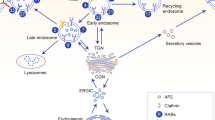
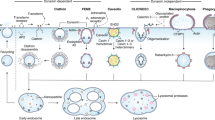
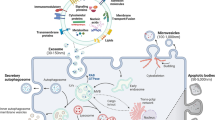
Abstract
Endocytosis is a complex process whereby cell surface proteins, lipids and fluid from the extracellular environment are packaged, sorted and internalized into cells. Endocytosis is also a mechanism of drug internalization into cells. There are multiple routes of endocytosis that determine the fate of molecules, from degradation in the lysosomes to recycling back to the plasma membrane. The overall rates of endocytosis and temporal regulation of molecules transiting through endocytic pathways are also intricately linked with signalling outcomes. This process relies on an array of factors, such as intrinsic amino acid motifs and post-translational modifications. Endocytosis is frequently disrupted in cancer. These disruptions lead to inappropriate retention of receptor tyrosine kinases on the tumour cell membrane, changes in the recycling of oncogenic molecules, defective signalling feedback loops and loss of cell polarity. In the past decade, endocytosis has emerged as a pivotal regulator of nutrient scavenging, response to and regulation of immune surveillance and tumour immune evasion, tumour metastasis and therapeutic drug delivery. This Review summarizes and integrates these advances into the understanding of endocytosis in cancer. The potential to regulate these pathways in the clinic to improve cancer therapy is also discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mellman, I. & Yarden, Y. Endocytosis and cancer. Cold Spring Harb. Perspect. Biol. 5, a016949 (2013).
Goh, L. K. & Sorkin, A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 5, a017459 (2013).
Dacks, J. B., Poon, P. P. & Field, M. C. Phylogeny of endocytic components yields insight into the process of nonendosymbiotic organelle evolution. Proc. Natl Acad. Sci. USA 105, 588–593 (2008).
Uribe-Querol, E. & Rosales, C. Phagocytosis: our current understanding of a universal biological process. Front. Immunol. 11, 1066 (2020).
Stow, J. L., Hung, Y. & Wall, A. A. Macropinocytosis: insights from immunology and cancer. Curr. Opin. Cell Biol. 65, 131–140 (2020). A comprehensive, topical and thought-provoking review on macropinocytosis.
Sandvig, K., Kavaliauskiene, S. & Skotland, T. Clathrin-independent endocytosis: an increasing degree of complexity. Histochem. Cell Biol. 150, 107–118 (2018).
Thottacherry, J. J., Sathe, M., Prabhakara, C. & Mayor, S. Spoiled for choice: diverse endocytic pathways function at the cell surface. Annu. Rev. Cell. Dev. Biol. 35, 55–84 (2019). A comprehensive and topical review of clathrin-dependent and clathrin-independent endocytic pathways.
Howes, M. T. et al. Clathrin-independent carriers form a high capacity endocytic sorting system at the leading edge of migrating cells. J. Cell Biol. 190, 675–691 (2010).
Elkin, S. R. et al. A systematic analysis reveals heterogeneous changes in the endocytic activities of cancer cells. Cancer Res. 75, 4640–4650 (2015).
Schmid, S. L. Reciprocal regulation of signaling and endocytosis: implications for the evolving cancer cell. J. Cell Biol. 216, 2623–2632 (2017). This review demonstrates the heterogeneity of endocytosis in cancer cells.
Sorkin, A. & Von Zastrow, M. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10, 609–622 (2009).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Lanzetti, L. & Di Fiore, P. P. Endocytosis and cancer: an ‘insider’ network with dangerous liaisons. Traffic 9, 2011–2021 (2008).
Sigismund, S., Lanzetti, L., Scita, G. & Di Fiore, P. P. Endocytosis in the context-dependent regulation of individual and collective cell properties. Nat. Rev. Mol. Cell Biol. 22, 625–643 (2021).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021). This review serves as a guide to studying endocytosis. Although focused on endocytosis of therapeutics, the principles outlined apply to all studies of endocytic pathways.
Pereira, P. M. R., Mandleywala, K., Ragupathi, A. & Lewis, J. S. Acute statin treatment improves antibody accumulation in EGFR- and PSMA-expressing tumors. Clin. Cancer Res. 26, 6215–6229 (2020).
Sigismund, S. et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl Acad. Sci. USA 102, 2760–2765 (2005).
Watanabe, S. & Boucrot, E. Fast and ultrafast endocytosis. Curr. Opin. Cell Biol. 47, 64–71 (2017). This review characterizes FEME.
Shah, C. et al. Structural insights into membrane interaction and caveolar targeting of dynamin-like EHD2. Structure 22, 409–420 (2014).
Renard, H.-F. et al. Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis. Nature 517, 493–496 (2015).
Yue, H.-Y. & Xu, J. Cholesterol regulates multiple forms of vesicle endocytosis at a mammalian central synapse. J. Neurochem. 134, 247–260 (2015). This study demonstrates the ubiquitous involvement of cholesterol in cellular trafficking pathways.
Larsson, E., Morén, B., McMahon, K.-A., Parton, R. G. & Lundmark, R. Dynamin2 functions as an accessory protein to reduce the rate of caveola internalization. J. Cell Biol. 222, e202205122 (2023). This paper addresses the role of endocytic proteins in caveolae.
MacDonald, P. E. & Rorsman, P. The ins and outs of secretion from pancreatic β-cells: control of single-vesicle exo- and endocytosis. Physiology 22, 113–121 (2007).
Ma, C.-I. J., Burgess, J. & Brill, J. A. Maturing secretory granules: where secretory and endocytic pathways converge. Adv. Biol. Regul. 80, 100807 (2021).
Sitaram, A. & Marks, M. S. Mechanisms of protein delivery to melanosomes in pigment cells. Physiology 27, 85–99 (2012).
Burgoyne, R. D. & Morgan, A. Secretory granule exocytosis. Physiol. Rev. 83, 581–632 (2003).
de Saint Basile, G., Ménasché, G. & Fischer, A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat. Rev. Immunol. 10, 568–579 (2010).
Biswas, S. et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature 591, 464–470 (2021). A topical review on endocytosis in antigen recognition and immune programming.
Birn, H., Nielsen, S. & Christensen, E. I. Internalization and apical-to-basolateral transport of folate in rat kidney proximal tubule. Am. J. Physiol. Ren. Physiol. 272, F70–F78 (1997).
Dunn, K. W. et al. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am. J. Physiol. Cell Physiol. 283, 905–916 (2002). A rare study examining endocytosis in vivo.
Khandelwal, P., Ruiz, W. G. & Apodaca, G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J. 29, 1961–1975 (2010).
Nakhoul, N. & Batuman, V. Role of proximal tubules in the pathogenesis of kidney disease. Contrib. Nephrol. 169, 37–50 (2011).
Rosendale, M. & Perrais, D. Imaging in focus: Imaging the dynamics of endocytosis. Int. J. Biochem. Cell Biol. 93, 41–45 (2017).
Amornphimoltham, P., Masedunskas, A. & Weigert, R. Intravital microscopy as a tool to study drug delivery in preclinical studies. Adv. Drug Del. Rev. 63, 119–128 (2011).
Pinilla-Macua, I., Grassart, A., Duvvuri, U., Watkins, S. C. & Sorkin, A. EGF receptor signaling, phosphorylation, ubiquitylation and endocytosis in tumors in vivo. eLife 6, e31993 (2017).
Schuh, C. D. et al. Combined structural and functional imaging of the kidney reveals major axial differences in proximal tubule endocytosis. J. Am. Soc. Nephrol. 29, 2696–2712 (2018).
Chen, P.-H. et al. Crosstalk between CLCb/Dyn1-mediated adaptive clathrin-mediated endocytosis and epidermal growth factor receptor signaling increases metastasis. Dev. Cell 40, 278–288 (2017).
Schuuring, E., Verhoeven, E., Mooi, W. J. & Michalides, R. J. A. M. Identification and cloning of two overexpressed genes, U21B31/PRAD1 and EMS1, within the amplified chromosome 11q13 region in human carcinomas. Oncogene 7, 355–361 (1992).
Sargin, B. et al. Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood 110, 1004–1012 (2007).
Koo, B.-K. et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nature 488, 665–669 (2012).
Robinson, B. S. & Moberg, K. H. Drosophila endocytic neoplastic tumor suppressor genes regulate Sav/Wts/Hpo signaling and the c-Jun N-terminal kinase pathway. Cell Cycle 10, 4110–4118 (2011).
Gupta, R., Toufaily, C. & Annabi, B. Caveolin and cavin family members: dual roles in cancer. Biochimie 107, 188–202 (2014).
Li, D., Guo, Y., Tian, S., Zhu, C. & Sun, C. CAV2 regulates Mir-4723/Wnt7A signalling axis through endocytosis and epithelial-mesenchymal transition to promote proliferation, invasion, and metastasis of pancreatic cancer cells. J. Cancer 13, 2200–2212 (2022).
Liu, F., Shangli, Z. & Hu, Z. CAV2 promotes the growth of renal cell carcinoma through the EGFR/PI3K/Akt pathway. OncoTargets Ther. 11, 6209–6216 (2018).
Li, G. & Marlin, M. C. Rab family of GTPases. Methods Mol. Biol. 1298, 1–15 (2015).
Johnson, I. R. D. et al. Endosomal gene expression: a new indicator for prostate cancer patient prognosis? Oncotarget 6, 37919–37929 (2015).
Thomas, J. D. et al. Rab1A Is an mTORC1 activator and a colorectal oncogene. Cancer Cell 26, 754–769 (2014).
Wheeler, D. B., Zoncu, R., Root, D. E., Sabatini, D. M. & Sawyers, C. L. Identification of an oncogenic RAB protein. Science 350, 211–217 (2015).
Jin, H. et al. Rab GTPases: central coordinators of membrane trafficking in cancer. Front. Cell Dev. Biol. 9, 648384 (2021).
Qin, X. et al. Targeting Rabs as a novel therapeutic strategy for cancer therapy. Drug. Discov. Today 22, 1139–1147 (2017).
Raffaniello, R. D. Rab3 proteins and cancer: exit strategies. J. Cell. Biochem. 122, 1295–1301 (2021).
Finicle, B. T., Jayashankar, V. & Edinger, A. L. Nutrient scavenging in cancer. Nat. Rev. Cancer 18, 619–633 (2018).
Forster, J. C., Harriss-Phillips, W. M., Douglass, M. J. & Bezak, E. A review of the development of tumor vasculature and its effects on the tumor microenvironment. Hypoxia 5, 21–32 (2017).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Lum, J. J. et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120, 237–248 (2005).
Su, H. et al. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell 39, 678–693 (2021).
Zhang, M. S. et al. Hypoxia-induced macropinocytosis represents a metabolic route for liver cancer. Nat. Commun. 13, 954 (2022).
Byun, J.-K. et al. Macropinocytosis is an alternative pathway of cysteine acquisition and mitigates sorafenib-induced ferroptosis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 41, 98 (2022).
Delikatny, E. J., Chawla, S., Leung, D.-J. & Poptani, H. MR-visible lipids and the tumor microenvironment. NMR Biomed. 24, 592–611 (2011).
Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019).
Gharpure, K. M. et al. FABP4 as a key determinant of metastatic potential of ovarian cancer. Nat. Commun. 9, 2923 (2018).
Zhang, M. et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 8, 1006–1025 (2018).
Watt, M. J. et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl. Med. 11, eaau5758 (2019).
Bi, J. et al. Altered cellular metabolism in gliomas — an emerging landscape of actionable co-dependency targets. Nat. Rev. Cancer 20, 57–70 (2020).
Mulcahy, L. A., Pink, R. C. & Carter, D. R. F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 3, 24641 (2014).
Cerezo-Magaña, M., Christianson, H. C., Van Kuppevelt, T. H., Forsberg-Nilsson, K. & Belting, M. Hypoxic induction of exosome uptake through proteoglycan-dependent endocytosis fuels the lipid droplet phenotype in glioma. Mol. Cancer Res. 19, 528–540 (2021).
Li, P. et al. Lung mesenchymal cells elicit lipid storage in neutrophils that fuel breast cancer lung metastasis. Nat. Immunol. 21, 1444–1455 (2020). This study demonstrates metabolic crosstalk between neutrophils and metastatic tumour cells in the form of endocytosis of lipids, which in turn supports cancer cell growth.
Zhang, Y. et al. Macropinocytosis in cancer-associated fibroblasts is dependent on CaMKK2/ARHGEF2 signaling and functions to support tumor and stromal cell fitness. Cancer Discov. 11, 1808–1825 (2021).
Bar-Sagi, D. & Feramisco, J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068 (1986).
Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. & Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 (1992).
Ryan, M. B. & Corcoran, R. B. Therapeutic strategies to target RAS-mutant cancers. Nat. Rev. Clin. Oncol. 15, 709–720 (2018).
Raphael, B. J. et al. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell 32, 185–203 (2017).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013).
Prior, I. A., Hood, F. E. & Hartley, J. L. The frequency of Ras mutations in cancer. Cancer Res. 80, 2969–2974 (2020).
Davidson, S. M. et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 23, 235–241 (2017).
Hobbs, G. A. & Der, C. J. Binge drinking: macropinocytosis promotes tumorigenic growth of RAS-mutant cancers. Trends Biochem. Sci. 45, 459–461 (2020).
Hobbs, G. A. et al. Atypical KRASG12R mutant is impaired in PI3K signaling and macropinocytosis in pancreatic cancer. Cancer Discov. 10, 104–123 (2020).
King, B., Araki, J., Palm, W. & Thompson, C. B. Yap/Taz promote the scavenging of extracellular nutrients through macropinocytosis. Genes Dev. 34, 1345–1358 (2020).
O’Bryan, J. P. et al. axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell. Biol. 11, 5016–5031 (1991).
Goyette, M.-A. & Côté, J.-F. AXL receptor tyrosine kinase as a promising therapeutic target directing multiple aspects of cancer progression and metastasis. Cancers 14, 466 (2022).
Zdżalik-Bielecka, D. et al. The GAS6-AXL signaling pathway triggers actin remodeling that drives membrane ruffling, macropinocytosis, and cancer-cell invasion. Proc. Natl Acad. Sci. USA 118, e2024596118 (2021). This paper provides mechanistic detail of the signalling that drives macropinocytosis and cancer cell invasion.
Liu, H. et al. KRAS-enhanced macropinocytosis and reduced FcRn-mediated recycling sensitize pancreatic cancer to albumin-conjugated drugs. J. Control. Release 296, 40–53 (2019).
Kratz, F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 132, 171–183 (2008).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 46, 6387–6392 (1986).
Nazha, B., Moussaly, E., Zaarour, M., Weerasinghe, C. & Azab, B. Hypoalbuminemia in colorectal cancer prognosis: nutritional marker or inflammatory surrogate? World J. Gastrointest. Surg. 7, 370–377 (2015).
Fujii, T. et al. Implications of low serum albumin as a prognostic factor of long-term outcomes in patients with breast cancer. Vivo 34, 2033–2036 (2020).
Kang, B. et al. Effect of hypoalbuminemia on short-term outcomes after colorectal cancer surgery: a propensity score matching analysis. Front. Nutr. 9, 925086 (2022).
Sutton, M. N. et al. RAS-driven macropinocytosis of albumin or dextran reveals mutation-specific target engagement of RAS p.G12C inhibitor ARS-1620 by NIR-fluorescence imaging. Mol. Imag. Biol. 24, 498–509 (2022).
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015).
Bern, M., Sand, K. M. K., Nilsen, J., Sandlie, I. & Andersen, J. T. The role of albumin receptors in regulation of albumin homeostasis: implications for drug delivery. J. Control. Release 211, 144–162 (2015).
Swiercz, R. et al. Loss of expression of the recycling receptor, FcRn, promotes tumor cell growth by increasing albumin consumption. Oncotarget 8, 3528–3541 (2017).
Baker, K. et al. Neonatal Fc receptor for IgG (FcRn) regulates cross-presentation of IgG immune complexes by CD8-CD11b+ dendritic cells. Proc. Natl Acad. Sci. USA 108, 9927–9932 (2011).
Baker, K. et al. Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer. Immunity 39, 1095–1107 (2013).
Dalloneau, E. et al. Downregulation of the neonatal Fc receptor expression in non-small cell lung cancer tissue is associated with a poor prognosis. Oncotarget 7, 54415–54429 (2016).
Torphy, R. J. et al. GPR182 limits antitumor immunity via chemokine scavenging in mouse melanoma models. Nat. Commun. 13, 97 (2022).
Xing, Q. et al. Scavenger receptor MARCO contributes to macrophage phagocytosis and clearance of tumor cells. Exp. Cell Res. 408, 112862–112871 (2021).
Plygawko, A. T., Kan, S. & Campbell, K. Epithelial–mesenchymal plasticity: emerging parallels between tissue morphogenesis and cancer metastasis. Philos. Trans. R. Soc. B Biol. Sci. 375, 20200087 (2020).
Bhatia, S., Wang, P., Toh, A. & Thompson, E. W. New insights into the role of phenotypic plasticity and EMT in driving cancer progression. Front. Mol. Biosci. 7, 71 (2020).
Mosesson, Y., Mills, G. B. & Yarden, Y. Derailed endocytosis: an emerging feature of cancer. Nat. Rev. Cancer 8, 835–850 (2008).
Goossens, S., Vandamme, N., Van Vlierberghe, P. & Berx, G. EMT transcription factors in cancer development re-evaluated: beyond EMT and MET. Biochim. Biophys. Acta Rev. Cancer 1868, 584–591 (2017).
Castosa, R. et al. Hakai overexpression effectively induces tumour progression and metastasis in vivo. Sci. Rep. 8, 3466 (2018).
Fujita, Y. et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4, 222–231 (2002).
Katsuno-Kambe, H. & Yap, A. S. Endocytosis, cadherins and tissue dynamics. Traffic 21, 268–273 (2020).
Schiano Lomoriello, I. et al. A self-sustaining endocytic-based loop promotes breast cancer plasticity leading to aggressiveness and pro-metastatic behavior. Nat. Commun. 11, 3020 (2020).
Choi, H. J. et al. Transforming growth factor β1 enhances adhesion of endometrial cells to mesothelium by regulating integrin expression. BMB Rep. 50, 429–434 (2017).
Wang, S. E. et al. Transforming growth factor β induces clustering of HER2 and integrins by activating Src-focal adhesion kinase and receptor association to the cytoskeleton. Cancer Res. 69, 475–482 (2009).
Manshouri, R. et al. ZEB1/NuRD complex suppresses TBC1D2b to stimulate E-cadherin internalization and promote metastasis in lung cancer. Nat. Commun. 10, 5125 (2019).
Deng, Y. et al. Prognostic value of flotillins (flotillin-1 and flotillin-2) in human cancers: a meta-analysis. Clin. Chim. Acta 481, 90–98 (2018).
Greenlee, J. D., Subramanian, T., Liu, K. & King, M. R. Rafting down the metastatic cascade: the role of lipid rafts in cancer metastasis, cell death, and clinical outcomes. Cancer Res. 81, 5–17 (2021).
Doherty, S. D., Prieto, V. G., George, S., Hazarika, P. & Duvic, M. High flotillin-2 expression is associated with lymph node metastasis and Breslow depth in melanoma. Melanoma Res. 16, 461–463 (2006).
Glebov, O. O., Bright, N. A. & Nichols, B. J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 8, 46–54 (2006).
Gauthier-Rouvière, C., Bodin, S., Comunale, F. & Planchon, D. Flotillin membrane domains in cancer. Cancer Metastasis Rev. 39, 361–374 (2020).
Genest, M. et al. Upregulated flotillins and sphingosine kinase 2 derail AXL vesicular traffic to promote epithelial-mesenchymal transition. J. Cell Sci. 135, jcs259178 (2022).
Antony, J. & Huang, R. Y.-J. AXL-driven EMT state as a targetable conduit in cancer. Cancer Res. 77, 3725–3732 (2017).
Rodriguez-Boulan, E. & Macara, I. G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225–242 (2014).
Thottacherry, J. J., Chen, J. & Johnston, D. S. Apical–basal polarity in the gut. Semin. Cell Dev. Biol. https://doi.org/10.1016/j.semcdb.2022.12.007 (2023).
Shivas, J. M., Morrison, H. A., Bilder, D. & Skop, A. R. Polarity and endocytosis: reciprocal regulation. Trends Cell Biol. 20, 445–452 (2010).
Ballestrem, C., Hinz, B., Imhof, B. A. & Wehrle-Halle, B. Marching at the front and dragging behind differential αVβ3-integrin turnover regulates focal adhesion behavior. J. Cell Biol. 155, 1319–1332 (2001).
Partridge, M. A. & Marcantonio, E. E. Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol. Biol. Cell 17, 4237–4248 (2006).
Nader, G. P. F., Ezratty, E. J. & Gundersen, G. G. FAK, talin and PIPKIγ regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat. Cell Biol. 18, 491–503 (2016).
Banerjee, P. et al. The EMT activator ZEB1 accelerates endosomal trafficking to establish a polarity axis in lung adenocarcinoma cells. Nat. Commun. 12, 6354 (2021).
Zhong, Y. et al. MYH9-dependent polarization of ATG9B promotes colorectal cancer metastasis by accelerating focal adhesion assembly. Cell Death Differ. 28, 3251–3269 (2021).
Cong, M. et al. MTSS1 suppresses mammary tumor-initiating cells by enhancing RBCK1-mediated p65 ubiquitination. Nat. Cancer 1, 222–234 (2020).
Wu, Z. et al. RNF180 mediates STAT3 activity by regulating the expression of RhoC via the proteasomal pathway in gastric cancer cells. Cell Death Dis. 11, 881 (2020).
Zhu, J. et al. The ubiquitin ligase RNF181 stabilizes ERα and modulates breast cancer progression. Oncogene 39, 6776–6788 (2020).
Tsunoda, T. et al. ENTREP/FAM189A2 encodes a new ITCH ubiquitin ligase activator that is downregulated in breast cancer. EMBO Rep. 23, e51182 (2022).
Sposini, S. et al. Imaging endocytic vesicle formation at high spatial and temporal resolutions with the pulsed-pH protocol. Nat. Protoc. 15, 3088–3104 (2020).
Ranftler, C. et al. in Cell Imaging Techniques: Methods and Protocols (eds Taatjes, D. J. & Roth, J.) 437–447 (Humana Press, 2013).
Baek, J. et al. Imaging galectin-3 dependent endocytosis with lattice light-sheet microscopy. SPIE Proc. Int. Conf. Biophotonics V 10340, 103400J, https://doi.org/10.1117/12.2275706 (2017).
Charpentier, J. C. & King, P. D. Mechanisms and functions of endocytosis in T cells. Cell Commun. Signal. 19, 92 (2021).
Burgdorf, S. & Kurts, C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 20, 89–95 (2008).
Compeer, E. B. et al. A mobile endocytic network connects clathrin-independent receptor endocytosis to recycling and promotes T cell activation. Nat. Commun. 9, 1597 (2018). This study describes the interplay between endocytosis and T cell responses.
Evnouchidou, I. et al. IRAP-dependent endosomal T cell receptor signalling is essential for T cell responses. Nat. Commun. 11, 2779 (2020).
Masilamani, M., Peruzzi, G., Borrego, F. & Coligan, J. E. Endocytosis and intracellular trafficking of human natural killer cell receptors. Traffic 10, 1735–1744 (2009).
Béguin, E. P. et al. Endocytosis by macrophages: interplay of macrophage scavenger receptor-1 and LDL receptor-related protein-1. Haematologica 105, e133–e137 (2020).
McShane, A. N. & Malinova, D. The ins and outs of antigen uptake in B cells. Front. Immunol. 13, 892169 (2022).
Lanzetti, L. & Di Fiore, P. P. Behind the scenes: endo/exocytosis in the acquisition of metastatic traits. Cancer Res. 77, 1813–1817 (2017). A comprehensive and topical review of endocytosis involvement in metastasis.
Khan, I. & Steeg, P. S. Endocytosis: a pivotal pathway for regulating metastasis. Br. J. Cancer 124, 66–75 (2021).
Safaei, S. et al. Overexpression of cytoplasmic dynamin 2 is associated with worse outcomes in patients with clear cell renal cell carcinoma. Cancer Biomark. 35, 27–45 (2022).
Wang, X. et al. lncRNA HITT inhibits metastasis by attenuating Rab5-mediated endocytosis in lung adenocarcinoma. Mol. Ther. 30, 1071–1088 (2022).
Wang, D., Liu, S. & Wang, G. Establishment of an endocytosis-related prognostic signature for patients with low-grade glioma. Front. Genet. 12, 709666 (2021).
Abu-Thuraia, A. et al. AXL confers cell migration and invasion by hijacking a PEAK1-regulated focal adhesion protein network. Nat. Commun. 11, 3586 (2020).
He, Z. et al. Dynamic regulation of KIF15 phosphorylation and acetylation promotes focal adhesions disassembly in pancreatic cancer. Cell Death Dis. 13, 896 (2022).
Ramsay, A. G. et al. HS1-associated protein X-1 regulates carcinoma cell migration and invasion via clathrin-mediated endocytosis of integrin αvβ6. Cancer Res. 67, 5275–5284 (2007).
de Beco, S., Gueudry, C., Amblard, F. & Coscoy, S. Endocytosis is required for E-cadherin redistribution at mature adherens junctions. Proc. Natl Acad. Sci. USA 106, 7010–7015 (2009).
Chen, H.-R. et al. DDR1 promotes E-cadherin stability via inhibition of integrin-β1-Src activation-mediated E-cadherin endocytosis. Sci. Rep. 6, 36336 (2016).
Diggins, N. L., Kang, H., Weaver, A. & Webb, D. J. α5β1 integrin trafficking and Rac activation are regulated by APPL1 in a Rab5-dependent manner to inhibit cell migration. J. Cell Sci. 131, jcs207019 (2018).
Cheung, K. J. et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863 (2016).
Ring, A., Spataro, M., Wicki, A. & Aceto, N. Clinical and biological aspects of disseminated tumor cells and dormancy in breast cancer. Front. Cell Dev. Biol. 10, 929893 (2022).
Elkhatib, N., Porshneva, K. & Montagnac, G. Migration cues interpretation by clathrin-coated structures. Curr. Opin. Cell Biol. 72, 100–105 (2021).
Wang, L.-T., Rajah, A., Brown, C. M. & McCaffrey, L. CD13 orients the apical-basal polarity axis necessary for lumen formation. Nat. Commun. 12, 4697 (2021).
Bretscher, M. S. Endocytosis and recycling of the fibronectin receptor in CHO cells. EMBO J. 8, 1341–1348 (1989).
Rappoport, J. Z. & Simon, S. M. Real-time analysis of clathrin-mediated endocytosis during cell migration. J. Cell Sci. 116, 847–855 (2003).
Powelka, A. M. et al. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic 5, 20–36 (2004).
Moreno-Layseca, P. et al. Cargo-specific recruitment in clathrin- and dynamin-independent endocytosis. Nat. Cell Biol. 23, 1073–1084 (2021). An elegant study of cargo selection in endocytic pathways.
Xu, H. et al. Hypoxia stimulates invasion and migration of human cervical cancer cell lines HeLa/SiHa through the Rab11 trafficking of integrin αvβ3/FAK/PI3K pathway-mediated Rac1 activation. J. Biosci. 42, 491–499 (2017).
Tehran, D. A., López-Hernández, T. & Maritzen, T. Endocytic adaptor proteins in health and disease: lessons from model organisms and human mutations. Cells 8, 1345 (2019).
Raman, D., Sai, J., Hawkins, O. & Richmond, A. Adaptor protein2 (AP2) orchestrates CXCR2-mediated cell migration. Traffic 15, 451–469 (2014).
Xu, B. et al. The significance of dynamin 2 expression for prostate cancer progression, prognostication, and therapeutic targeting. Cancer Med. 3, 14–24 (2014).
Chen, P.-W., Gasilina, A., Yadav, M. P. & Randazzo, P. A. Control of cell signaling by Arf GTPases and their regulators: focus on links to cancer and other GTPase families. Biochim. Biophys. Acta Mol. Cell Res. 1869, 119171 (2022).
Qi, S. et al. Arf6-driven endocytic recycling of CD147 determines HCC malignant phenotypes. J. Exp. Clin. Cancer Res. 38, 471 (2019).
Parton, R. G., Tillu, V., McMahon, K.-A. & Collins, B. M. Key phases in the formation of caveolae. Curr. Opin. Cell Biol. 71, 7–14 (2021).
Singh, V. & Lamaze, C. Membrane tension buffering by caveolae: a role in cancer? Cancer Metastasis Rev. 39, 505–517 (2020).
Joshi, B. et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 68, 8210–8220 (2008).
Combedazou, A. et al. Small GTPases orchestrate cell-cell communication during collective cell movement. Small GTPases 11, 103–112 (2020).
Olayioye, M. A., Noll, B. & Hausser, A. Spatiotemporal control of intracellular membrane trafficking by Rho GTPases. Cells 8, 1478 (2019).
Wang, T. et al. RHO GTPase family in hepatocellular carcinoma. Exp. Hematol. Oncol. 11, 91 (2022).
Zhang, J., Jiang, Z. & Shi, A. Rab GTPases: the principal players in crafting the regulatory landscape of endosomal trafficking. Comput. Struct. Biotechnol. J. 20, 4464–4472 (2022).
Sun, L. et al. Rab34 regulates adhesion, migration, and invasion of breast cancer cells. Oncogene 37, 3698–3714 (2018).
Yu, M. H., Luo, Y., Qin, S. L. & Zhong, M. Increased expression of Rab5A predicts metastasis and poor prognosis in colorectal cancer patients. Int. J. Exp. Pathol. 8, 6974–6980 (2015).
Wen, F. et al. Characterization of prognostic value and immunological roles of RAB22A in hepatocellular carcinoma. Front. Immunol. 14, 1086342 (2023).
Liu, Q. et al. High RAS-related protein Rab-7a (RAB7A) expression is a poor prognostic factor in pancreatic adenocarcinoma. Sci. Rep. 12, 17492 (2022).
Jiang, Y. et al. Rab23 is overexpressed in human bladder cancer and promotes cancer cell proliferation and invasion. Tumor Biol. 37, 8131–8138 (2016).
Luo, X. et al. Rab22a promotes epithelial-mesenchymal transition in papillary thyroid carcinoma by activating PI3K/AKT/mTOR signaling pathway. Biomed. Res. Int. 2022, 1874550 (2022).
Mavrakis, M. & Juanes, M. A. The compass to follow: focal adhesion turnover. Curr. Opin. Cell Biol. 80, 102152 (2023).
Lundmark, R. et al. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr. Biol. 8, 1802–1808 (2008).
Sathe, M. et al. Small GTPases and BAR domain proteins regulate branched actin polymerisation for clathrin and dynamin-independent endocytosis. Nat. Commun. 9, 1835 (2018).
Bergers, G. & Fendt, S.-M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Zhang, S. et al. Long-chain fatty acyl coenzyme A inhibits NME1/2 and regulates cancer metastasis. Proc. Natl Acad. Sci. USA 119, e2117013119 (2022).
Boissan, M. et al. Nucleoside diphosphate kinases fuel dynamin superfamily proteins with GTP for membrane remodeling. Science 344, 1510–1515 (2014).
Dammai, V. Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev. 17, 2812–2824 (2003).
Ignesti, M. et al. Notch signaling during development requires the function of awd, the Drosophila homolog of human metastasis suppressor gene Nm23. BMC Biol. 12, 12 (2014).
Khan, I., Gril, B. & Steeg, P. S. Metastasis suppressors NME1 and NME2 promote dynamin 2 oligomerization and regulate tumor cell endocytosis, motility, and metastasis. Cancer Res. 79, 4689–4702 (2019).
Nallamothu, G., Woolworth, J. A., Dammai, V. & Hsu, T. Awd, the homolog of metastasis suppressor gene Nm23, regulates Drosophila epithelial cell invasion. Mol. Cell. Biol. 28, 1964–1973 (2008).
Woolworth, J. A., Nallamothu, G. & Hsu, T. The Drosophila metastasis suppressor gene nm23 homolog, awd, regulates epithelial integrity during oogenesis. Mol. Cell. Biol. 29, 4679–4690 (2009).
Schlattner, U. The complex functions of the NME family — a matter of location and molecular activity. Int. J. Mol. Sci. 22, 13083 (2021).
Kaźmierczak, Z. et al. Endocytosis in cellular uptake of drug delivery vectors: molecular aspects in drug development. Bioorg. Med. Chem. 28, 115556 (2020).
Ge, Y., Huang, M. & Yao, Y.-M. The effect and regulatory mechanism of high mobility group box-1 protein on immune cells in inflammatory diseases. Cells 10, 1044 (2021).
Gao, Q. et al. High mobility group protein B1 decreases surface localization of PD-1 to augment T-cell activation. Cancer Immunol. Res. 10, 844–855 (2022).
Scott, A. M., Wolchok, J. D. & Old, L. J. Antibody therapy of cancer. Nat. Rev. Cancer 12, 278–287 (2012). A perspective on applying knowledge of endocytosis to drug delivery design.
Roghanian, A. et al. Antagonistic human FcγRIIB (CD32B) antibodies have anti-tumor activity and overcome resistance to antibody therapy in vivo. Cancer Cell 27, 473–488 (2015).
Chew, H. Y. et al. Endocytosis inhibition in humans to improve responses to ADCC-mediating antibodies. Cell 180, 895–914 (2020). This study demonstrates that endocytosis can be temporarily and reversibly inhibited in humans in the clinical setting.
Pereira, P. M. R. et al. Caveolin-1 mediates cellular distribution of HER2 and affects trastuzumab binding and therapeutic efficacy. Nat. Commun. 9, 5137 (2018). This study demonstrates effects of endocytosis on trastuzumab binding and efficacy.
Scaltriti, M. et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 28, 803–814 (2009).
Tibbetts, R. et al. Anti-disialoganglioside antibody internalization by neuroblastoma cells as a mechanism of immunotherapy resistance. Cancer Immunol. Immunother. 71, 153–164 (2022).
Ritchie, M., Tchistiakova, L. & Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. mAbs 5, 13–21 (2013).
Tsurutani, J. et al. Targeting HER2 with trastuzumab deruxtecan: a dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 10, 688–701 (2020).
Yan, H., Yu, K., Zhang, K., Liu, L. & Li, Y. Efficacy and safety of trastuzumab emtansine (T-DM1) in the treatment of HER2-positive metastatic breast cancer (MBC): a meta-analysis of randomized controlled trial. Oncotarget 8, 102458–102467 (2017).
Li, B. T. et al. HER2-mediated internalization of cytotoxic agents in ERBB2 amplified or mutant lung cancers. Cancer Discov. 10, 674–687 (2020).
Robichaux, J. P. et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 36, 444–457 (2019).
Joseph, S. R. et al. Antibody/ligand-target receptor internalization assay protocol using fresh human or murine tumor ex vivo samples. Star. Protoc. 1, 100087 (2020).
Joseph, S. R. et al. An ex vivo human tumor assay shows distinct patterns of EGFR trafficking in squamous cell carcinoma correlating to therapeutic outcomes. J. Invest. Dermatol. 139, 213–223 (2019). This study demonstrates in ex vivo tumour samples from patients that innate endocytosis is disrupted in squamous cell carcinoma.
Lum, B. et al. Why does endocytosis inhibition lead to increased endosomal delivery of ADC/RLTs: pharmacological reversal. Cell Symposia: Hallmarks of Cancer (Fraunhofer ITEM, 2022).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Zhang, J. et al. A prostate-specific membrane antigen activated molecular rotor for real-time fluorescence imaging. Nat. Commun. 12, 5460 (2021).
Yang, J. et al. Endocytosis pathway self-regulation for precise image-guided therapy through an enzyme-responsive modular peptide probe. Anal. Chem. 94, 7960–7969 (2022).
Australian Clinical Trials. australianclinicaltrials.gov.au, https://www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12619001051134 (2019a).
Australian Clinical Trials. australianclinicaltrials.gov.au, https://www.australianclinicaltrials.gov.au/anzctr/trial/ACTRN12619001527156 (2019b).
Trochet, D. & Bitoun, M. A review of Dynamin 2 involvement in cancers highlights a promising therapeutic target. J. Exp. Clin. Cancer Res. 40, 238 (2021).
Tremblay, C. S. et al. Small molecule inhibition of Dynamin-dependent endocytosis targets multiple niche signals and impairs leukemia stem cells. Nat. Commun. 11, 6211 (2020).
Feng, M. et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 19, 568–586 (2019).
Van Der Bliek, A. M. & Meyerowrtz, E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature 351, 411–414 (1991).
Pereira, P. M. R. et al. Caveolin-1 temporal modulation enhances antibody drug efficacy in heterogeneous gastric cancer. Nat. Commun. 13, 2526 (2022). This study demonstrates the involvement of caveolin-1 in antibody drug efficacy.
Daniel, J. A. et al. Phenothiazine-derived antipsychotic drugs inhibit dynamin and clathrin-mediated endocytosis. Traffic 16, 635–654 (2015).
McCluskey, A. et al. Building a better dynasore: the dyngo compounds potently inhibit dynamin and endocytosis. Traffic 14, 1272–1289 (2013).
Bridge, J. A. et al. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am. J. Pathol. 159, 411–415 (2001).
Reis, C. R. et al. Crosstalk between Akt/GSK3β signaling and dynamin-1 regulates clathrin-mediated endocytosis. EMBO J. 34, 2132–2146 (2015).
Eppinga, R. D. et al. Increased expression of the large GTPase dynamin 2 potentiates metastatic migration and invasion of pancreatic ductal carcinoma. Oncogene 31, 1228–1241 (2012).
Lee, Y. Y. et al. Low dynamin 2 expression is associated with tumor invasion and metastasis in invasive squamous cell carcinoma of cervix. Cancer Biol. Ther. 10, 329–335 (2010).
Inokawa, Y. et al. Dynamin 3: a new candidate tumor suppressor gene in hepatocellular carcinoma detected by triple combination array analysis. OncoTargets Ther. 6, 1417–1424 (2013).
Fa, J. Dynamin 3 overexpression suppresses the proliferation, migration and invasion of cervical cancer cells. Oncol. Lett. 22, 524 (2021).
Lu, Q. et al. Dynamin 3 inhibits the proliferation of non-small-cell lung cancer cells by suppressing c-MET-GBR2-STAT3 complex formation. Front. Cell Dev. Biol. 9, 641403 (2021).
Jiao, J., Jiang, L. & Luo, Y. N6-methyladenosine-related RNA signature predicting the prognosis of ovarian cancer. Recent. Pat. Anticancer Drug Discov. 16, 407–416 (2021).
Huang, P., Guo, Y.-D. & Zhang, H.-W. Identification of Hub genes in pediatric medulloblastoma by multiple-microarray analysis. J. Mol. Neurosci. 70, 522–531 (2020).
Saafan, H. et al. Utilising the EGFR interactome to identify mechanisms of drug resistance in non-small cell lung cancer – Proof of concept towards a systems pharmacology approach. Eur. J. Pharm. Sci. 94, 20–32 (2016).
Kaikkonen, E., Takala, A., Pursiheimo, J.-P., Wahlström, G. & Schleutker, J. The interactome of the prostate-specific protein Anoctamin 7. Cancer Biomark. 28, 91–100 (2020).
Rangel, R. et al. Identification of new tumor suppressor genes in triple-negative breast cancer. Cancer Res. 77, 4089–4101 (2017).
Cho, S. H. et al. The AP2M1 gene expression is a promising biomarker for predicting survival of patients with hepatocellular carcinoma. J. Cell. Biochem. 120, 4140–4146 (2019).
Wu, C.-C., Li, H., Xiao, Y., Deng, W.-W. & Sun, Z.-J. Expression levels of SIX1, ME2, and AP2M1 in adenoid cystic carcinoma and mucoepidermoid carcinoma. Oral. Dis. 26, 1687–1695 (2020).
Buser, D. P. et al. Quantitative proteomics reveals reduction of endocytic machinery components in gliomas. EBioMedicine 46, 32–41 (2019).
Rogaia, D. et al. The localization of the HRX/ALL1 protein to specific nuclear subdomains is altered by fusion with its eps15 translocation partner. Cancer Res. 57, 799–802 (1997).
Yamamoto, K. et al. Expression of a novel type of KMT2A/EPS15 fusion transcript in FLT3 mutation-positive B-lymphoblastic leukemia with t(1;11)(p32;q23). Cancer Genet. 254-255, 92–97 (2021).
Coon, B. G., Burgner, J., Camonis, J. H. & Aguilar, R. C. The epsin family of endocytic adaptors promotes fibrosarcoma migration and invasion. J. Biol. Chem. 285, 33073–33081 (2010).
Spradling, K. D., McDaniel, A. E., Lohi, J. & Pilcher, B. K. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. J. Biol. Chem. 276, 29257–29267 (2001).
Wang, Y., Dai, Z., Sadee, W. & Hancock, W. S. A pharmacoproteomics study of the cancer cell line EKVX using capillary-LC/MS/MS. Mol. Pharm. 3, 566–578 (2006).
Tessneer, K. L. et al. Endocytic adaptor protein epsin is elevated in prostate cancer and required for cancer progression. ISRN Oncol. 2013, 420597 (2013).
Pawlowski, K. M. et al. Comparison of cellular and tissue transcriptional profiles in canine mammary tumor. J. Physiol. Pharmacol. 60, 85–94 (2009).
Caligiuri, M. A. et al. Novel c-CBL and CBL-b ubiquitin ligase mutations in human acute myeloid leukemia. Blood 110, 1022–1024 (2007).
Belizaire, R. et al. CBL mutations drive PI3K/AKT signaling via increased interaction with LYN and PIK3R1. Blood 137, 2209–2220 (2021).
Davidson, B. et al. Caveolin-1 expression in advanced-stage ovarian carcinoma — a clinicopathologic study. Gynecol. Oncol. 81, 166–171 (2001).
Savage, K. et al. Caveolin 1 Is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin. Cancer Res. 13, 90–101 (2007).
Yan, J., Lu, Q., Cai, L., Li, X. W. & Dong, J. H. Expression of caveolin-1 gene in hepatitis B-related hepatocellular carcinoma and the clinical significance thereof. Zhonghua Yi Xue Za Zhi 88, 3272–3274 (2008).
Hung, K.-F. et al. The biphasic differential expression of the cellular membrane protein, caveolin-1, in oral carcinogenesis. J. Oral. Pathol. Med. 32, 461–467 (2003).
Cantiani, L. et al. Caveolin-1 reduces osteosarcoma metastases by inhibiting c-Src activity and Met signaling. Cancer Res. 67, 7675–7685 (2007).
Campbell, L. et al. Caveolin-1 in renal cell carcinoma promotes tumour cell invasion, and in co-operation with pERK predicts metastases in patients with clinically confined disease. J. Transl. Med. 11, 255 (2013).
Chatterjee, M. et al. Caveolin-1 is associated with tumor progression and confers a multi-modality resistance phenotype in pancreatic cancer. Sci. Rep. 5, 10867 (2015).
Ando, T. et al. The overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinoma. Oncol. Rep. 18, 601–609 (2007).
Iwaya, K., Norio, K. & Mukai, K. Coexpression of Arp2 and WAVE2 predicts poor outcome in invasive breast carcinoma. Mod. Pathol. 20, 339–343 (2007).
Iwaya, K. et al. Correlation between liver metastasis of the colocalization of actin-related protein 2 and 3 complex and WAVE2 in colorectal carcinoma. Cancer Sci. 98, 992–999 (2007).
Semba, S. et al. Coexpression of actin-related protein 2 and Wiskott-Aldrich syndrome family verproline-homologous protein 2 in adenocarcinoma of the lung. Clin. Cancer Res. 12, 2449–2454 (2006).
Liu, Z. et al. Expression of the Arp2/3 complex in human gliomas and its role in the migration and invasion of glioma cells. Oncol. Rep. 30, 2127–2136 (2013).
Gligorijevic, B. et al. N-WASP-mediated invadopodium formation is involved in intravasation and lung metastasis of mammary tumors. J. Cell Sci. 125, 724–734 (2012).
Ishihara, D. et al. Wiskott-Aldrich syndrome protein regulates leukocyte-dependent breast cancer metastasis. Cell Rep. 4, 429–436 (2013).
Fernando, H. S., Sanders, A. J., Kynaston, H. G. & Jiang, W. G. WAVE1 is associated with invasiveness and growth of prostate cancer cells. J. Urol. 180, 1515–1521 (2008).
Fernando, H. S., Sanders, A. J., Kynaston, H. G. & Jiang, W. G. WAVE3 is associated with invasiveness in prostate cancer cells. Urol. Oncol.: Semin. Orig. Investig. 28, 320–327 (2010).
Taylor, M. A. et al. Upregulated WAVE3 expression is essential for TGF-β-mediated EMT and metastasis of triple-negative breast cancer cells. Breast Cancer Res. Treat. 142, 341–353 (2013).
Sossey-Alaoui, K. et al. Down-regulation of WAVE3, a metastasis promoter gene, inhibits invasion and metastasis of breast cancer cells. Am. J. Pathol. 170, 2112–2121 (2007).
Kang, R. et al. WAVE1 regulates Bcl-2 localization and phosphorylation in leukemia cells. Leukemia 24, 177–186 (2010).
Kurisu, S., Suetsugu, S., Yamazaki, D., Yamaguchi, H. & Takenawa, T. Rac-WAVE2 signaling is involved in the invasive and metastatic phenotypes of murine melanoma cells. Oncogene 24, 1309–1319 (2005).
Sossey-Alaoui, K., Su, G., Malaj, E., Roe, B. & Cowell, J. K. WAVE3, an actin-polymerization gene, is truncated and inactivated as a result of a constitutional t(1;13)(q21;q12) chromosome translocation in a patient with ganglioneuroblastoma. Oncogene 21, 5967–5974 (2002).
Clark, E. S., Whigham, A. S., Yarbrough, W. G. & Weaver, A. M. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 67, 4227–4235 (2007).
Clark, E. S. & Weaver, A. M. A new role for cortactin in invadopodia: regulation of protease secretion. Eur. J. Cell Biol. 87, 581–590 (2008).
Luo, M.-L. et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 66, 11690–11699 (2006).
Hui, R. et al. EMS1 gene expression in primary breast cancer: relationship to cyclin D1 and oestrogen receptor expression and patient survival. Oncogene 17, 1053–1059 (1998).
Yuan, B.-Z., Zhou, X., Zimonjic, D. B., Durkin, M. E. & Popescu, N. C. Amplification and overexpression of the EMS 1 oncogene, a possible prognostic marker, in human hepatocellular carcinoma. J. Mol. Diagn. 5, 48–53 (2003).
Zaharieva, B. M. et al. High-throughput tissue microarray analysis of 11q13 gene amplification (CCND1, FGF3, FGF4, EMS1) in urinary bladder cancer. J. Pathol. 201, 603–608 (2003).
Wang, L. et al. Expression of cortactin in human gliomas and its effect on migration and invasion of glioma cells. Oncol. Rep. 34, 1815–1824 (2015).
Timpson, P., Lynch, D. K., Schramek, D., Walker, F. & Daly, R. J. Cortactin overexpression inhibits ligand-induced down-regulation of the epidermal growth factor receptor. Cancer Res. 65, 3273–3280 (2005).
Kalpana, G., Figy, C., Yeung, M. & Yeung, K. C. Reduced RhoA expression enhances breast cancer metastasis with a concomitant increase in CCR5 and CXCR4 chemokines signaling. Sci. Rep. 9, 16351 (2019).
Kamai, T. et al. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin. Cancer Res. 10, 4799–4805 (2004).
Fritz, G., Brachetti, C., Bahlmann, F., Schmidt, M. & Kaina, B. Rho GTPases in human breast tumours: expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer 87, 635–644 (2002).
Horiuchi, A. et al. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab. Invest. 83, 861–870 (2003).
Pan, Y. et al. Expression of seven main Rho family members in gastric carcinoma. Biochem. Biophys. Res. Commun. 315, 686–691 (2004).
Kamai, T. et al. Significant association of Rho/ROCK pathway with invasion and metastasis of bladder cancer. Clin. Cancer Res. 9, 2632–2641 (2003).
Gómez del Pulgar, T., Benitah, S. A., Valerón, P. F., Espina, C. & Lacal, J. C. Rho GTPase expression in tumourigenesis: evidence for a significant link. Bioessays 27, 602–613 (2005).
So, C. W. et al. EEN encodes for a member of a new family of proteins containing an Src homology 3 domain and is the third gene located on chromosome 19p13 that fuses to MLL in human leukemia. Proc. Natl Acad. Sci. USA 94, 2563–2568 (1997).
Baldassarre, T. et al. Endophilin A2 promotes TNBC cell invasion and tumor metastasis. Mol. Cancer Res. 13, 1044–1055 (2015).
Baldassarre, T., Truesdell, P. & Craig, A. W. Endophilin A2 promotes HER2 internalization and sensitivity to trastuzumab-based therapy in HER2-positive breast cancers. Breast Cancer Res 19, 110 (2017).
Fan, H., Zhao, X., Sun, S., Luo, M. & Guan, J. L. Function of focal adhesion kinase scaffolding to mediate endophilin A2 phosphorylation promotes epithelial-mesenchymal transition and mammary cancer stem cell activities in vivo. J. Biol. Chem. 288, 3322–3333 (2013).
Wu, X., Gan, B., Yoo, Y. & Guan, J.-L. FAK-mediated Src phosphorylation of endophilin A2 inhibits endocytosis of MT1-MMP and promotes ECM degradation. Dev. Cell 9, 185–196 (2005).
Yang, X., Ren, H., Yao, L., Chen, X. & He, A. Role of EHD2 in migration and invasion of human breast cancer cells. Tumor Biol. 36, 3717–3726 (2015).
Liu, J. et al. Decreased expression of EHD2 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Dig. Dis. Sci. 61, 2554–2567 (2016).
Guan, C., Lu, C., Xiao, M. & Chen, W. EHD2 overexpression suppresses the proliferation, migration, and invasion in human colon cancer. Cancer Invest. 39, 297–309 (2021).
Li, M. et al. Effects of EHD2 interference on migration of esophageal squamous cell carcinoma. Med. Oncol. 30, 396 (2013).
Kim, Y. et al. Prognostic implication of histological features associated with EHD2 expression in papillary thyroid carcinoma. PLoS ONE 12, e0174737 (2017).
Liu, C. et al. Effect of EH domain containing protein 2 on the biological behavior of clear cell renal cell carcinoma. Hum. Exp. Toxicol. 38, 927–937 (2019).
Huh, Y. H. et al. Swiprosin-1 stimulates cancer invasion and metastasis by increasing the Rho family of GTPase signaling. Oncotarget 6, 13060–13071 (2015).
Fan, C. C. et al. EFHD2 contributes to non-small cell lung cancer cisplatin resistance by the activation of NOX4-ROS-ABCC1 axis. Redox Biol. 34, 101571 (2020).
Boulay, P. L. et al. ARF1 controls proliferation of breast cancer cells by regulating the retinoblastoma protein. Oncogene 30, 3846–3861 (2011).
Schlienger, S., Ramirez, R. A. & Claing, A. ARF1 regulates adhesion of MDA-MB-231 invasive breast cancer cells through formation of focal adhesions. Cell. Signal. 27, 403–415 (2015).
Schlienger, S., Campbell, S. & Claing, A. ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol. Biol. Cell. 25, 17–29 (2014).
Rainero, E. et al. Ligand-occupied integrin internalization links nutrient signaling to invasive migration. Cell Rep. 10, 398–413 (2015).
Palacios, F., Price, L., Schweitzer, J., Collard, J. G. & D’Souza-Schorey, C. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 20, 4973–4986 (2001).
Morishige, M. et al. GEP100 links epidermal growth factor receptor signalling to Arf6 activation to induce breast cancer invasion. Nat. Cell Biol. 10, 85–92 (2008).
Schlienger, S., Campbell, S., Pasquin, S., Gaboury, L. & Claing, A. ADP-ribosylation factor 1 expression regulates epithelial-mesenchymal transition and predicts poor clinical outcome in triple-negative breast cancer. Oncotarget 7, 15811–15827 (2016).
Zhao, H. et al. Coexpression of IQ-domain GTPase-activating protein 1 (IQGAP1) and dishevelled (Dvl) is correlated with poor prognosis in non-small cell lung cancer. PLoS ONE 9, e113713 (2014).
Yang, Z. et al. Overexpression of PAK1 correlates with aberrant expression of EMT markers and poor prognosis in non-small cell lung cancer. J. Cancer 8, 1484–1491 (2017).
Lin, C.-H. et al. Connective tissue growth factor induces collagen I expression in human lung fibroblasts through the Rac1/MLK3/JNK/AP-1 pathway. Biochim. Biophys. Acta - Mol. Cell Res. 1833, 2823–2833 (2013).
Wang, G. et al. PAK1 regulates RUFY3-mediated gastric cancer cell migration and invasion. Cell Death Dis. 6, e1682 (2015).
Wang, G. et al. PAK1-mediated MORC2 phosphorylation promotes gastric tumorigenesis. Oncotarget 6, 9877–9886 (2015).
Mishra, P., Senthivinayagam, S., Rangasamy, V., Sondarva, G. & Rana, B. Mixed lineage kinase-3/JNK1 axis promotes migration of human gastric cancer cells following gastrin stimulation. Mol. Endocrinol. 24, 598–607 (2010).
Pichot, C. S. et al. Cdc42-interacting protein 4 promotes breast cancer cell invasion and formation of invadopodia through activation of N-WASp. Cancer Res. 70, 8347–8356 (2010).
Sanchez, A. M. et al. Estrogen receptor-α promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP. Mol. Endocrinol. 24, 2114–2125 (2010).
Martin, T. A., Pereira, G., Watkins, G., Mansel, R. E. & Jiang, W. G. N-WASP is a putative tumour suppressor in breast cancer cells, in vitro and in vivo, and is associated with clinical outcome in patients with breast cancer. Clin. Exp. Metastasis 25, 97–108 (2008).
Arias-Romero, L. E., Villamar-Cruz, O., Huang, M., Hoeflich, K. P. & Chernoff, J. Pak1 kinase links ErbB2 to β-catenin in transformation of breast epithelial cells. Cancer Res. 73, 3671–3682 (2013).
Rattanasinchai, C., Llewellyn, B. J., Conrad, S. E. & Gallo, K. A. MLK3 regulates FRA-1 and MMPs to drive invasion and transendothelial migration in triple-negative breast cancer cells. Oncogenesis 6, e345 (2017).
Blessing, N. A., Brockman, A. L. & Chadee, D. N. The E3 ligase CHIP mediates ubiquitination and degradation of mixed-lineage kinase 3. Mol. Cell. Biol. 34, 3132–3143 (2014).
Wang, W.-S. et al. The expression of CFL1 and N-WASP in esophageal squamous cell carcinoma and its correlation with clinicopathological features. Dis. Esophagus 23, 512–521 (2010).
Zhang, Y. et al. Involvement of microRNA-224 in cell proliferation, migration, invasion, and anti-apoptosis in hepatocellular carcinoma. J. Gastroenterol. Hepatol. 28, 565–575 (2013).
Aguilar, B. J., Zhou, H. & Lu, Q. Cdc42 signaling pathway inhibition as a therapeutic target in Ras- related cancers. Curr. Med. Chem. 24, 3485–3507 (2017).
Frittoli, E. et al. A RAB5/RAB4 recycling circuitry induces a proteolytic invasive program and promotes tumor dissemination. J. Cell Biol. 206, 307–328 (2014).
Karthikeyan, S., Casey, P. J. & Wang, M. RAB4A GTPase regulates epithelial-to-mesenchymal transition by modulating RAC1 activation. Breast Cancer Res. 24, 72 (2022).
Tubbesing, K. et al. Complex Rab4-mediated regulation of endosomal size and EGFR activation. Mol. Cancer Res. 18, 757–773 (2020).
Yang, P.-S. et al. Rab5A is associated with axillary lymph node metastasis in breast cancer patients. Cancer Sci. 102, 2172–2178 (2011).
Díaz, J. et al. Rab5 is required in metastatic cancer cells for caveolin-1-enhanced Rac1 activation, migration and invasion. J. Cell Sci. 127, 2401–2406 (2014).
Silva, P. et al. Hypoxia promotes Rab5 activation, leading to tumor cell migration, invasion and metastasis. Oncotarget 7, 29548–29562 (2016).
Margiotta, A., Progida, C., Bakke, O. & Bucci, C. Rab7a regulates cell migration through Rac1 and vimentin. Biochim. Biophys. Acta Mol. Cell Res. 1864, 367–381 (2017).
Wang, T. et al. A role of Rab7 in stabilizing EGFR-Her2 and in sustaining Akt survival signal. J. Cell. Physiol. 227, 2788–2797 (2012).
Williams, K. C. & Coppolino, M. G. Phosphorylation of membrane type 1-matrix metalloproteinase (MT1-MMP) and its vesicle-associated membrane protein 7 (VAMP7)-dependent trafficking facilitate cell invasion and migration. J. Biol. Chem. 286, 43405–43416 (2011).
Steffan, J. J. & Cardelli, J. A. Thiazolidinediones induce Rab7–RILP–MAPK-dependent juxtanuclear lysosome aggregation and reduce tumor cell invasion. Traffic 11, 274–286 (2010).
Steffan, J. J. et al. Supporting a role for the GTPase Rab7 in prostate cancer progression. PLoS ONE 9, e87882 (2014).
Wang, W. et al. Internalized CD44s splice isoform attenuates EGFR degradation by targeting Rab7A. Proc. Natl Acad. Sci. USA 114, 8366–8371 (2017).
Alonso-Curbelo, D. et al. RAB7 controls melanoma progression by exploiting a lineage-specific wiring of the endolysosomal pathway. Cancer Cell 26, 61–76 (2014).
Howe, E. N. et al. Rab11b-mediated integrin recycling promotes brain metastatic adaptation and outgrowth. Nat. Commun. 11, 3017 (2020).
Palmieri, D., Bouadis, A., Ronchetti, R., Merino, M. J. & Steeg, P. S. Rab11a differentially modulates epidermal growth factor-induced proliferation and motility in immortal breast cells. Breast Cancer Res. Treat. 100, 127–137 (2006).
Yu, L., Li, X., Li, H., Chen, H. & Liu, H. Rab11a sustains GSK3β/Wnt/β-catenin signaling to enhance cancer progression in pancreatic cancer. Tumor Biol. 37, 13821–13829 (2016).
Dong, Q. et al. Rab11a promotes proliferation and invasion through regulation of YAP in non-small cell lung cancer. Oncotarget 8, 27800–27811 (2017).
Zhang, Z.-Y. et al. Rab11a regulates MMP2 expression by activating the PI3K/AKT pathway in human hepatocellular carcinoma cells. Pathol. Res. Pract. 216, 153046 (2020).
D’Agostino, L. et al. Recycling endosomes in mature epithelia restrain tumorigenic signaling. Cancer Res. 79, 4099–4112 (2019).
Yoon, S.-O., Shin, S. & Mercurio, A. M. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the α6β4 integrin. Cancer Res. 65, 2761–2769 (2005).
Zhang, J. et al. Overexpression of Rab25 contributes to metastasis of bladder cancer through induction of epithelial-mesenchymal transition and activation of Akt/GSK-3β/Snail signaling. Carcinogenesis 34, 2401–2408 (2013).
Cao, C. et al. Expression of Rab25 correlates with the invasion and metastasis of gastric cancer. Chin. J. Cancer Res. 25, 192–199 (2013).
Geng, D., Zhao, W., Feng, Y. & Liu, J. Overexpression of Rab25 promotes hepatocellular carcinoma cell proliferation and invasion. Tumor Biol. 37, 7713–7718 (2016).
Ma, Y. F. et al. Expression of Ras-related protein 25 predicts chemotherapy resistance and prognosis in advanced non-small cell lung cancer. Gen. Mol. Res. 14, 13998–14008 (2015).
Cheng, K. W. et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat. Med. 10, 1251–1256 (2004).
Jeong, B. Y. et al. Rab25 augments cancer cell invasiveness through a β1 integrin/EGFR/VEGF-A/Snail signaling axis and expression of fascin. Exp. Mol. Med. 50, e435 (2018).
Kessler, D. et al. The action of small GTPases Rab11 and Rab25 in vesicle trafficking during cell migration. Cell. Physiol. Biochem. 29, 647–656 (2012).
Hu, C., Chen, B., Zhou, Y. & Shan, Y. High expression of Rab25 contributes to malignant phenotypes and biochemical recurrence in patients with prostate cancer after radical prostatectomy. Cancer Cell Int. 17, 45 (2017).
Ding, B. et al. Knockdown of Ras-related protein 25 (Rab25) inhibits the in vitro cytotoxicity and in vivo antitumor activity of human glioblastoma multiforme cells. Oncol. Res. Feat. Preclin. Clin. Cancer Ther. 25, 331–340 (2017).
Nam, K. T. et al. Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J. Clin. Investig. 120, 840–849 (2010).
Tong, M. et al. Rab25 is a tumor suppressor gene with antiangiogenic and anti-invasive activities in esophageal squamous cell carcinoma. Cancer Res. 72, 6024–6035 (2012).
Cheng, J.-M., Ding, M., Aribi, A., Shah, P. & Rao, K. Loss of RAB25 expression in breast cancer. Int. J. Cancer 118, 2957–2964 (2006).
Amornphimoltham, P. et al. Rab25 regulates invasion and metastasis in head and neck cancer. Clin. Cancer Res. 19, 1375–1388 (2013).
Hansen, S. H., Sandvig, K. & van Deurs, B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J. Cell Biol. 123, 89–97 (1993).
Carpentier, J. L. et al. Potassium depletion and hypertonic medium reduce “non-coated” and clathrin-coated pit formation, as well as endocytosis through these two gates. J. Cell. Physiol. 138, 519–526 (1989).
Larkin, J. M., Brown, M. S., Goldstein, J. L. & Anderson, R. G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 33, 273–285 (1983).
Sasso, L., Purdie, L., Grabowska, A., Jones, A. T. & Alexander, C. Time and cell-dependent effects of endocytosis inhibitors on the internalization of biomolecule markers and nanomaterials. J. Interdiscip. Nanomed. 3, 67–81 (2018).
Boucrot, E. et al. Endophilin marks and controls a clathrin-independent endocytic pathway. Nature 517, 460–465 (2015).
Wang, L. H., Rothberg, K. G. & Anderson, R. G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107–1117 (1993).
Chen, C. L. et al. Inhibitors of clathrin-dependent endocytosis enhance TGFbeta signaling and responses. J. Cell Sci. 122, 1863–1871 (2009).
von Kleist, L. et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146, 471–484 (2011).
Dutta, D., Williamson, C. D., Cole, N. B. & Donaldson, J. G. Pitstop 2 is a potent inhibitor of clathrin-independent endocytosis. PLoS ONE 7, e45799 (2012).
Willox, A. K., Sahraoui, Y. M. & Royle, S. J. Non-specificity of Pitstop 2 in clathrin-mediated endocytosis. Biol. Open 3, 326–331 (2014).
Macia, E. et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 (2006).
Park, R. J. et al. Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. J. Cell Sci. 126, 5305–5312 (2013).
Kilsdonk, E. P. et al. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 270, 17250–172566 (1995).
Hao, M., Mukherjee, S., Sun, Y. & Maxfield, F. R. Effects of cholesterol depletion and increased lipid unsaturation on the properties of endocytic membranes. J. Biol. Chem. 279, 14171–14178 (2004).
McKenney, J. M. Pharmacologic characteristics of statins. Clin. Cardiol. 26, 32–38 (2003).
Chaudhary, N. et al. Endocytic crosstalk: cavins, caveolins, and caveolae regulate clathrin-independent endocytosis. PLoS Biol. 12, e1001832 (2014).
Rentero, C. et al. Functional implications of plasma membrane condensation for T cell activation. PLoS ONE 3, e2262 (2008).
Thottacherry, J. J. et al. Mechanochemical feedback control of dynamin independent endocytosis modulates membrane tension in adherent cells. Nat. Commun. 9, 4217 (2018).
Akiyama, T. et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 262, 5592–5595 (1987).
Parton, R. G., Joggerst, B. & Simons, K. Regulated internalization of caveolae. J. Cell Biol. 127, 1199–1215 (1994).
Brenner, S. L. & Korn, E. D. Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. Additional evidence for an F-actin treadmill. J. Biol. Chem. 254, 9982–9985 (1979).
Fujimoto, L. M., Roth, R., Heuser, J. E. & Schmid, S. L. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1, 161–171 (2000).
Gladhaug, I. P. & Christoffersen, T. Amiloride inhibits constitutive internalization and increases the surface number of epidermal growth factor receptors in intact rat hepatocytes. J. Cell. Physiol. 143, 188–195 (1990).
Kleyman, T. R. & Cragoe, E. J. Jr. Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 105, 1–21 (1988).
Henriksen, L., Grandal, M. V., Knudsen, S. L., van Deurs, B. & Grøvdal, L. M. Internalization mechanisms of the epidermal growth factor receptor after activation with different ligands. PLoS ONE 8, e58148 (2013).
Le Roy, C. & Wrana, J. L. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nat. Rev. Mol. Cell Biol. 6, 112–126 (2005).
Polo, S. Signaling-mediated control of ubiquitin ligases in endocytosis. BMC Biol. 10, 25 (2012).
Ford, M. G. J. et al. Curvature of clathrin-coated pits driven by epsin. Nature 419, 361–366 (2002).
Pearse, B. M. F. Coated vesicles from pig brain: purification and biochemical characterization. J. Mol. Biol. 97, 93–98 (1975).
Schmid, S. L. & Frolov, V. A. Dynamin: functional design of a membrane fission catalyst. Annu. Rev. Cell. Dev. Biol. 27, 79–105 (2011).
Yarar, D., Waterman-Storer, C. M. & Schmid, S. L. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol. Biol. Cell. 16, 964–975 (2005).
Kaksonen, M., Toret, C. P. & Drubin, D. G. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell 123, 305–320 (2005).
Parton, R. G. & Simons, K. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8, 185–194 (2007).
Pelkmans, L., Bürli, T., Zerial, M. & Helenius, A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell 118, 767–780 (2004).
Hill, M. M. et al. PTRF-cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132, 113–124 (2008).
Kovtun, O., Tillu, V. A., Ariotti, N., Parton, R. G. & Collins, B. M. Cavin family proteins and the assembly of caveolae. J. Cell Sci. 128, 1269–1278 (2015).
Vinten, J., Johnsen, A. H., Roepstorff, P., Harpøth, J. & Tranum-Jensen, J. Identification of a major protein on the cytosolic face of caveolae. Biochim. Biophys. Acta Biomembr. 1717, 34–40 (2005).
Torrino, S. et al. EHD2 is a mechanotransducer connecting caveolae dynamics with gene transcription. J. Cell Biol. 217, 4092–4105 (2018).
Casamento, A. & Boucrot, E. Molecular mechanism of fast endophilin-mediated endocytosis. Biochem. J. 477, 2327–2345 (2020).
Hinze, C. & Boucrot, E. Local actin polymerization during endocytic carrier formation. Biochem. Soc. Trans. 46, 565–576 (2018).
Massol, P., Montcourrier, P., Guillemot, J.-C. & Chavrier, P. Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J. 17, 6219–6229 (1998).
Schlam, D. et al. Phosphoinositide 3-kinase enables phagocytosis of large particles by terminating actin assembly through Rac/Cdc42 GTPase-activating proteins. Nat. Commun. 6, 8623 (2015).
Park, H. & Cox, D. Cdc42 regulates Fcγ receptor-mediated phagocytosis through the activation and phosphorylation of Wiskott-Aldrich Syndrome protein (WASP) and neural-WASP. Mol. Biol. Cell. 20, 4500–4508 (2009).
Lee, D. J., Cox, D., Li, J. & Greenberg, S. Rac1 and Cdc42 are required for phagocytosis, but not NF-κB-dependent gene expression, in macrophages challenged with Pseudomonas aeruginosa. J. Biol. Chem. 275, 141–146 (2000).
Rotty, J. D. et al. Arp2/3 complex is required for macrophage integrin functions but is dispensable for FcR phagocytosis and in vivo motility. Dev. Cell 42, 498–513 (2017).
May, R. C., Caron, E., Hall, A. & Machesky, L. M. Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat. Cell Biol. 2, 246–248 (2000).
Velle, K. B. & Fritz-Laylin, L. K. Conserved actin machinery drives microtubule-independent motility and phagocytosis in Naegleria. J. Cell Biol. 219, e202007158 (2020).
Jaumouillé, V., Cartagena-Rivera, A. X. & Waterman, C. M. Coupling of β2 integrins to actin by a mechanosensitive molecular clutch drives complement receptor-mediated phagocytosis. Nat. Cell Biol. 21, 1357–1369 (2019).
Castellano, F., Chavrier, P. & Caron, E. Actin dynamics during phagocytosis. Semin. Immunol. 13, 347–355 (2001).
Haga, Y., Miwa, N., Jahangeer, S., Okada, T. & Nakamura, S.-i CtBP1/BARS is an activator of phospholipase D1 necessary for agonist-induced macropinocytosis. EMBO J. 28, 1197–1207 (2009).
Liberali, P. et al. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 27, 970–981 (2008).
Condon, N. D. et al. Macropinosome formation by tent pole ruffling in macrophages. J. Cell Biol. 217, 3873–3885 (2018).
Veltman, D. M. et al. A plasma membrane template for macropinocytic cups. eLife 5, e20085 (2016).
Williamson, C. D. & Donaldson, J. G. Arf6, JIP3, and dynein shape and mediate macropinocytosis. Mol. Biol. Cell. 30, 1477–1489 (2019).
Junemann, A. et al. A Diaphanous-related formin links Ras signaling directly to actin assembly in macropinocytosis and phagocytosis. Proc. Natl Acad. Sci. USA 113, 7464–7473 (2016).
Kumari, S. & Mayor, S. ARF1 is directly involved in dynamin-independent endocytosis. Nat. Cell Biol. 10, 30–41 (2008).
Sabharanjak, S., Sharma, P., Parton, R. G. & Mayor, S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2, 411–423 (2002).
Chadda, R. et al. Cholesterol-sensitive Cdc42 activation regulates actin polymerization for endocytosis via the GEEC pathway. Traffic 8, 702–717 (2007).
Schnatwinkel, C. et al. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2, E261 (2004).
Donaldson, J. G. Macropinosome formation, maturation and membrane recycling: lessons from clathrin-independent endosomal membrane systems. Philos. Trans. R. Soc. B: Biol. Sci. 374, 20180148 (2019).
Burr, M. L. et al. CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity. Nature 549, 101–105 (2017).
Mezzadra, R. et al. Identification of CMTM6 and CMTM4 as PD-L1 protein regulators. Nature 549, 106–110 (2017).
Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat. Med. 6, 443–446 (2000).
Cartron, G. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99, 754–758 (2002).
Beano, A. et al. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J. Transl. Med. 6, 25 (2008).
Blomberg, O. S. et al. IL-5-producing CD4+ T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell 41, 106–123 (2023).
Shi, Y. et al. Trastuzumab triggers phagocytic killing of high HER2 cancer cells in vitro and in vivo by interaction with Fcγ receptors on macrophages. J. Immun. 194, 4379–4386 (2015).
Acknowledgements
F.S. acknowledges funding from the National Health and Medical Research Council Ideas Grant APP2001396 and philanthropic funding from Princess Alexandra Hospital Research Fund and Aveo communities.
Author information
Authors and Affiliations
Contributions
B.B., S.R.J. and F.S. researched data for the article. F.S. contributed substantially to discussion of the content and wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
F.S. holds several patents relevant to the field of this Review: PAT-02100-JP-01; 2015-538212; 02100-GB-01; 13848409.2; 02100-DE-01; 60 2013059561.5; 02100-AU-02; 2013334493; 02100-US-01 14/438440; 02100-JP-01 2015-538212. F.S. declares that she completed a 2019 contract with Merck (published as part of her group’s work in Cell 2020) and has presented at a conference sponsored by Telix (Melbourne, Australia). All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Sharad S. Singhal, Yosef Yarden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Antibody-dependent cellular cytotoxicity
-
(ADCC). A cytotoxic reaction mediated by immune cells (effector cells) against target cells sensitized by antibodies that are specific to the surface antigens of the target cell.
- Antibody–drug conjugates
-
(ADCs). A class of anticancer drugs in which monoclonal antibodies are conjugated to toxins, forming a single complex that can specifically target cancer cells and, after being internalized, deliver a cytotoxic payload.
- Autophagy
-
The process by which cells recycle, degrade and reuse their own components.
- Bin–amphiphysin–Rvs (BAR) domain
-
Banana-shaped protein dimerization domain that binds to and bends membranes.
- Caveolae
-
Pit-like invaginations of the plasma membrane that contain caveolin proteins, which form a subdomain of lipid rafts.
- Cavins
-
Peripheral membrane proteins that coat the caveolar surface in the interior membrane layer and specifically associate with caveolae in heteromeric complexes.
- Cell polarity
-
Spatial and directional differences in shape, structure and function within a cell.
- Clathrin-independent endocytosis
-
(CIE). This term refers to all endocytic pathways that do not require clathrin. CIE includes clathrin-independent carriers (CLICs), caveolae, fast endophilin-mediated endocytosis and micropinocytosis.
- Contact inhibition
-
A regulatory mechanism whereby cells are unable to move or proliferate when in contact with one another. This inhibitory mechanism is either aberrant or absent in cancer.
- Epithelial to mesenchymal plasticity
-
(EMP). The reversible process whereby epithelial cells go through a phenotype transition from a polarized adherent state to an unpolarized mesenchymal state.
- Fast endocytosis
-
Clathrin-independent endocytosis that takes place on a millisecond to second timescale in response to stimuli such as stress, chemotaxis or synaptic vesicle exocytosis. It includes macropinocytosis, activity-dependent bulk endocytosis, fast endophilin-mediated endocytosis, kiss-and-run and ultrafast endocytosis.
- Fast endophilin-mediated endocytosis
-
(FEME). A rapid endocytic pathway (a few seconds) that is triggered by ligand–receptor interactions and is regulated by endophilin A2 and dynamin recruitment as well as actin polymerization.
- Ferroptosis
-
A type of programmed cell death that is characterized by the accumulation of lipid peroxides and is dependent on iron accumulation.
- Glycosylphosphatidylinositol-anchored proteins (GPI-AP)-enriched early endosomal compartment
-
(GEEC). A specialized early endosomal compartment that results from the endocytosis, via a dynamin-independent endocytic route, of a class of lipid-anchored proteins called GPI-APs. They result from fusion of uncoated clathrin-independent tubulovesicular carriers called clathrin-independent carriers (CLICs), which originate directly from the cell surface. This process is named the CLIC/GEEC pathway.
- Innervation
-
The process by which nerves infiltrate tissues.
- Phagocytosis
-
The process by which a cell membrane extends to engulf particulate matter larger than about 0.5 μm in diameter, generating intracellular phagosomes.
- Pinocytosis
-
The process by which cells internalize fluids from the extracellular environment. This process may lead to the internalization of small particles suspended in the extracellular fluid, but this is a result of internalizing the fluid, and is not phagocytosis. Macropinocytosis is a form of endocytosis in which a large fluid-filled vesicle, or macropinosome, is pinched off from the cell membrane and brought into the interior of the cell, and micropinocytosis is a synonym of pinocytosis.
- Plasticity
-
Ability of cells to change their phenotypes in response to environmental signals.
- Radioligands
-
Radioactively labelled compounds, which bind at the target binding site.
- Residence time
-
The total duration of the ligand–receptor interaction.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Banushi, B., Joseph, S.R., Lum, B. et al. Endocytosis in cancer and cancer therapy. Nat Rev Cancer 23, 450–473 (2023). https://doi.org/10.1038/s41568-023-00574-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-023-00574-6
This article is cited by
-
Decoding of the surfaceome and endocytome in primary glioblastoma cells identifies potential target antigens in the hypoxic tumor niche
Acta Neuropathologica Communications (2024)
-
Reduced Proteolipid Protein 2 promotes endoplasmic reticulum stress-related apoptosis and increases drug sensitivity in acute myeloid leukemia
Molecular Biology Reports (2024)
-
Considerations for the design of antibody drug conjugates (ADCs) for clinical development: lessons learned
Journal of Hematology & Oncology (2023)