonic crypt stem cells. J. Clin. Invest. 112, 1351–1360 (2003).
Nag, A. C. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios 28, 41–61 (1980).
Vliegen, H. W., van der Laarse, A., Cornelisse, C. J. & Eulderink, F. Myocardial changes in pressure overload-induced left ventricular hypertrophy. A study on tissue composition, polyploidization and multinucleation. Eur. Heart J. 12, 488–494 (1991).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
Bates, M. G. D. et al. Cardiac involvement in mitochondrial DNA disease: clinical spectrum, diagnosis, and management. Eur. Heart J. 33, 3023–3033 (2012).
Fischer, F., Hamann, A. & Osiewacz, H. D. Mitochondrial quality control: an integrated network of pathways. Trends Biochem. Sci. 37, 284–292 (2012).
Szklarczyk, R., Nooteboom, M. & Osiewacz, H. D. Control of mitochondrial integrity in ageing and disease. Phil. Trans. R. Soc. B Biol. Sci. 369, 20130439 (2014).
Twig, G., Hyde, B. & Shirihai, O. S. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim. Biophys. Acta 1777, 1092–1097 (2008).
Calvani, R. et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol. Chem. 394, 393–414 (2013).
Boateng, S. Y. & Goldspink, P. H. Assembly and maintenance of the sarcomere night and day. Cardiovasc. Res. 77, 667–675 (2008).
Klein, I., Samarel, A. M., Welikson, R. & Hong, C. Heterotopic cardiac transplantation decreases the capacity for rat myocardial protein synthesis. Circ. Res. 68, 1100–1107 (1991).
Razeghi, P. et al. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation 108, 2536–2541 (2003).
Patterson, C., Portbury, A. L., Schisler, J. C. & Willis, M. S. Tear me down: role of calpain in the development of cardiac ventricular hypertrophy. Circ. Res. 109, 453–462 (2011).
Portbury, A. L., Willis, M. S. & Patterson, C. Tearin’ up my heart: proteolysis in the cardiac sarcomere. J. Biol. Chem. 286, 9929–9934 (2011).
Powell, S. R. The ubiquitin-proteasome system in cardiac physiology and pathology. Am. J. Physiol. Circ. Physiol. 291, H1–H19 (2006).
Quirós, P. M., Langer, T. & López-Otín, C. New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 16, 345–359 (2015).
Voos, W. Chaperone-protease networks in mitochondrial protein homeostasis. Biochim. Biophys. Acta 1833, 388–399 (2013).
Ngo, J. K. & Davies, K. J. A. Importance of the lon protease in mitochondrial maintenance and the significance of declining lon in aging. Ann. NY Acad. Sci. 1119, 78–87 (2007).
Gispert, S. et al. Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum. Mol. Genet. 22, 4871–4887 (2013).
Cipolat, S. et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126, 163–175 (2006).
Maltecca, F. et al. The mitochondrial protease AFG3L2 is essential for axonal development. J. Neurosci. 28, 2827–2836 (2008).
Narendra, D., Tanaka, A., Suen, D.-F. & Youle, R. J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 183, 795–803 (2008).
Calì, T., Ottolini, D., Negro, A. & Brini, M. Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca2+ transfer to sustain cell bioenergetics. Biochim. Biophys. Acta 1832, 495–508 (2013).
Verfaillie, T. et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 19, 1880–1891 (2012).
Santos, C. X. C., Anilkumar, N., Zhang, M., Brewer, A. C. & Shah, A. M. Redox signaling in cardiac myocytes. Free Radic. Biol. Med. 50, 777–793 (2011).
Sumandea, M. P. & Steinberg, S. F. Redox signaling and cardiac sarcomeres. J. Biol. Chem. 286, 9921–9927 (2011).
Divald, A. et al. Myocardial ischemic preconditioning preserves postischemic function of the 26S proteasome through diminished oxidative damage to 19S regulatory particle subunits. Circ. Res. 106, 1829–1838 (2010).
Yuan, H. et al. LPS-induced autophagy is mediated by oxidative signaling in cardiomyocytes and is associated with cytoprotection. Am. J. Physiol. Heart Circ. Physiol. 296, H470–H479 (2009).
Haberland, M., Montgomery, R. L. & Olson, E. N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 10, 32–42 (2009).
Tepp, K. et al. Changes in the mitochondrial function and in the efficiency of energy transfer pathways during cardiomyocyte aging. Mol. Cell. Biochem. 432, 141–158 (2017).
Tatarková, Z. et al. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol. Res. 60, 281–289 (2011).
Esterhammer, R. et al. Cardiac high-energy phosphate metabolism alters with age as studied in 196 healthy males with the help of 31-phosphorus 2-dimensional chemical shift imaging. PLoS ONE 9, e97368 (2014).
Yaniv, Y., Juhaszova, M. & Sollott, S. J. Age-related changes of myocardial ATP supply and demand mechanisms. Trends Endocrinol. Metab. 24, 495–505 (2013).
Nathania, M. et al. Impact of age on the association between cardiac high-energy phosphate metabolism and cardiac power in women. Heart 104, 111–118 (2018).
Klepinin, A. et al. Simple oxygraphic analysis for the presence of adenylate kinase 1 and 2 in normal and tumor cells. J. Bioenerg. Biomembr. 48, 531–548 (2016).
Huss, J. M. & Kelly, D. P. Nuclear receptor signaling and cardiac energetics. Circ. Res. 95, 568–578 (2004).
Rattanasopa, C., Phungphong, S., Wattanapermpool, J. & Bupha-Intr, T. Significant role of estrogen in maintaining cardiac mitochondrial functions. J. Steroid Biochem. Mol. Biol. 147, 1–9 (2015).
Huss, J. M., Torra, I. P., Staels, B., Giguère, V. & Kelly, D. P. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol. Cell. Biol. 24, 9079–9091 (2004).
Dorn, G. W., Vega, R. B. & Kelly, D. P. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 29, 1981–1991 (2015).
Kubli, D. A. & Gustafsson, Å. B. Mitochondria and mitophagy: the yin and yang of cell death control. Circ. Res. 111, 1208–1221 (2012).
Fernandez-Marcos, P. J. & Auwerx, J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93, 884S–890S (2011).
Leone, T. C. & Kelly, D. P. Transcriptional control of cardiac fuel metabolism and mitochondrial function. Cold Spring Harb. Symp. Quant. Biol. 76, 175–182 (2011).
Karamanlidis, G. et al. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ. Res. 106, 1541–1548 (2010).
Bayeva, M., Gheorghiade, M. & Ardehali, H. Mitochondria as a therapeutic target in heart failure. J. Am. Coll. Cardiol. 61, 599–610 (2013).
Faerber, G. et al. Induction of heart failure by minimally invasive aortic constriction in mice: reduced peroxisome proliferator-activated receptor γ coactivator levels and mitochondrial dysfunction. J. Thorac. Cardiovasc. Surg. 141, 492–500.e1 (2011).
Shimizu, Y. et al. Hydrogen sulfide regulates cardiac mitochondrial biogenesis via the activation of AMPK. J. Mol. Cell. Cardiol. 116, 29–40 (2018).
Picca, A. & Lezza, A. M. S. Regulation of mitochondrial biogenesis through TFAM-mitochondrial DNA interactions. Useful insights from aging and calorie restriction studies. Mitochondrion 25, 67–75 (2015).
Anmann, T. et al. Formation of highly organized intracellular structure and energy metabolism in cardiac muscle cells during postnatal development of rat heart. Biochim. Biophys. Acta 1837, 1350–1361 (2014).
Palmer, J. W., Tandler, B. & Hoppel, C. L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 252, 8731–8739 (1977).
Palmer, J. W., Tandler, B. & Hoppel, C. L. Biochemical differences between subsarcolemmal and interfibrillar mitochondria from rat cardiac muscle: effects of procedural manipulations. Arch. Biochem. Biophys. 236, 691–702 (1985).
Ichas, F., Jouaville, L. S. & Mazat, J. P. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89, 1145–1153 (1997).
Glancy, B. et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature 523, 617–620 (2015).
Amchenkova, A. A., Bakeeva, L. E., Chentsov, Y. S., Skulachev, V. P. & Zorov, D. B. Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J. Cell Biol. 107, 481–495 (1988).
Glancy, B. et al. Power grid protection of the muscle mitochondrial reticulum. Cell Rep. 19, 487–496 (2017).
El’darov, C. M., Vays, V. B., Vangeli, I. M., Kolosova, N. G. & Bakeeva, L. E. Morphometric examination of mitochondrial ultrastructure in aging cardiomyocytes. Biochemistry (Mosc.) 80, 604–609 (2015).
Tate, E. L. & Herbener, G. H. A morphometric study of the density of mitochondrial cristae in heart and liver of aging mice. J. Gerontol. 31, 129–134 (1976).
Chen, H. et al. Titration of mitochondrial fusion rescues Mff-deficient cardiomyopathy. J. Cell Biol. 211, 795–805 (2015).
Song, M., Mihara, K., Chen, Y., Scorrano, L. & Dorn, G. W. Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 21, 273–286 (2015).
Song, M., Franco, A., Fleischer, J. A., Zhang, L. & Dorn, G. W. Abrogating mitochondrial dynamics in mouse hearts accelerates mitochondrial senescence. Cell Metab. 26, 872–883.e5 (2017).
Burman, J. L. et al. Mitochondrial fission facilitates the selective mitophagy of protein aggregates. J. Cell Biol. 216, 3231–3247 (2017).
Parone, P. A. et al. Preventing mitochondrial fission impairs mitochondrial function and leads to loss of mitochondrial DNA. PLoS ONE 3, e3257 (2008).
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Sun, N. et al. Measuring in vivo mitophagy. Mol. Cell 60, 685–696 (2015).
Lemasters, J. J. Variants of mitochondrial autophagy: types 1 and 2 mitophagy and micromitophagy (type 3). Redox Biol. 2, 749–754 (2014).
Youle, R. J. & Narendra, D. P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14 (2011).
Jin, S. M. et al. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 191, 933–942 (2010).
Chen, Y. & Dorn, G. W. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340, 471–475 (2013).
Okatsu, K. et al. Phosphorylated ubiquitin chain is the genuine Parkin receptor. J. Cell Biol. 209, 111–128 (2015).
Koyano, F. et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature 510, 162–166 (2014).
Sarraf, S. A. et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496, 372–376 (2013).
Pankiv, S. et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 282, 24131–24145 (2007).
Lazarou, M. et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314 (2015).
Murakawa, T. et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 6, 7527 (2015).
Chen, Y. et al. Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc. Natl Acad. Sci. USA 107, 9035–9042 (2010).
Hanna, R. A. et al. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J. Biol. Chem. 287, 19094–19104 (2012).
Liu, L. et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185 (2012).
Soubannier, V. et al. A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Curr. Biol. 22, 135–141 (2012).
McLelland, G.-L., Soubannier, V., Chen, C. X., McBride, H. M. & Fon, E. A. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 33, 282–295 (2014).
Zhou, J. et al. Changes in macroautophagy, chaperone-mediated autophagy, and mitochondrial metabolism in murine skeletal and cardiac muscle during aging. Aging (Albany, NY) 9, 583–599 (2017).
Peng, L. et al. Changes in cell autophagy and apoptosis during age-related left ventricular remodeling in mice and their potential mechanisms. Biochem. Biophys. Res. Commun. 430, 822–826 (2013).
Zhang, Y. et al. Complex inhibition of autophagy by mitochondrial aldehyde dehydrogenase shortens lifespan and exacerbates cardiac aging. Biochim. Biophys. Acta 1863, 1919–1932 (2017).
Shirakabe, A., Ikeda, Y., Sciarretta, S., Zablocki, D. K. & Sadoshima, J. Aging and autophagy in the heart. Circ. Res. 118, 1563–1576 (2016).
Blice-Baum, A. C. et al. Modest overexpression of FOXO maintains cardiac proteostasis and ameliorates age-associated functional decline. Aging Cell 16, 93–103 (2017).
Chun, S. K. et al. Autophagy in ischemic livers: a critical role of sirtuin 1/mitofusin 2 axis in autophagy induction. Toxicol. Res. 32, 35–46 (2016).
Huang, R. et al. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol. Cell 57, 456–466 (2015).
Ferrara, N. et al. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 11, 139–150 (2008).
Ren, J. et al. Akt2 ablation prolongs life span and improves myocardial contractile function with adaptive cardiac remodeling: role of Sirt1-mediated autophagy regulation. Aging Cell 16, 976–987 (2017).
Hsu, Y.-J. et al. Sirtuin 1 protects the aging heart from contractile dysfunction mediated through the inhibition of endoplasmic reticulum stress-mediated apoptosis in cardiac-specific Sirtuin 1 knockout mouse model. Int. J. Cardiol. 228, 543–552 (2017).
Hoshino, A. et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 4, 2308 (2013).
Edwards, M. G. et al. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics 8, 80 (2007).
Tan, V. P. & Miyamoto, S. Nutrient-sensing mTORC1: Integration of metabolic and autophagic signals. J. Mol. Cell. Cardiol. 95, 31–41 (2016).
Egan, D., Kim, J., Shaw, R. J. & Guan, K.-L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7, 643–644 (2011).
Martina, J. A., Chen, Y., Gucek, M. & Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 8, 903–914 (2012).
Maejima, Y., Isobe, M. & Sadoshima, J. Regulation of autophagy by Beclin 1 in the heart. J. Mol. Cell. Cardiol. 95, 19–25 (2016).
Zalckvar, E. et al. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 10, 285–292 (2009).
Gurkar, A. U. et al. Identification of ROCK1 kinase as a critical regulator of Beclin1-mediated autophagy during metabolic stress. Nat. Commun. 4, 2189 (2013).
Wei, Y., Pattingre, S., Sinha, S., Bassik, M. & Levine, B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell 30, 678–688 (2008).
Ikeda, Y. et al. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ. Res. 116, 264–278 (2015).
Troncoso, R. et al. Energy-preserving effects of IGF-1 antagonize starvation-induced cardiac autophagy. Cardiovasc. Res. 93, 320–329 (2012).
Sciarretta, S. et al. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 125, 1134–1146 (2012).
Cuervo, A. M. Calorie restriction and aging: the ultimate “cleansing diet”. J. Gerontol. A Biol. Sci. Med. Sci. 63, 547–549 (2008).
Tóth, M. L. et al. Longevity pathways converge on autophagy genes to regulate life span in Caenorhabditis elegans. Autophagy 4, 330–338 (2008).
Wohlgemuth, S. E. et al. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 10, 281–292 (2007).
Shinmura, K. et al. Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J. Mol. Cell. Cardiol. 50, 117–127 (2011).
Han, X. et al. Influence of long-term caloric restriction on myocardial and cardiomyocyte contractile function and autophagy in mice. J. Nutr. Biochem. 23, 1592–1599 (2012).
Delbridge, L. M. D., Mellor, K. M., Taylor, D. J. & Gottlieb, R. A. Myocardial stress and autophagy: mechanisms and potential therapies. Nat. Rev. Cardiol. 14, 412–425 (2017).
Acknowledgements
The authors acknowledge support from Fondazione Roma (NCDs Call for Proposals 2013), Innovative Medicine Initiative-Joint Undertaking (IMI-JU 115621), intramural research grants from the Catholic University of the Sacred Heart (D3.2 2013 and D3.2 2015), the non-profit research foundation “Centro Studi Achille e Linda Lorenzon”, and the Claude D. Pepper Older Americans Independence Center at the University of Florida’s Institute on Aging (NIA 1P30AG028740).
Reviewer information
Nature Reviews Cardiology thanks E. Lesnefsky and the other anonymous reviewers for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
A.P. and R.T.M. researched data for the article. A.P., J.L.B., and J.-S.K. discussed the content of the article. A.P., R.T.M., L.D., and E.M. wrote the manuscript. E.M. and C.L. revised and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Picca, A., Mankowski, R.T., Burman, J.L. et al. Mitochondrial quality control mechanisms as molecular targets in cardiac ageing. Nat Rev Cardiol 15, 543–554 (2018). https://doi.org/10.1038/s41569-018-0059-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-018-0059-z
This article is cited by
-
Exploring the potential link between MitoEVs and the immune microenvironment of periodontitis based on machine learning and bioinformatics methods
BMC Oral Health (2024)
-
The clarithromycin-binding proteins NIPSNAP1 and 2 regulate cytokine production through mitochondrial quality control
Scientific Reports (2024)
-
Renal aging and mitochondrial quality control
Biogerontology (2024)
-
ATF5 regulates tubulointerstitial injury in diabetic kidney disease via mitochondrial unfolded protein response
Molecular Medicine (2023)
-
SS-31 alleviated nociceptive responses and restored mitochondrial function in a headache mouse model via Sirt3/Pgc-1α positive feedback loop
The Journal of Headache and Pain (2023)