Abstract
In patients with manifestations of cardiovascular disease, acetylsalicylic acid (popularly known as aspirin) has been the mainstay of treatment for decades owing to its capacity to reduce the risk of ischaemic events. Accordingly, novel antithrombotic therapies have been traditionally tested on a background of acetylsalicylic acid therapy. Although the adjunctive use of such antithrombotic therapies can potentially further reduce the risk of ischaemic events, these agents are also inevitably associated with an increased risk of bleeding. However, acetylsalicylic acid also increases the risk of bleeding, challenging the paradigm that this agent should remain the cornerstone of antiplatelet treatment when alternative antithrombotic agents are also used. Many antithrombotic compounds are characterized by increased potency and consistent efficacy, which might lessen the need for concomitant acetylsalicylic acid. Accordingly, numerous investigations are testing the hypothesis that acetylsalicylic acid-sparing regimens based on newer antithrombotic agents might have an increased net benefit for individual patients owing to the reduction in bleeding risk, without a trade-off in efficacy. This Review summarizes the state of the art relating to antithrombotic approaches with and without acetylsalicylic acid for the prevention of cardiovascular disease and cardioembolic stroke. Discussion of the scientific rationale, from bench to bedside, for ongoing studies of acetylsalicylic acid-free pharmacological strategies is included.
Key points
-
Most new antithrombotic treatment strategies aimed at further outcome improvement have been developed with acetylsalicylic acid as background therapy.
-
Given that acetylsalicylic acid increases bleeding risk, a number of studies are exploring the possibility of avoiding this drug in the presence of other antithrombotic agents.
-
Pharmacodynamic investigations indicate that no other antithrombotic agent can replace the cyclooxygenase 1-selective, platelet-inhibitory effects of acetylsalicylic acid; however, many newer antithrombotic therapies might have greater antithrombotic efficacy.
-
Given the established role of acetylsalicylic acid in cardiovascular disease management and prevention, favourable results from large-scale clinical trials are warranted before acetylsalicylic acid-free strategies are recommended for routine clinical practice.
-
Acetylsalicylic acid is cost effective and has favourable noncardiac effects, which are under ongoing investigation and need to be taken into account when considering acetylsalicylic acid-free approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




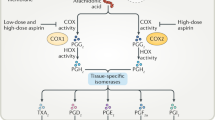
Adapted with permission from ref.81, John Wiley and Sons.


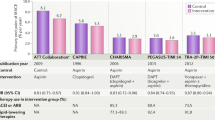
Adapted from ref.131, CC-BY-4.0.
Similar content being viewed by others
References
Desborough, M. J. R. & Keeling, D. M. The aspirin story — from willow to wonder drug. Br. J. Haematol. 177, 674–683 (2017).
Lafont, O. From the willow to aspirin [French]. Rev. Hist. Pharm. 55, 209–216 (2007).
Weiss, H. J. The discovery of the antiplatelet effect of aspirin: a personal reminiscence. J. Thromb. Haemost. 1, 1869–1875 (2003).
Patrono, C., García Rodríguez, L. A., Landolfi, R. & Baigent, C. Low-dose aspirin for the prevention of atherothrombosis. N. Engl. J. Med. 353, 2373–2383 (2005).
Patrono, C. & Rocca, B. Aspirin: promise and resistance in the new millennium. Arterioscler. Thromb. Vasc. Biol. 28, s25–s32 (2008).
Graham, M. M. et al. Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery. Ann. Intern. Med. 168, 237–244 (2018).
Sundström, J. et al. Low-dose aspirin discontinuation and risk of cardiovascular events. Circulation 136, 1183–1192 (2017).
Roffi, M. et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 37, 267–315 (2016).
Amsterdam, E. A. et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 130, e344–e426 (2014).
Ibanez, B. et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 39, 119–177 (2018).
O’Gara, P. T. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 127, e362–e425 (2013).
Task Force Members et al. 2013 ESC Guidelines on the management of stable coronary artery disease. Eur. Heart J. 34, 2949–3003 (2013).
Fihn, S. D. et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 126, e354–e471 (2012).
Authors/Task Force members et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 35, 2541–2619 (2014).
Levine, G. N. et al. 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 124, 2574–2609 (2011).
Piepoli, M. F. et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 37, 2315–2381 (2016).
Smith, S. C. et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 124, 2458–2473 (2011).
Capodanno, D. & Angiolillo, D. J. Aspirin for primary cardiovascular risk prevention and beyond in diabetes mellitus. Circulation 134, 1579–1594 (2016).
Moon, J. Y., Franchi, F., Rollini, F. & Angiolillo, D. J. The quest for safer antithrombotic treatment regimens in patients with coronary artery disease: new strategies and paradigm shifts. Expert Rev. Hematol. 11, 5–12 (2018).
Miyazaki, Y. et al. Single or dual antiplatelet therapy after PCI. Nat. Rev. Cardiol. 14, 294–303 (2017).
Gargiulo, G. et al. A Critical appraisal of aspirin in secondary prevention. Circulation 134, 1881–1906 (2016).
Bhatt, D. L. Antithrombotic therapy in 2017: Advances in atherosclerosis, atrial fibrillation, and valvular disease. Nat. Rev. Cardiol. 15, 71–72 (2018).
Antithrombotic Trialists’ (ATT) Collaboration. et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 373, 1849–1860 (2009).
Patrono, C. The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. J. Am. Coll. Cardiol. 66, 74–85 (2015).
Guirguis-Blake, J. M., Evans, C. V., Senger, C. A., O’Connor, E. A. & Whitlock, E. P. Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U. S. Preventive Services Task Force. Ann. Intern. Med. 164, 804–813 (2016).
Whitlock, E. P., Burda, B. U., Williams, S. B., Guirguis-Blake, J. M. & Evans, C. V. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U. S. Preventive Services Task Force. Ann. Intern. Med. 164, 826 (2016).
Hart, R. G., Pearce, L. A. & Aguilar, M. I. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann. Intern. Med. 146, 857–867 (2007).
[No authors listed]. Stroke prevention in atrial fibrillation study. Final results. Circulation 84, 527–539 (1991).
Miller, V. T. et al. Ischemic stroke in patients with atrial fibrillation: effect of aspirin according to stroke mechanism. Stroke Prevention in Atrial Fibrillation Investigators. Neurology 43, 32–36 (1993).
Gargiulo, G., Capodanno, D., Longo, G., Capranzano, P. & Tamburino, C. Updates on NSAIDs in patients with and without coronary artery disease: pitfalls, interactions and cardiovascular outcomes. Expert Rev. Cardiovasc. Ther. 12, 1185–1203 (2014).
Patrignani, P. & Patrono, C. Aspirin and cancer. J. Am. Coll. Cardiol. 68, 967–976 (2016).
Chubak, J. et al. Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U. S. Preventive Services Task Force. Ann. Intern. Med. 164, 814 (2016).
Bibbins-Domingo, K. et al. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U. S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 164, 836 (2016).
Yusuf, S. et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 345, 494–502 (2001).
Valgimigli, M. et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. Heart J. 39, 213–260 (2018).
Sabatine, M. S. et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N. Engl. J. Med. 352, 1179–1189 (2005).
Chen, Z. M. et al. Addition of clopidogrel to aspirin in 45 852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 366, 1607–1621 (2005).
Steinhubl, S. R. et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 288, 2411–2420 (2002).
Levine, G. N. et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. J. Thorac. Cardiovasc. Surg. 152, 1243–1275 (2016).
CURRENT-OASIS 7 Investigators. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N. Engl. J. Med. 363, 930–942 (2010).
Mehta, S. R. et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 376, 1233–1243 (2010).
Angiolillo, D. J. et al. Variability in individual responsiveness to clopidogrel. J. Am. Coll. Cardiol. 49, 1505–1516 (2007).
Wiviott, S. D. et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 357, 2001–2015 (2007).
Wallentin, L. et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 361, 1045–1057 (2009).
Roe, M. T. et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N. Engl. J. Med. 367, 1297–1309 (2012).
Wiviott, S. D. et al. Prasugrel versus clopidogrel for patients with unstable angina or non-ST-segment elevation myocardial infarction with or without angiography: a secondary, prespecified analysis of the TRILOGY ACS trial. Lancet 382, 605–613 (2013).
Tricoci, P. et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N. Engl. J. Med. 366, 20–33 (2012).
Valgimigli, M. et al. Usefulness and safety of vorapaxar in patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary intervention (from the TRACER trial). Am. J. Cardiol. 114, 665–673 (2014).
Mega, J. L. et al. Rivaroxaban in patients with a recent acute coronary syndrome. N. Engl. J. Med. 366, 9–19 (2012).
Alexander, J. H. et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N. Engl. J. Med. 365, 699–708 (2011).
Bhatt, D. L. et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N. Engl. J. Med. 354, 1706–1717 (2006).
Morrow, D. A. et al. Vorapaxar in the secondary prevention of atherothrombotic events. N. Engl. J. Med. 366, 1404–1413 (2012).
Bonaca, M. P. et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 372, 1791–1800 (2015).
Udell, J. A. et al. Long-term dual antiplatelet therapy for secondary prevention of cardiovascular events in the subgroup of patients with previous myocardial infarction: a collaborative meta-analysis of randomized trials. Eur. Heart J. 37, 390–399 (2016).
Diener, H.-C. et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet 364, 331–337 (2004).
Eikelboom, J. W. et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N. Engl. J. Med. 377, 1319–1330 (2017).
ACTIVE Investigators. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N. Engl. J. Med. 360, 2066–2078 (2009).
ACTIVE Writing Group of the ACTIVE Investigators. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 367, 1903–1912 (2006).
Ruff, C. T. et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 383, 955–962 (2014).
Connolly, S. J. et al. Apixaban in patients with atrial fibrillation. N. Engl. J. Med. 364, 806–817 (2011).
Sørensen, R. et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet 374, 1967–1974 (2009).
Angiolillo, D. J. et al. Antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention: a North American perspective — 2016 update. Circ. Cardiovasc. Interv. 9, e004395 (2016).
Angiolillo, D. J. et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective – 2018 update. Circulation https://doi.org/10.1161/CIRCULATIONAHA.118.034722 (2018).
Vranckx, P. et al. Thrombo-embolic prevention after transcatheter aortic valve implantation. Eur. Heart J. 38, 3341–3350 (2017).
Rodés-Cabau, J. et al. Aspirin versus aspirin plus clopidogrel as antithrombotic treatment following transcatheter aortic valve replacement with a balloon-expandable valve: the ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) randomized clinical trial. JACC Cardiovasc. Interv. 10, 1357–1365 (2017).
Capodanno, D. & Angiolillo, D. J. Antithrombotic therapy for prevention of cerebral thromboembolic events after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 10, 1366–1369 (2017).
Siller-Matula, J. M. et al. Inter-patient variability of platelet reactivity in patients treated with prasugrel and ticagrelor. Platelets 27, 373–377 (2016).
Cadroy, Y. et al. Early potent antithrombotic effect with combined aspirin and a loading dose of clopidogrel on experimental arterial thrombogenesis in humans. Circulation 101, 2823–2828 (2000).
Angiolillo, D. J. & Ferreiro, J. L. Platelet adenosine diphosphate P2Y12 receptor antagonism: benefits and limitations of current treatment strategies and future directions. Rev. Esp. Cardiol. 63, 60–76 (2010).
Storey, R. F. Biology and pharmacology of the platelet P2Y12 receptor. Curr. Pharm. Des. 12, 1255–1259 (2006).
McFadyen, J. D., Schaff, M. & Peter, K. Current and future antiplatelet therapies: emphasis on preserving haemostasis. Nat. Rev. Cardiol. 15, 181–191 (2018).
Armstrong, P. C. J., Dhanji, A.-R. A., Tucker, A. T., Mitchell, J. A. & Warner, T. D. Reduction of platelet thromboxane A2 production ex vivo and in vivo by clopidogrel therapy. J. Thromb. Haemost. 8, 613–615 (2010).
Li, Z. et al. Two waves of platelet secretion induced by thromboxane A2 receptor and a critical role for phosphoinositide 3-kinases. J. Biol. Chem. 278, 30725–30731 (2003).
Rollini, F. et al. Cigarette smoking and antiplatelet effects of aspirin monotherapy versus clopidogrel monotherapy in patients with atherosclerotic disease: results of a prospective pharmacodynamic study. J. Cardiovasc. Transl Res. 7, 53–63 (2014).
Franchi, F. et al. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease: the OPTIMUS (Optimizing Antiplatelet Therapy in Diabetes Mellitus)-4 study. Circulation 134, 780–792 (2016).
Angiolillo, D. J. et al. Impact of P2Y12 inhibitory effects induced by clopidogrel on platelet procoagulant activity in type 2 diabetes mellitus patients. Thromb. Res. 124, 318–322 (2009).
Storey, R. F. et al. The central role of the P2T receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br. J. Haematol. 110, 925–934 (2000).
Scavone, M., Femia, E. A., Caroppo, V. & Cattaneo, M. Inhibition of the platelet P2Y12 receptor for adenosine diphosphate does not impair the capacity of platelet to synthesize thromboxane A2. Eur. Heart J. 37, 3347–3356 (2016).
Storey, R. F. et al. Platelet inhibition with ticagrelor 60 mg versus 90 mg twice daily in the PEGASUS-TIMI 54 trial. J. Am. Coll. Cardiol. 67, 1145–1154 (2016).
Gurbel, P. A. et al. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-induced Platelet Effect (ASPECT) study. Circulation 115, 3156–3164 (2007).
Armstrong, P. C. J. et al. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J. Thromb. Haemost. 9, 552–561 (2011).
Traby, L. et al. Effects of P2Y12 receptor inhibition with or without aspirin on hemostatic system activation: a randomized trial in healthy subjects. J. Thromb. Haemost. 14, 273–281 (2016).
Mahaffey, K. W. et al. Ticagrelor compared with clopidogrel by geographic region in the Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation 124, 544–554 (2011).
Tello-Montoliu, A. et al. Impact of aspirin dose on adenosine diphosphate-mediated platelet activities. Results of an in vitro pilot investigation. Thromb. Haemost 110, 777–784 (2013).
Teng, R., Maya, J. & Butler, K. Evaluation of the pharmacokinetics and pharmacodynamics of ticagrelor co-administered with aspirin in healthy volunteers. Platelets 24, 615–624 (2013).
Cattaneo, M., Schulz, R. & Nylander, S. Adenosine-mediated effects of ticagrelor. J. Am. Coll. Cardiol. 63, 2503–2509 (2014).
Storey, R. F. et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets 25, 517–525 (2014).
Varenhorst, C. et al. Causes of mortality with ticagrelor compared with clopidogrel in acute coronary syndromes. Heart 100, 1762–1769 (2014).
Capodanno, D. Oral antithrombotic therapy after acute coronary syndromes: “dual antiplatelet” or “dual pathway”? EuroIntervention 13, 773–775 (2017).
Angiolillo, D. J., Capodanno, D. & Goto, S. Platelet thrombin receptor antagonism and atherothrombosis. Eur. Heart J. 31, 17–28 (2010).
Esmon, C. T. Targeting factor Xa and thrombin: impact on coagulation and beyond. Thromb. Haemost. 111, 625–633 (2014).
Becker, E. M. et al. Effects of rivaroxaban, acetylsalicylic acid and clopidogrel as monotherapy and in combination in a porcine model of stent thrombosis. J. Thromb. Haemost. 10, 2470–2480 (2012).
Perzborn, E., Heitmeier, S. & Laux, V. Effects of rivaroxaban on platelet activation and platelet-coagulation pathway interaction: in vitro and in vivo studies. J. Cardiovasc. Pharmacol. Ther. 20, 554–562 (2015).
CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 348, 1329–1339 (1996).
Hirsh, J. & Bhatt, D. L. Comparative benefits of clopidogrel and aspirin in high-risk patient populations. Arch. Intern. Med. 164, 2106 (2004).
Ringleb, P. A. et al. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 35, 528–532 (2004).
Park, T. K. et al. Clopidogrel versus aspirin as an antiplatelet monotherapy after 12-month dual-antiplatelet therapy in the era of drug-eluting stents. Circ. Cardiovasc. Interv. 9, e002816 (2016).
Hiatt, W. R. et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N. Engl. J. Med. 376, 32–40 (2017).
Capodanno, D., Alberts, M. & Angiolillo, D. J. Antithrombotic therapy for secondary prevention of atherothrombotic events in cerebrovascular disease. Nat. Rev. Cardiol. 13, 609–622 (2016).
Hass, W. K. et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N. Engl. J. Med. 321, 501–507 (1989).
Bousser, M.-G. et al. Terutroban versus aspirin in patients with cerebral ischaemic events (PERFORM): a randomised, double-blind, parallel-group trial. Lancet 377, 2013–2022 (2011).
Johnston, S. C. et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N. Engl. J. Med. 375, 35–43 (2016).
Ohman, E. M. et al. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in acute coronary syndromes (GEMINI-ACS-1): a double-blind, multicentre, randomised trial. Lancet 389, 1799–1808 (2017).
Dewilde, W. J. M. et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet 381, 1107–1115 (2013).
Gibson, C. M. et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N. Engl. J. Med. 375, 2423–2434 (2016).
Gibson, C. M. et al. Recurrent hospitalization among patients with atrial fibrillation undergoing intracoronary stenting treated with 2 treatment strategies of rivaroxaban or a dose-adjusted oral vitamin K antagonist treatment strategyclinical perspective. Circulation 135, 323–333 (2017).
Cannon, C. P. et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N. Engl. J. Med. 377, 1513–1524 (2017).
Cavallari, I. & Patti, G. Meta-analysis comparing the safety and efficacy of dual versus triple antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am. J. Cardiol. 121, 718–724 (2017).
Agarwal, N. et al. Safety and efficacy of dual versus triple antithrombotic therapy in patients undergoing percutaneous coronary intervention. Am. J. Med. 130, 1280–1289 (2017).
Mehta, S. R. et al. 2018 Canadian Cardiovascular Society (CCS)/Canadian Association of Interventional Cardiology (CAIC) focused update of the guidelines for the use of antiplatelet therapy. Can. J. Cardiol. 34, 214–233 (2018).
Vranckx, P. et al. Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation: rationale and design of the GLOBAL LEADERS trial. EuroIntervention 12, 1239–1245 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03231059 (2017).
Baber, U. et al. Ticagrelor with aspirin or alone in high-risk patients after coronary intervention: rationale and design of the TWILIGHT study. Am. Heart J. 182, 125–134 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02079194 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02494895 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02619760 (2017).
Lopes, R. D. et al. An open-label, 2 × 2 factorial, randomized controlled trial to evaluate the safety of apixaban versus vitamin K antagonist and aspirin versus placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: rationale and design of the AUGUSTUS trial. Am. Heart J. 200, 17–23 (2018).
Vranckx, P. et al. Evaluation of the safety and efficacy of an edoxaban-based antithrombotic regimen in patients with atrial fibrillation following successful percutaneous coronary intervention (PCI) with stent placement: Rationale and design of the ENTRUST-AF PCI trial. Am. Heart J. 196, 105–112 (2018).
Windecker, S. et al. Trial design: rivaroxaban for the prevention of major cardiovascular events after transcatheter aortic valve replacement. Rationale and design of the GALILEO study. Am. Heart J. 184, 81–87 (2017).
Dangas, G. D., Weitz, J. I., Giustino, G., Makkar, R. & Mehran, R. Prosthetic heart valve thrombosis. J. Am. Coll. Cardiol. 68, 2670–2689 (2016).
Collet, J.-P. et al. Oral anti-Xa anticoagulation after trans-aortic valve implantation for aortic stenosis: the randomized ATLANTIS trial. Am. Heart J. 200, 44–50 (2018).
Nijenhuis, V. J. et al. Rationale and design of POPULAR-TAVI: antiplatelet therapy for patients undergoing transcatheter aortic valve implantation. Am. Heart J. 173, 77–85 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02817789 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02943785 (2018).
Bliden, K. P., Tantry, U. S., Chaudhary, R., Byun, S. & Gurbel, P. A. Extended-release acetylsalicylic acid for secondary prevention of stroke and cardiovascular events. Expert Rev. Cardiovasc. Ther. 14, 779–791 (2016).
Bhatt, D. L. et al. Enteric coating and aspirin nonresponsiveness in patients with type 2 diabetes mellitus. J. Am. Coll. Cardiol. 69, 603–612 (2017).
De Berardis, G. et al. Aspirin and simvastatin combination for cardiovascular events prevention trial in diabetes (ACCEPT-D): design of a randomized study of the efficacy of low-dose aspirin in the prevention of cardiovascular events in subjects with diabetes mellitus treated with statins. Trials 8, 21 (2007).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00501059 (2017).
ASPREE Investigator Group. Study design of Aspirin in Reducing Events in the Elderly (ASPREE): a randomized, controlled trial. Contemp. Clin. Trials 36, 555–564 (2013).
Bowman, L. et al. ASCEND: A Study of Cardiovascular Events in Diabetes: characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am. Heart J. 198, 135–144 (2018).
Coyle, C. et al. ADD-ASPIRIN: a phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp. Clin. Trials 51, 56–64 (2016).
Johnston, A., Jones, W. S. & Hernandez, A. F. The ADAPTABLE trial and aspirin dosing in secondary prevention for patients with coronary artery disease. Curr. Cardiol. Rep. 18, 81 (2016).
Ferreiro, J. L. & Angiolillo, D. J. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation 123, 798–813 (2011).
Capodanno, D. et al. Pharmacodynamic effects of different aspirin dosing regimens in type 2 diabetes mellitus patients with coronary artery disease. Circ. Cardiovasc. Interv. 4, 180–187 (2011).
Dillinger, J.-G. et al. Biological efficacy of twice daily aspirin in type 2 diabetic patients with coronary artery disease. Am. Heart J. 164, 600.e1–606.e1 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02520921 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01813435 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02270242 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02415400 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02866175 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02556203 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02664649 (2017).
Acknowledgements
D.J.A. is the recipient of funding from the Scott R. MacKenzie Foundation and the US National Institutes of Health (NIH) National Center for Advancing Translational Sciences Clinical and Translational Science Award to the University of Florida UL1 TR000064 and NIH National Human Genome Research Institute U01 HG007269.
Reviewer information
Nature Reviews Cardiology thanks G. Lip, L. Wallentin, and U. Zeymer for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
D.J.A. and D.C. researched data for the article and wrote the manuscript. All authors contributed substantially to discussions of the article content and undertook reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D.J.A. declares that he has received consulting fees or honoraria from Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Janssen, Merck, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical. D.J.A. also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, and Renal Guard Solutions. D.L.B. declares that he has received research funding from Abbott, Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, and The Medicines Company. He also declares that he is an advisory board member for Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences and was on the Board of Directors of the Boston VA Research Institute and the Society of Cardiovascular Patient Care; he was Chair of the American Heart Association Quality Oversight Committee and is on the Data Monitoring Committees of the Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, and Population Health Research Institute; he was a Trustee of the American College of Cardiology (ACC), from whom he receives honoraria for his roles as Senior Associate Editor (Clinical Trials and News) at ACC.org and Vice Chair of the ACC Accreditation Committee. He is a member of the clinical trial steering committees of Duke Clinical Research Institute Population Health Research Institute and Harvard Clinical Research Institute. He was Secretary and Treasurer of the Society of Cardiovascular Patient Care, a member of the Continuing Medical Education steering committee for WebMD, and Chair of the National Cardiovascular Data Registry ACTION Registry Steering Committee and of the VA Clinical Assessment, Reporting, and Tracking Research and Publications Committee. He also acts as site co-investigator for Biotronik, Boston Scientific, and St Jude Medical (now Abbott) and conducts unfunded research for FlowCo, Merck, PLx Pharma, and Takeda. Finally, he is Editor in Chief of the Harvard Heart Letter and the Journal of Invasive Cardiology, a guest editor and associate editor of the Journal of the American College of Cardiology, Chief Medical Editor of Cardiology Today’s Intervention, and Deputy Editor of Clinical Cardiology; he receives royalties from Elsevier for his role as Editor of Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease. D.C. declares that he has received speaker’s fees or consulting honoraria from AstraZeneca, Bayer, and Sanofi. J.-P.C. declares that he has received research grants from Bristol-Myers Squibb, Boston Scientific, Federation Française de Cardiologie, Medtronic, and Société Française de Cardiologie; consulting fees from Bristol-Myers Squibb and Sanofi Aventis; and lecture fees from AstraZeneca, Bayer Health Care, Bristol-Myers Squibb, and Daiichi-Sankyo. G.D. declares that he has received honoraria from Bayer. C.M.G. declares that he has received honoraria from Bayer, Janssen Pharmaceuticals, Johnson and Johnson, and Portola Pharmaceuticals. H.-C.G. declares that he has received consulting fees from Medtronic and research grants from Abbott Vascular, Biotronik, Boston Scientific, and Medtronic. T.K. declares that he has received honoraria from Sanofi and research grants from Abbott Vascular. R.D.L. declares that he has received research support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, and Pfizer and consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, and Portola. R.M. declares that she has received consulting fees from Abbott Vascular, Abiomed, Boston Scientific, Bristol-Myers Squibb, Cardiovascular Systems, Elixir, Medscape, Shanghai Bracco Sine Pharmaceutical, and The Medicines Company and executive committee fees from Janssen Pharmaceuticals and Osprey Medical. She also declares that her institution receives funding from AstraZeneca, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, CardioKinetix, Claret Medical, CSL Behring, Eli Lilly/DSI, Medtronic, Novartis Pharmaceuticals, OrbusNeich, Spectranetics, and Watermark Research Partners. P.W.S. declares that he has received consulting fees and honoraria from Abbott Vascular, Biosensors, Medtronic, Micell Technologies, QualiMed, Sinomed, St Jude Medical, Stentys, Svelte, Philips/Volcano, and Xeltis. P.G.S. declares that he has received research grants from Bayer, Merck, Sanofi, and Servier and has received speaker’s fees or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, and Servier. R.F.S. declares that he has received consultancy fees from Actelion, Avacta, AstraZeneca, Bayer, Bristol-Myers Squibb/Pfizer, Idorsia, Novartis, PlaqueTec, and The Medicines Company; research grants from AstraZeneca and PlaqueTec; and honoraria from AstraZeneca and Bayer. M.V. declares that he has received research grants from AstraZeneca and Terumo and has received honoraria from Abbott Vascular, Amgen, AstraZeneca, Bayer, Biosensors, Cardinal Health, Daiichi-Sankyo, and Terumo. P.V. declares that he has received speaker’s fees or consulting fees from AstraZeneca, Bayer Health Care, and Daiichi-Sankyo. S.W. declares that he has received institutional research funding from Abbott, Amgen, Boston Scientific, Biotronik, and St Jude Medical. U.B., F.F., and F.R. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Capodanno, D., Mehran, R., Valgimigli, M. et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol 15, 480–496 (2018). https://doi.org/10.1038/s41569-018-0049-1
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-018-0049-1
This article is cited by
-
The protective effect of rivaroxaban with or without aspirin on inflammation, oxidative stress, and platelet reactivity in isoproterenol-induced cardiac injury in rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Bleeding avoidance strategies in percutaneous coronary intervention
Nature Reviews Cardiology (2022)
-
Antiplatelet Therapy in Patients Undergoing Elective Percutaneous Coronary Intervention
Current Cardiology Reports (2022)
-
P2Y12 inhibitor monotherapy in patients undergoing percutaneous coronary intervention
Nature Reviews Cardiology (2022)
-
Safety assessment of subtilisin QK in rats
BMC Pharmacology and Toxicology (2021)