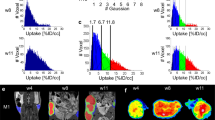
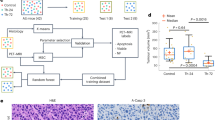
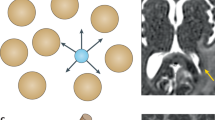
Abstract
Molecular imaging has experienced enormous advancements in the areas of imaging technology, imaging probe and contrast development, and data quality, as well as machine learning-based data analysis. Positron emission tomography (PET) and its combination with computed tomography (CT) or magnetic resonance imaging (MRI) as a multimodality PET–CT or PET–MRI system offer a wealth of molecular, functional and morphological data with a single patient scan. Despite the recent technical advances and the availability of dozens of disease-specific contrast and imaging probes, only a few parameters, such as tumour size or the mean tracer uptake, are used for the evaluation of images in clinical practice. Multiparametric in vivo imaging data not only are highly quantitative but also can provide invaluable information about pathophysiology, receptor expression, metabolism, or morphological and functional features of tumours, such as pH, oxygenation or tissue density, as well as pharmacodynamic properties of drugs, to measure drug response with a contrast agent. It can further quantitatively map and spatially resolve the intertumoural and intratumoural heterogeneity, providing insights into tumour vulnerabilities for target-specific therapeutic interventions. Failure to exploit and integrate the full potential of such powerful imaging data may lead to a lost opportunity in which patients do not receive the best possible care. With the desire to implement personalized medicine in the cancer clinic, the full comprehensive diagnostic power of multiplexed imaging should be utilized.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Marusyk, A. & Polyak, K. Tumor heterogeneity: causes and consequences. Biochim. Biophys. Acta 1805, 105–117 (2010).
Dagogo-Jack, I. & Shaw, A. T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018).
Marusyk, A., Janiszewska, M. & Polyak, K. Intratumor heterogeneity: the Rosetta stone of therapy resistance. Cancer Cell 37, 471–484 (2020).
Saunders, N. A. et al. Role of intratumoural heterogeneity in cancer drug resistance: molecular and clinical perspectives. EMBO Mol. Med. 4, 675–684 (2012).
Hyman, D. M., Taylor, B. S. & Baselga, J. Implementing genome-driven oncology. Cell 168, 584–599 (2017).
Keller, L. & Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 19, 553–567 (2019). This review discusses how tumour heterogeneity can be analysed by profiling tumour cells in circulation.
Joosse, S. A. & Pantel, K. Circulating DNA and liquid biopsies in the management of patients with cancer. Cancer Res. 82, 2213–2215 (2022).
Mannheim, J. G. et al. PET/MRI hybrid systems. Semin. Nucl. Med. 48, 332–347 (2018). This review summarizes the different PET–MRI systems for multimodal preclinical and clinical imaging.
Seifert, R. et al. Clinical use of PET/MR in oncology: an update. Semin. Nucl. Med. 52, 356–364 (2022).
Wehrl, H. F., Sauter, A. W., Divine, M. R. & Pichler, B. J. Combined PET/MR: a technology becomes mature. J. Nucl. Med. 56, 165–168 (2015).
Herrmann, K. et al. Radiotheranostics: a roadmap for future development. Lancet Oncol. 21, e146–e156 (2020).
Weissleder, R., Schwaiger, M. C., Gambhir, S. S. & Hricak, H. Imaging approaches to optimize molecular therapies. Sci. Transl. Med. 8, 355ps316 (2016).
Divine, M. R. et al. A population-based Gaussian mixture model incorporating 18F-FDG PET and diffusion-weighted MRI quantifies tumor tissue classes. J. Nucl. Med. 57, 473–479 (2016).
Katiyar, P. et al. Spectral clustering predicts tumor tissue heterogeneity using dynamic 18F-FDG PET: a complement to the standard compartmental modeling approach. J. Nucl. Med. 58, 651–657 (2017).
Katiyar, P. et al. A novel unsupervised segmentation approach quantifies tumor tissue populations using multiparametric MRI: first results with histological validation. Mol. Imaging Biol. 19, 391–397 (2017).
Zaharchuk, G. & Davidzon, G. Artificial intelligence for optimization and interpretation of PET/CT and PET/MR images. Semin. Nucl. Med. 51, 134–142 (2021). This review describes the use of MI and AI for multimodal imaging.
Beyer, T. et al. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 41, 1369–1379 (2000).
Judenhofer, M. S. et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat. Med. 14, 459–465 (2008).
Shao, Y. et al. Simultaneous PET and MR imaging. Phys. Med. Biol. 42, 1965–1970 (1997).
Wehrl, H. F. et al. Preclinical and translational PET/MR imaging. J. Nucl. Med. 55, 11S–18S (2014).
Provost, J. et al. Simultaneous positron emission tomography and ultrafast ultrasound for hybrid molecular, anatomical and functional imaging. Nat. Biomed. Eng. 2, 85–94 (2018).
Wehrl, H. F. et al. Assessment of MR compatibility of a PET insert developed for simultaneous multiparametric PET/MR imaging on an animal system operating at 7 T. Magn. Reson. Med. 65, 269–279 (2011).
Judenhofer, M. S. & Cherry, S. R. Applications for preclinical PET/MRI. Semin. Nucl. Med. 43, 19–29 (2013).
Sauter, A. W., Wehrl, H. F., Kolb, A., Judenhofer, M. S. & Pichler, B. J. Combined PET/MRI: one step further in multimodality imaging. Trends Mol. Med. 16, 508–515 (2010).
Asa, S. et al. Hybrid Ga-68 prostate-specific membrane antigen PET/MRI in the detection of skeletal metastasis in patients with newly diagnosed prostate cancer: contribution of each part to the diagnostic performance. Nucl. Med. Commun. 44, 65–73 (2023).
Wehrl, H. F. et al. Multimodal elucidation of choline metabolism in a murine glioma model using magnetic resonance spectroscopy and 11C-choline positron emission tomography. Cancer Res. 73, 1470–1480 (2013).
Andreou, C., Weissleder, R. & Kircher, M. F. Multiplexed imaging in oncology. Nat. Biomed. Eng. 6, 527–540 (2022).
Disselhorst, J. A. et al. Linking imaging to omics utilizing image-guided tissue extraction. Proc. Natl Acad. Sci. USA 115, E2980–E2987 (2018).
Trautwein, C. et al. Tissue metabolites in diffuse glioma and their modulations by IDH1 mutation, histology, and treatment. JCI Insight https://doi.org/10.1172/jci.insight.153526 (2022).
Mu, W. et al. Non-invasive decision support for NSCLC treatment using PET/CT radiomics. Nat. Commun. 11, 5228 (2020).
Stumpo, V. et al. Feasibility of glioblastoma tissue response mapping with physiologic BOLD imaging using precise oxygen and carbon dioxide challenge. MAGMA 35, 29–44 (2022).
Zhang, L. et al. Lanthanide-based T2ex and CEST complexes provide insights into the design of pH sensitive MRI agents. Angew. Chem. Int. Ed. Engl. 56, 16626–16630 (2017).
Giger, M. L. Machine learning in medical imaging. J. Am. Coll. Radiol. 15, 512–520 (2018).
Hosny, A., Parmar, C., Quackenbush, J., Schwartz, L. H. & Aerts, H. Artificial intelligence in radiology. Nat. Rev. Cancer 18, 500–510 (2018).
Gillies, R. J., Kinahan, P. E. & Hricak, H. Radiomics: images are more than pictures, they are data. Radiology 278, 563–577 (2016). This review provides an overview of radiomics — the convergence of imaging and genetic profiling.
Tomaszewski, M. R. & Gillies, R. J. The biological meaning of radiomic features. Radiology 298, 505–516 (2021).
Mu, W., Schabath, M. B. & Gillies, R. J. Images are data: challenges and opportunities in the clinical translation of radiomics. Cancer Res. 82, 2066–2068 (2022).
Sharma, A., Lelic, D., Brock, C., Paine, P. & Aziz, Q. New technologies to investigate the brain–gut axis. World J. Gastroenterol. 15, 182–191 (2009).
Rosen, S. D. & Camici, P. G. The brain–heart axis in the perception of cardiac pain: the elusive link between ischaemia and pain. Ann. Med. 32, 350–364 (2000).
Badawi, R. D. et al. First human imaging studies with the EXPLORER Total-Body PET scanner. J. Nucl. Med. 60, 299–303 (2019). This study is one of the first publications of total-body human PET imaging.
Lammertsma, A. A. Quantification of PET studies. J. Nucl. Cardiol. 26, 2045–2047 (2019).
Cherry, S. R. et al. Total-body imaging: transforming the role of positron emission tomography. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aaf6169 (2017). This review describes total-body PET, from initial ideas to applications.
Prenosil, G. A. et al. Performance characteristics of the biograph vision quadra PET/CT system with long axial field of view using the NEMA NU 2-2018 standard. J. Nucl. Med. https://doi.org/10.2967/jnumed.121.261972 (2021).
Ibaraki, M. et al. Brain partial volume correction with point spreading function reconstruction in high-resolution digital PET: comparison with an MR-based method in FDG imaging. Ann. Nucl. Med. 36, 717–727 (2022).
Grimm, J., Kiessling, F. & Pichler, B. J. Quo vadis, molecular imaging? J. Nucl. Med. 61, 1428–1434 (2020). This review describes molecular imaging methods in general and provides an overview of imaging PET tracers, from small molecules to biologicals.
Boellaard, R. et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur. J. Nucl. Med. Mol. Imaging 42, 328–354 (2015).
Jadvar, H. Is there use for FDG-PET in prostate cancer? Semin. Nucl. Med. 46, 502–506 (2016).
Sharma, P. et al. Comparison of the prognostic values of 68Ga-DOTANOC PET/CT and 18F-FDG PET/CT in patients with well-differentiated neuroendocrine tumor. Eur. J. Nucl. Med. Mol. Imaging 41, 2194–2202 (2014).
Kayani, I. et al. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1,Tyr3-octreotate) and 18F-FDG. Cancer 112, 2447–2455 (2008).
Schuster, D. M., Nanni, C. & Fanti, S. PET tracers beyond FDG in prostate cancer. Semin. Nucl. Med. 46, 507–521 (2016).
Buteau, J. P. et al. PSMA and FDG-PET as predictive and prognostic biomarkers in patients given [177Lu]Lu-PSMA-617 versus cabazitaxel for metastatic castration-resistant prostate cancer (TheraP): a biomarker analysis from a randomised, open-label, phase 2 trial. Lancet Oncol. 23, 1389–1397 (2022).
Squires, M. H. 3rd et al. Octreoscan versus FDG-PET for neuroendocrine tumor staging: a biological approach. Ann. Surg. Oncol. 22, 2295–2301 (2015).
Alevroudis, E. et al. Clinical utility of 18F-FDG PET in neuroendocrine tumors prior to peptide receptor radionuclide therapy: a systematic review and meta-analysis. Cancers https://doi.org/10.3390/cancers13081813 (2021).
Bhandari, V. et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 51, 308–318 (2019).
Liu, T. et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 12, 86 (2019).
Petrova, V., Annicchiarico-Petruzzelli, M., Melino, G. & Amelio, I. The hypoxic tumour microenvironment. Oncogenesis 7, 10 (2018).
Wilson, W. R. & Hay, M. P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 11, 393–410 (2011).
Masaki, Y. et al. The accumulation mechanism of the hypoxia imaging probe ‘FMISO’ by imaging mass spectrometry: possible involvement of low-molecular metabolites. Sci. Rep. 5, 16802 (2015).
Reischl, G. et al. Preparation of the hypoxia imaging PET tracer [18F]FAZA: reaction parameters and automation. Appl. Radiat. Isot. 62, 897–901 (2005).
Fleming, I. N. et al. Imaging tumour hypoxia with positron emission tomography. Br. J. Cancer 112, 238–250 (2015).
Busk, M., Overgaard, J. & Horsman, M. R. Imaging of tumor hypoxia for radiotherapy: current status and future directions. Semin. Nucl. Med. 50, 562–583 (2020).
Anemone, A., Consolino, L., Arena, F., Capozza, M. & Longo, D. L. Imaging tumor acidosis: a survey of the available techniques for mapping in vivo tumor pH. Cancer Metast. Rev. 38, 25–49 (2019).
Noman, M. Z. et al. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 309, C569–C579 (2015).
Lopes, S., Ferreira, S. & Caetano, M. PET/CT in the evaluation of hypoxia for radiotherapy planning in head and neck tumors: systematic literature review. J. Nucl. Med. Technol. 49, 107–113 (2021).
Gerard, M. et al. Hypoxia imaging and adaptive radiotherapy: a state-of-the-art approach in the management of glioma. Front. Med. 6, 117 (2019).
Dirix, P. et al. Dose painting in radiotherapy for head and neck squamous cell carcinoma: value of repeated functional imaging with 18F-FDG PET, 18F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J. Nucl. Med. 50, 1020–1027 (2009).
Melsens, E. et al. Hypoxia imaging with 18F-FAZA PET/CT predicts radiotherapy response in esophageal adenocarcinoma xenografts. Radiat. Oncol. 13, 39 (2018).
Carmona-Bozo, J. C. et al. Hypoxia and perfusion in breast cancer: simultaneous assessment using PET/MR imaging. Eur. Radiol. 31, 333–344 (2021).
Stegmayr, C. et al. Current trends in the use of O-(2-[18F]fluoroethyl)-l-tyrosine ([18F]FET) in neurooncology. Nucl. Med. Biol. 92, 78–84 (2021).
Stegmayr, C., Willuweit, A., Lohmann, P. & Langen, K. J. O-(2-[18F]-fluoroethyl)-l-tyrosine (FET) in neurooncology: a review of experimental results. Curr. Radiopharm. 12, 201–210 (2019).
Zanoni, L. et al. Role of 18F-FLT PET/CT in suspected recurrent or residual lymphoma: final results of a pilot prospective trial. Eur. J. Nucl. Med. Mol. Imaging 46, 1661–1671 (2019).
Bashir, A. et al. PET imaging of meningioma with 18F-FLT: a predictor of tumour progression. Brain 143, 3308–3317 (2020).
Buck, A. K. et al. Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J. Nucl. Med. 44, 1426–1431 (2003).
Vesselle, H. et al. In vivo validation of 3′deoxy-3′-[18F]fluorothymidine ([18F]FLT) as a proliferation imaging tracer in humans: correlation of [18F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin. Cancer Res. 8, 3315–3323 (2002).
Shinomiya, A. et al. Evaluation of 3′-deoxy-3′-[18F]-fluorothymidine (18F-FLT) kinetics correlated with thymidine kinase-1 expression and cell proliferation in newly diagnosed gliomas. Eur. J. Nucl. Med. Mol. Imaging 40, 175–185 (2013).
Brockenbrough, J. S. et al. Tumor 3′-deoxy-3′-18F-fluorothymidine (18F-FLT) uptake by PET correlates with thymidine kinase 1 expression: static and kinetic analysis of 18F-FLT PET studies in lung tumors. J. Nucl. Med. 52, 1181–1188 (2011).
Schwenck, J. et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging 44, 92–101 (2017).
Ambrosini, V., Campana, D., Tomassetti, P. & Fanti, S. 68Ga-labelled peptides for diagnosis of gastroenteropancreatic NET. Eur. J. Nucl. Med. Mol. Imaging 39, S52–S60 (2012).
Sartor, O. & de Bono, J. S. Metastatic prostate cancer. N. Engl. J. Med. 378, 1653–1654 (2018).
Oberg, K., Knigge, U., Kwekkeboom, D., Perren, A. & Group, E. G. W. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 23, vii124–vii130 (2012).
Langbein, T., Weber, W. A. & Eiber, M. Future of theranostics: an outlook on precision oncology in nuclear medicine. J. Nucl. Med. 60, 13S–19S (2019). Together with Hermann et al. (2020), this review discusses theranostics in nuclear medicine and precision oncology.
Hofman, M. S. et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet 397, 797–804 (2021).
Sartor, O. et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 385, 1091–1103 (2021).
Strosberg, J. et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-dotatate in the phase III NETTER-1 trial. J. Clin. Oncol. 36, 2578–2584 (2018).
Hofman, M. S. et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 19, 825–833 (2018).
Strosberg, J. et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 376, 125–135 (2017).
Backhaus, P. et al. Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 45, 860–877 (2018).
Gao, Y. et al. Prostate-specific membrane antigen (PSMA) promotes angiogenesis of glioblastoma through interacting with ITGB4 and regulating NF-κB signaling pathway. Front. Cell Dev. Biol. 9, 598377 (2021).
Silver, D. A., Pellicer, I., Fair, W. R., Heston, W. D. & Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 3, 81–85 (1997).
Schwenck, J. et al. In vivo visualization of prostate-specific membrane antigen in glioblastoma. Eur. J. Nucl. Med. Mol. Imaging 42, 170–171 (2015).
Wernicke, A. G. et al. Prostate-specific membrane antigen as a potential novel vascular target for treatment of glioblastoma multiforme. Arch. Pathol. Lab. Med. 135, 1486–1489 (2011).
Wernicke, A. G. et al. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS 122, 482–489 (2014).
Sollini, M. et al. PSMA expression level predicts differentiated thyroid cancer aggressiveness and patient outcome. EJNMMI Res. 9, 93 (2019).
Schmidt, L. H. et al. Prostate specific membrane antigen (PSMA) expression in non-small cell lung cancer. PLoS ONE 12, e0186280 (2017).
Hirmas, N. et al. 68Ga-PSMA-11 PET/CT improves tumor detection and impacts management in patients with hepatocellular carcinoma. J. Nucl. Med. 62, 1235–1241 (2021).
Kesler, M. et al. 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: a prospective pilot study. J. Nucl. Med. 60, 185–191 (2019).
Jiao, D. et al. Expression of prostate-specific membrane antigen in tumor-associated vasculature predicts poor prognosis in hepatocellular carcinoma. Clin. Transl. Gastroenterol. 10, e00041 (2019).
Conway, R. E. et al. Prostate specific membrane antigen produces pro-angiogenic laminin peptides downstream of matrix metalloprotease-2. Angiogenesis 16, 847–860 (2013).
Conway, R. E. et al. Prostate-specific membrane antigen (PSMA)-mediated laminin proteolysis generates a pro-angiogenic peptide. Angiogenesis 19, 487–500 (2016).
Papetti, M. & Herman, I. M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell Physiol. 282, C947–C970 (2002).
Holzgreve, A. et al. PSMA expression in glioblastoma as a basis for theranostic approaches: a retrospective, correlational panel study including immunohistochemistry, clinical parameters and PET imaging. Front. Oncol. 11, 646387 (2021).
Rizzo, A. et al. Can PSMA-targeting radiopharmaceuticals be useful for detecting hepatocellular carcinoma using positron emission tomography? An updated systematic review and meta-analysis. Pharmaceuticals 15, 1368 (2022).
Derlin, T., Kreipe, H. H., Schumacher, U. & Soudah, B. PSMA expression in tumor neovasculature endothelial cells of follicular thyroid adenoma as identified by molecular imaging using 68Ga-PSMA ligand PET/CT. Clin. Nucl. Med. 42, e173–e174 (2017).
Kunikowska, J. et al. Tumor uptake in glioblastoma multiforme after IV injection of [177Lu]Lu-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 47, 1605–1606 (2020).
Uijen, M. J. M. et al. PSMA radioligand therapy for solid tumors other than prostate cancer: background, opportunities, challenges, and first clinical reports. Eur. J. Nucl. Med. Mol. Imaging 48, 4350–4368 (2021).
Schulz, G. et al. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Res. 44, 5914–5920 (1984).
Schmitt, J. et al. Translational immunoPET imaging using a radiolabeled GD2-specific antibody in neuroblastoma. Theranostics https://doi.org/10.7150/thno.56736 (2022).
Butch, E. R. et al. Positron emission tomography detects in vivo expression of disialoganglioside GD2 in mouse models of primary and metastatic osteosarcoma. Cancer Res. 79, 3112–3124 (2019).
Trautwein, N. F. et al. First in human PET/MRI imaging of in vivo GD2 expression in osteosarcoma. J. Nucl. Med. https://doi.org/10.2967/jnumed.122.264626 (2022).
Navid, F., Santana, V. M. & Barfield, R. C. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr. Cancer Drug Targets 10, 200–209 (2010).
Ploessl, C., Pan, A., Maples, K. T. & Lowe, D. K. Dinutuximab: an anti-GD2 monoclonal antibody for high-risk neuroblastoma. Ann. Pharmacother. 50, 416–422 (2016).
Schumacher-Kuckelkorn, R. et al. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr. Blood Cancer 64, 46–56 (2017).
Anderson, N. M. & Simon, M. C. The tumor microenvironment. Curr. Biol. 30, R921–R925 (2020).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016).
Kim, I., Choi, S., Yoo, S., Lee, M. & Kim, I. S. Cancer-associated fibroblasts in the hypoxic tumor microenvironment. Cancers https://doi.org/10.3390/cancers14143321 (2022).
Madsen, C. D. et al. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. EMBO Rep. 16, 1394–1408 (2015).
Pure, E. & Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene 37, 4343–4357 (2018).
Loktev, A. et al. A tumor-imaging method targeting cancer-associated fibroblasts. J. Nucl. Med. 59, 1423–1429 (2018).
Lindner, T. et al. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 59, 1415–1422 (2018).
Kratochwil, C. et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J. Nucl. Med. 60, 801–805 (2019).
Koerber, S. A. et al. The role of 68Ga-FAPI PET/CT for patients with malignancies of the lower gastrointestinal tract: first clinical experience. J. Nucl. Med. 61, 1331–1336 (2020).
Komek, H. et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 PET/CT and [18F]FDG PET/CT in colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 49, 3898–3909 (2022).
Gu, B. et al. Head-to-head evaluation of [18F]FDG and [68Ga]Ga-DOTA-FAPI-04 PET/CT in recurrent soft tissue sarcoma. Eur. J. Nucl. Med. Mol. Imaging 49, 2889–2901 (2022).
Lindner, T. et al. Design and development of 99mTc-labeled FAPI tracers for SPECT imaging and 188Re therapy. J. Nucl. Med. 61, 1507–1513 (2020).
Ballal, S. et al. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: new frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging https://doi.org/10.1007/s00259-020-04990-w (2020).
Vasan, N., Baselga, J. & Hyman, D. M. A view on drug resistance in cancer. Nature 575, 299–309 (2019).
Wang, L., Lankhorst, L. & Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 22, 340–355 (2022). This review discusses tumour senescence and novel treatment options based on this phenomenon.
Bodnar, A. G. et al. Extension of life-span by introduction of telomerase into normal human cells. Science 279, 349–352 (1998).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Liao, E. C. et al. Radiation induces senescence and a bystander effect through metabolic alterations. Cell Death Dis. 5, e1255 (2014).
Chang, B. D. et al. Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene 18, 4808–4818 (1999).
Munoz-Espin, D. & Serrano, M. Cellular senescence: from physiology to pathology. Nat. Rev. Mol. Cell Biol. 15, 482–496 (2014).
Birch, J. & Gil, J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 34, 1565–1576 (2020).
Coppe, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853–2868 (2008).
Schmitt, C. A., Wang, B. & Demaria, M. Senescence and cancer — role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 19, 619–636 (2022).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
Faheem, M. M. et al. Convergence of therapy-induced senescence (TIS) and EMT in multistep carcinogenesis: current opinions and emerging perspectives. Cell Death Discov. 6, 51 (2020).
Faget, D. V., Ren, Q. & Stewart, S. A. Unmasking senescence: context-dependent effects of SASP in cancer. Nat. Rev. Cancer 19, 439–453 (2019).
Zhao, B. et al. Topoisomerase 1 cleavage complex enables pattern recognition and inflammation during senescence. Nat. Commun. 11, 908 (2020).
Wang, C. et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature 574, 268–272 (2019).
Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 5, 99–118 (2010).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J. Clin. Invest. 123, 966–972 (2013).
Zhang, L., Pitcher, L. E., Prahalad, V., Niedernhofer, L. J. & Robbins, P. D. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. FEBS J. https://doi.org/10.1111/febs.16350 (2022).
Laberge, R. M. et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 17, 1049–1061 (2015).
Wolter, K. & Zender, L. Therapy-induced senescence — an induced synthetic lethality in liver cancer? Nat. Rev. Gastroenterol. Hepatol. 17, 135–136 (2020).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Lawson, D. A., Kessenbrock, K., Davis, R. T., Pervolarakis, N. & Werb, Z. Tumour heterogeneity and metastasis at single-cell resolution. Nat. Cell Biol. 20, 1349–1360 (2018).
Ding, L. et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 464, 999–1005 (2010).
Krueger, M. A. et al. Abstract 1146: [18F]FPyGal: a novel ß-galactosidase specific PET tracer for in vivo imaging of tumor senescence. Cancer Res. 79, 1146–1146 (2019).
Lee, B. Y. et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 5, 187–195 (2006).
Short, S., Fielder, E., Miwa, S. & von Zglinicki, T. Senolytics and senostatics as adjuvant tumour therapy. eBioMedicine 41, 683–692 (2019).
Lord, C. J. & Ashworth, A. PARP inhibitors: synthetic lethality in the clinic. Science 355, 1152–1158 (2017).
Pommier, Y., O’Connor, M. J. & de Bono, J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 8, 362ps317 (2016).
Audeh, M. W. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Maughan, B. L. & Antonarakis, E. S. Olaparib and rucaparib for the treatment of DNA repair-deficient metastatic castration-resistant prostate cancer. Exp. Opin. Pharmacother. 22, 1625–1632 (2021).
Rose, M., Burgess, J. T., O’Byrne, K., Richard, D. J. & Bolderson, E. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front. Cell Dev. Biol. 8, 564601 (2020).
Ashworth, A. & Lord, C. J. Synthetic lethal therapies for cancer: what’s next after PARP inhibitors? Nat. Rev. Clin. Oncol. 15, 564–576 (2018).
Michels, J. et al. Cisplatin resistance associated with PARP hyperactivation. Cancer Res. 73, 2271–2280 (2013).
Lord, C. J., Tutt, A. N. & Ashworth, A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu. Rev. Med. 66, 455–470 (2015).
Yi, M. et al. Advances and perspectives of PARP inhibitors. Exp. Hematol. Oncol. 8, 29 (2019).
Banerjee, S. et al. First-line PARP inhibitors in ovarian cancer: summary of an ESMO open — cancer horizons round-table discussion. ESMO Open 5, e001110 (2020).
Tu, Z. et al. Synthesis and in vivo evaluation of [11C]PJ34, a potential radiotracer for imaging the role of PARP-1 in necrosis. Nucl. Med. Biol. 32, 437–443 (2005).
Makvandi, M. et al. A PET imaging agent for evaluating PARP-1 expression in ovarian cancer. J. Clin. Invest. 128, 2116–2126 (2018).
Carney, B., Kossatz, S. & Reiner, T. Molecular imaging of PARP. J. Nucl. Med. 58, 1025–1030 (2017).
Carney, B. et al. Target engagement imaging of PARP inhibitors in small-cell lung cancer. Nat. Commun. 9, 176 (2018).
McDonald, E. S. et al. Positron emission tomography imaging of poly-(adenosine diphosphate-ribose) polymerase 1 expression in breast cancer: a nonrandomized clinical trial. JAMA Oncol. 6, 921–923 (2020).
Schoder, H. et al. Safety and feasibility of PARP1/2 imaging with 18F-PARPi in patients with head and neck cancer. Clin. Cancer Res. 26, 3110–3116 (2020).
Michel, L. S. et al. PET of poly (ADP-ribose) polymerase activity in cancer: preclinical assessment and first in-human studies. Radiology 282, 453–463 (2017).
Fan, Y. et al. Progress of immune checkpoint therapy in the clinic (Review). Oncol. Rep. 41, 3–14 (2019).
June, C. H., O’Connor, R. S., Kawalekar, O. U., Ghassemi, S. & Milone, M. C. CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018).
Freise, A. C. & Wu, A. M. In vivo imaging with antibodies and engineered fragments. Mol. Immunol. 67, 142–152 (2015). This review discusses PET imaging of the immune system with biologicals.
Bensch, F. et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 24, 1852–1858 (2018). This study presents PET immune imaging of PDL1.
Kristensen, L. K. et al. CD4+ and CD8a+ PET imaging predicts response to novel PD-1 checkpoint inhibitor: studies of Sym021 in syngeneic mouse cancer models. Theranostics 9, 8221–8238 (2019).
Tavaré, R. et al. Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proc. Natl Acad. Sci. USA 111, 1108–1113 (2014).
Tavaré, R. et al. Immuno-PET of murine T cell reconstitution postadoptive stem cell transplantation using anti-CD4 and anti-CD8 Cys-diabodies. J. Nucl. Med. 56, 1258–1264 (2015).
Lecocq, Q. et al. Theranostics in immuno-oncology using nanobody derivatives. Theranostics 9, 7772–7791 (2019).
van der Linden, R. H. et al. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim. Biophys. Acta 1431, 37–46 (1999).
Rashidian, M. & Ploegh, H. Nanobodies as non-invasive imaging tools. Immuno-Oncol. Technol. 7, 2–14 (2020).
Gide, T. N. et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 35, 238–255.e6 (2019).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830.e4 (2018).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Klein, O. et al. Melan-A-specific cytotoxic T cells are associated with tumor regression and autoimmunity following treatment with anti-CTLA-4. Clin. Cancer Res. 15, 2507–2513 (2009).
Tavare, R. et al. An effective immuno-PET imaging method to monitor CD8-dependent responses to immunotherapy. Cancer Res. 76, 73–82 (2016).
Rashidian, M. et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 214, 2243–2255 (2017).
Freise, A. C. et al. ImmunoPET imaging of murine CD4+ T cells using anti-CD4 Cys-diabody: effects of protein dose on T cell function and imaging. Mol. Imaging Biol. 19, 599–609 (2017).
Griessinger, C. M. et al. The PET-tracer 89Zr-Df-IAB22M2C enables monitoring of intratumoral CD8 T-cell infiltrates in tumor-bearing humanized mice after T-cell bispecific antibody treatment. Cancer Res. 80, 2903 (2020).
Pandit-Taskar, N. et al. First in human phase I imaging study with 89Zr-IAB22M2C anti-CD8 minibody in patients with solid tumors. J. Nucl. Med. 59, 596 (2018).
US National Library of Medicine. ClinicalTrials.gov, http://www.clinicaltrials.gov/ct2/show/NCT03802123 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT05397171 (2022).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT05744128 (2023).
EU Clinical Trials Register. https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-004328-004313/DE (2022).
Kist de Ruijter, L. et al. Whole-body CD8+ T cell visualization before and during cancer immunotherapy: a phase 1/2 trial. Nat. Med. 28, 2601–2610 (2022).
Ahrends, T. & Borst, J. The opposing roles of CD4+ T cells in anti-tumour immunity. Immunology 154, 582–592 (2018).
Di Mascio, M. et al. Noninvasive in vivo imaging of CD4 cells in simian-human immunodeficiency virus (SHIV)-infected nonhuman primates. Blood 114, 328–337 (2009).
Kanwar, B. et al. In vivo imaging of mucosal CD4+ T cells using single photon emission computed tomography in a murine model of colitis. J. Immunol. Methods 329, 21–30 (2008).
Rubin, R. H., Baltimore, D., Chen, B. K., Wilkinson, R. A. & Fischman, A. J. In vivo tissue distribution of CD4 lymphocytes in mice determined by radioimmunoscintigraphy with an 111In-labeled anti-CD4 monoclonal antibody. Proc. Natl Acad. Sci. USA 93, 7460–7463 (1996).
Choy, E. H. et al. Repeat-cycle study of high-dose intravenous 4162W94 anti-CD4 humanized monoclonal antibody in rheumatoid arthritis. A randomized placebo-controlled trial. Rheumatology 41, 1142–1148 (2002).
Harmand, T. J., Islam, A., Pishesha, N. & Ploegh, H. L. Nanobodies as in vivo, non-invasive, imaging agents. RSC Chem. Biol. 2, 685–701 (2021).
Moreland, L. W. et al. Double-blind, placebo-controlled multicenter trial using chimeric monoclonal anti-CD4 antibody, cM-T412, in rheumatoid arthritis patients receiving concomitant methotrexate. Arthritis Rheum. 38, 1581–1588 (1995).
Traenkle, B. et al. Single-domain antibodies for targeting, detection, and in vivo imaging of human CD4+ cells. Front. Immunol. 12, 799910 (2021).
Wilde, D. B., Marrack, P., Kappler, J., Dialynas, D. P. & Fitch, F. W. Evidence implicating L3T4 in class II MHC antigen reactivity; monoclonal antibody GK1.5 (anti-L3T4a) blocks class II MHC antigen-specific proliferation, release of lymphokines, and binding by cloned murine helper T lymphocyte lines. J. Immunol. 131, 2178–2183 (1983).
Kochenderfer, J. N. et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol. Ther. 25, 2245–2253 (2017).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Simonetta, F. et al. Molecular imaging of chimeric antigen receptor T cells by ICOS-ImmunoPET. Clin. Cancer Res. 27, 1058 (2021).
Weist, M. R. et al. PET of adoptively transferred chimeric antigen receptor T cells with 89Zr-oxine. J. Nucl. Med. 59, 1531–1537 (2018).
Minn, I. et al. Imaging CAR T cell therapy with PSMA-targeted positron emission tomography. Sci. Adv. 5, eaaw5096 (2019).
Sellmyer, M. A. et al. Imaging CAR T cell trafficking with eDHFR as a PET reporter gene. Mol. Ther. 28, 42–51 (2020).
Volpe, A. et al. Spatiotemporal PET imaging reveals differences in CAR-T tumor retention in triple-negative breast cancer models. Mol. Ther. 28, 2271–2285 (2020).
Keu, K. V. et al. Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Sci. Transl. Med. 9, eaag2196 (2017).
Di Gialleonardo, V., Signore, A., Glaudemans, A. W., Dierckx, R. A. & De Vries, E. F. N-(4-18F-fluorobenzoyl)interleukin-2 for PET of human-activated T lymphocytes. J. Nucl. Med. 53, 679–686 (2012).
Larimer, B. M. et al. Granzyme B PET imaging as a predictive biomarker of immunotherapy response. Cancer Res. 77, 2318–2327 (2017).
Gibson, H. M. et al. IFNgamma PET imaging as a predictive tool for monitoring response to tumor immunotherapy. Cancer Res. 78, 5706–5717 (2018).
Radu, C. G. et al. Molecular imaging of lymphoid organs and immune activation by positron emission tomography with a new [18F]-labeled 2’-deoxycytidine analog. Nat. Med. 14, 783–788 (2008).
Salas, J. R. et al. 18F-FAC PET selectively images hepatic infiltrating CD4 and CD8 T cells in a mouse model of autoimmune hepatitis. J. Nucl. Med. https://doi.org/10.2967/jnumed.118.210328 (2018).
Alam, I. S. et al. Imaging activated T cells predicts response to cancer vaccines. J. Clin. Invest. 128, 2569–2580 (2018).
Pan, Y., Yu, Y., Wang, X. & Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 11, 583084 (2020).
Xiang, X., Wang, J., Lu, D. & Xu, X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal. Transduct. Target. Ther. 6, 75 (2021).
Mukherjee, S., Sonanini, D., Maurer, A. & Daldrup-Link, H. E. The yin and yang of imaging tumor associated macrophages with PET and MRI. Theranostics 9, 7730–7748 (2019).
Blykers, A. et al. PET imaging of macrophage mannose receptor–expressing macrophages in tumor stroma using 18F-radiolabeled camelid single-domain antibody fragments. J. Nucl. Med. 56, 1265–1271 (2015).
Movahedi, K. et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 72, 4165 (2012).
Galli, F. et al. In vivo imaging of natural killer cell trafficking in tumors. J. Nucl. Med. 56, 1575–1580 (2015).
Griss, J. et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 10, 4186 (2019).
Krasniqi, A. et al. Theranostic radiolabeled anti-CD20 sdAb for targeted radionuclide therapy of non-Hodgkin’s lymphoma. Mol. Cancer Ther. 16, 2828 (2017).
Perez, C. R. & De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 10, 5408 (2019).
Ambrosini, V. et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur. J. Cancer 146, 56–73 (2021).
Maffey-Steffan, J. et al. The 68Ga/177Lu-theragnostic concept in PSMA-targeting of metastatic castration-resistant prostate cancer: impact of post-therapeutic whole-body scintigraphy in the follow-up. Eur. J. Nucl. Med. Mol. Imaging 47, 695–712 (2020).
Agdeppa, E. D. & Spilker, M. E. A review of imaging agent development. AAPS J. 11, 286–299 (2009).
Farsad, M. FDG PET/CT in the staging of lung cancer. Curr. Radiopharm. 13, 195–203 (2020).
Gandy, N., Arshad, M. A., Park, W. E., Rockall, A. G. & Barwick, T. D. FDG-PET imaging in cervical cancer. Semin. Nucl. Med. 49, 461–470 (2019).
Groheux, D. et al. 18F-FDG PET/CT for staging and restaging of breast cancer. J. Nucl. Med. 57, 17S–26S (2016).
Weber, W. A. Use of PET for monitoring cancer therapy and for predicting outcome. J. Nucl. Med. 46, 983–995 (2005). This review discusses PET imaging in oncology.
Reinfeld, B. I. et al. Cell-programmed nutrient partitioning in the tumour microenvironment. Nature 593, 282–288 (2021).
Schwenck, J. et al. Cancer immunotherapy is accompanied by distinct metabolic patterns in primary and secondary lymphoid organs observed by non-invasive in vivo 18F-FDG-PET. Theranostics 10, 925–937 (2020).
Lee, S. & Schmitt, C. A. The dynamic nature of senescence in cancer. Nat. Cell Biol. 21, 94–101 (2019).
Schmitz, J. et al. Decoding intratumoral heterogeneity of breast cancer by multiparametric in vivo imaging: a translational study. Cancer Res. 76, 5512–5522 (2016).
Hallqvist, A. et al. Positron emission tomography and computed tomographic imaging (PET/CT) for dose planning purposes of thoracic radiation with curative intent in lung cancer patients: a systematic review and meta-analysis. Radiother. Oncol. 123, 71–77 (2017).
Gehler, B. et al. [68Ga]-DOTATOC-PET/CT for meningioma IMRT treatment planning. Radiat. Oncol. 4, 56 (2009).
Rogowski, P. et al. Radiotherapy of oligometastatic prostate cancer: a systematic review. Radiat. Oncol. 16, 50 (2021).
Bashir, A. et al. Recurrent glioblastoma versus late posttreatment changes: diagnostic accuracy of O-(2-[18F]fluoroethyl)-l-tyrosine positron emission tomography (18F-FET PET). Neuro Oncol. 21, 1595–1606 (2019).
Langen, K. J., Galldiks, N., Hattingen, E. & Shah, N. J. Advances in neuro-oncology imaging. Nat. Rev. Neurol. 13, 279–289 (2017).
Pyka, T. et al. Diagnosis of glioma recurrence using multiparametric dynamic 18F-fluoroethyl-tyrosine PET-MRI. Eur. J. Radiol. 103, 32–37 (2018).
Waaijer, S. J. H. et al. Molecular imaging in cancer drug development. J. Nucl. Med. 59, 726–732 (2018).
Matthews, P. M., Rabiner, E. A., Passchier, J. & Gunn, R. N. Positron emission tomography molecular imaging for drug development. Br. J. Clin. Pharmacol. 73, 175–186 (2012).
Bahce, I. et al. Effects of erlotinib therapy on [11C]erlotinib uptake in EGFR mutated, advanced NSCLC. EJNMMI Res. 6, 10 (2016).
Oosting, S. F. et al. 89Zr-bevacizumab PET visualizes heterogeneous tracer accumulation in tumor lesions of renal cell carcinoma patients and differential effects of antiangiogenic treatment. J. Nucl. Med. 56, 63–69 (2015).
Contractor, K. B. & Aboagye, E. O. Monitoring predominantly cytostatic treatment response with 18F-FDG PET. J. Nucl. Med. 50, 97S–105S (2009).
Pandit-Taskar, N. et al. First-in-humans imaging with 89Zr-Df-IAB22M2C anti-CD8 minibody in patients with solid malignancies: preliminary pharmacokinetics, biodistribution, and lesion targeting. J. Nucl. Med. 61, 512–519 (2020).
Goggi, J. L. et al. Granzyme B PET imaging of combined chemotherapy and immune checkpoint inhibitor therapy in colon cancer. Mol. Imaging Biol. 23, 714–723 (2021).
Acknowledgements
The authors are supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy — EXC 2180-390900677: Cluster of Excellence iFIT ‘Image-Guided and Functionally Instructed Tumour Therapies’.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. B.J.P. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.M.C., J.S., C.la F., L.Z. and B.J.P. hold a patent for the senescence tracer mentioned in this Review. It has not been licensed yet. The Department of Preclinical Imaging and Radiopharmacy (B.J.P.), as well as the Department of Nuclear Medicine (C.la F.) and Department of Radiology at the University of Tübingen, have scientific collaborations with ImaginAb on CD8 imaging. The Department of Preclinical Imaging and Radiopharmacy (B.J.P.) performs contractual productions of the CD8+ tracer (sponsor: ImaginAb). D.S. and H.-G.R. have no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Abass Alavi, Sandip Basu and Rakesh Kumar for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Axial field of view
-
The active imaging field of view of a scanner where, for example, radiation is detected in PET.
- Base excision repair pathway
-
A DNA repair pathway that replaces missing or modified DNA bases, such as those produced by alkylating agents or in spontaneously degraded DNA, with the correct DNA base.
- Chemical exchange saturation transfer
-
MRI method based on protons of a targeted tissue exchanging with molecules of surrounding protons to enhance the MRI contrast.
- Computed tomography
-
(CT). X-ray-based tomographic imaging system.
- Dynamic contrast-enhanced (DCE)-MRI
-
Quantitative temporal measurement of the in vivo distribution of a contrast agent by MRI.
- Hyperpolarized imaging
-
MRI method to probe metabolic information in vivo by using exogenous labelled 13C substrates.
- Magnetic resonance imaging
-
(MRI). Non-radiation-based imaging using the contrast of proton density.
- Megabecquerels
-
Refers to one million becquerel; a becquerel is a unit of measurement of radioactivity equivalent to one nucleus decaying every second.
- Multiplexed imaging
-
Combining different in vivo and ex vivo imaging information.
- Nuclear magnetic resonance (NMR) spectroscopy
-
In vivo detection system revealing the distribution endogenous metabolites in tissue.
- Partial volume effect
-
Refers to an error or underestimation in absolute quantification if the object to be detected is smaller or at the same range of the pixel size of the detector.
- Positron emission tomography
-
(PET). Tomographic in vivo imaging system detecting quantitatively the distribution of an intravenously injected radiolabelled compound to reveal molecular information up to a picomolar sensitivity.
- PET–CT
-
Combination of PET and X-ray CT in one single device enabling image fusion of high spatial accuracy.
- PET–MRI
-
Combination of PET and MRI in one single device enabling simultaneous data acquisition to reveal metabolic, functional, morphological and anatomical parameters at high spatial and temporal fusion accuracy.
- Pharmacokinetics
-
Distribution dynamic of a pharmaceutical, for example, in PET, the kinetics of the radiolabelled tracer.
- Radiomics
-
Quantitative analysis of imaging features such as sphericity, shape and size.
- Raman imaging
-
Inelastic scattering of laser light on matter to reveal spectroscopic information.
- Spectral clustering
-
Analysis of multiparametric images using the spectrum of a similarity matrix.
- T1-weighted (T1W) contrast-enhanced (CE) MRI
-
Uses gadolinium-based contrast agents to enable a more detailed and accurate representation of patho-physiological change, for example, the disruption of the blood–brain barrier and thus helps in differential diagnoses.
- T2-weighted (T2W) axial MRI
-
Used to differentiate between water-bound protons and fat-bound protons providing a strong anatomical contrast in the brain.
- Theranostic approaches
-
The principle of combining diagnostics with therapy, ideally by using one molecule for therapy and diagnosis.
- Tracers
-
Radiolabelled biologically active compounds such as the radiolabelled glucose analogue [18F]FDG.
- Ultrasound
-
(US). Imaging system applying ultrasound waves to reveal soft tissue contrasts in vivo.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schwenck, J., Sonanini, D., Cotton, J.M. et al. Advances in PET imaging of cancer. Nat Rev Cancer 23, 474–490 (2023). https://doi.org/10.1038/s41568-023-00576-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00576-4
This article is cited by
-
Metabolic reprogramming in the tumor microenvironment of liver cancer
Journal of Hematology & Oncology (2024)
-
Single-cell low-pass whole genome sequencing accurately detects circulating tumor cells for liquid biopsy-based multi-cancer diagnosis
npj Precision Oncology (2024)
-
Noninvasive longitudinal PET/CT imaging of CAR T cells using PSMA reporter gene
European Journal of Nuclear Medicine and Molecular Imaging (2024)