Abstract
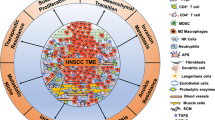
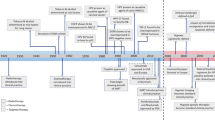
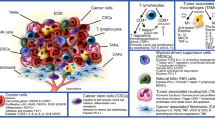
Targeted immunotherapy has improved patient survival in head and neck squamous cell carcinoma (HNSCC), but less than 20% of patients produce a durable response to these treatments. Thus, new immunotherapies that consider all key players of the complex HNSCC tumour microenvironment (TME) are necessary to further enhance tumour-specific T cell responses in patients. HNSCC is an ideal tumour type in which to evaluate immune and non-immune cell differences because of two distinct TME aetiologies (human papillomavirus (HPV)-positive and HPV-negative disease), multiple anatomic sites for tumour growth, and clear distinctions between patients with locally advanced disease and those with recurrent and/or metastatic disease. Recent technological and scientific advancements have provided a more complete picture of all cellular constituents within this complex TME and have evaluated the interplay of both immune and non-immune cells within HNSCC. Here, we include a comprehensive analysis of the complete ecosystem of the HNSCC TME, performed utilizing data-rich resources such as The Cancer Genome Atlas, and cutting-edge techniques, such as single-cell RNA sequencing, high-dimensional flow cytometry and spatial multispectral imaging, to generate improved treatment strategies for this diverse disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ferlay, J. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Johnson, D. E. et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 6, 92 (2020).
HHS.gov. Smoking Cessation: A Report of the Surgeon General — Key Findings https://www.hhs.gov/surgeongeneral/reports-and-publications/tobacco/2020-cessation-sgr-factsheet-key-findings/index.html#:~:Text=2020%20Surgeon%20General’s%20Report%20Findings,a%20decade%20to%20life%20expectancy (2020).
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Chaturvedi, A. K. et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 29, 4294–4301 (2011).
Powell, S. F., Vu, L., Spanos, W. C. & Pyeon, D. The key differences between human papillomavirus-positive and -negative head and neck cancers: biological and clinical implications. Cancers 13, 5206 (2021).
Ferris, R. L. et al. Nivolumab in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by prior cetuximab use. Clin. Cancer Res. 25, 5221–5230 (2019).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Cramer, J. D., Burtness, B. & Ferris, R. L. Immunotherapy for head and neck cancer: recent advances and future directions. Oral. Oncol. 99, 104460 (2019).
Li, L. et al. Comprehensive immunogenomic landscape analysis of prognosis-related genes in head and neck cancer. Sci. Rep. 10, 6395 (2020).
Mito, I. et al. Comprehensive analysis of immune cell enrichment in the tumor microenvironment of head and neck squamous cell carcinoma. Sci. Rep. 11, 16134 (2021).
Mandal, R. et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 1, e89829 (2016).
Jiang, A.-M. et al. Tumor mutation burden, immune cell infiltration, and construction of immune-related genes prognostic model in head and neck cancer. Int. J. Med. Sci. 18, 226–238 (2021).
Chen, X. et al. Immunological network analysis in HPV associated head and neck squamous cancer and implications for disease prognosis. Mol. Immunol. 96, 28–36 (2018).
Petitprez, F. et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020).
Ruffin, A. T. et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat. Commun. 12, 3349 (2021).
Wood, O. et al. Gene expression analysis of TIL rich HPV-driven head and neck tumors reveals a distinct B-cell signature when compared to HPV independent tumors. Oncotarget 7, 56781–56797 (2016).
Wuerdemann, N. et al. LAG-3, TIM-3 and VISTA expression on tumor-infiltrating lymphocytes in oropharyngeal squamous cell carcinoma-potential biomarkers for targeted therapy concepts. Int. J. Mol. Sci. 22, 379 (2020).
Lechner, A. et al. Characterization of tumor-associated T-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 8, 44418–44433 (2017).
Liu, Z. et al. Novel effector phenotype of Tim-3+ regulatory T cells leads to enhanced suppressive function in head and neck cancer patients. Clin. Cancer Res. 24, 4529–4538 (2018).
Liu, J.-F. et al. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J. Exp. Clin. Cancer Res. 37, 44 (2018).
Duhen, T. et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat. Commun. 9, 2724 (2018).
Hwang, B., Lee, J. H. & Bang, D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp. Mol. Med. 50, 1–14 (2018).
Kuksin, M. et al. Applications of single-cell and bulk RNA sequencing in onco-immunology. Eur. J. Cancer 149, 193–210 (2021).
Chaudhry, F. et al. Single-cell RNA sequencing of the cardiovascular system: new looks for old diseases. Front. Cardiovasc. Med. 6, 173 (2019).
Li, X. & Wang, C.-Y. From bulk, single-cell to spatial RNA sequencing. Int. J. Oral. Sci. 13, 36 (2021).
Kürten, C. H. L. et al. Investigating immune and non-immune cell interactions in head and neck tumors by single-cell RNA sequencing. Nat. Commun. 12, 7338 (2021).
Cillo, A. R. et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity 52, 183–199.e9 (2020).
Puram, S. V. et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624.e24 (2017).
Zhang, Q. et al. Integrated analysis of single-cell RNA-seq and bulk RNA-seq reveals distinct cancer-associated fibroblasts in head and neck squamous cell carcinoma. Ann. Transl. Med. 9, 1017 (2021).
Nordfors, C. et al. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur. J. Cancer 49, 2522–2530 (2013).
Wuerdemann, N. et al. PD-L1 expression and a high tumor infiltrate of CD8+ lymphocytes predict outcome in patients with oropharyngeal squamous cells carcinoma. Int. J. Mol. Sci. 21, 5228 (2020).
Zhou, Z. et al. PD-L1 in combination with CD8+ TIL and HIF-1α are promising prognosis predictors of head and neck squamous cell carcinoma. Cancer Manag. Res. 12, 13233–13239 (2020).
Xiao, Y. et al. CD103+ T and dendritic cells indicate a favorable prognosis in oral cancer. J. Dent. Res. 98, 1480–1487 (2019).
McKinney, E. F. & Smith, K. G. T cell exhaustion and immune-mediated disease-the potential for therapeutic exhaustion. Curr. Opin. Immunol. 43, 74–80 (2016).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Wherry, E. J. T cell exhaustion. Nat. Immunol. 12, 492–499 (2011).
Beltra, J.-C. et al. Developmental relationships of four exhausted CD8+ T cell subsets reveals underlying transcriptional and epigenetic landscape control mechanisms. Immunity 52, 825–841.e8 (2020).
Kansy, B. A. et al. PD-1 status in CD8+ T cells associates with survival and anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 77, 6353–6364 (2017).
Eberhardt, C. S. et al. Functional HPV-specific PD-1+ stem-like CD8 T cells in head and neck cancer. Nature 597, 279–284 (2021).
Wang, D. et al. A comprehensive profile of TCF1+ progenitor and TCF1- terminally exhausted PD-1+CD8+ T cells in head and neck squamous cell carcinoma: implications for prognosis and immunotherapy. Int. J. Oral. Sci. 14, 8 (2022).
Borst, J., Ahrends, T., Bąbała, N., Melief, C. J. M. & Kastenmüller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647 (2018).
Tay, R. E., Richardson, E. K. & Toh, H. C. Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 28, 5–17 (2021).
Tesmer, L. A., Lundy, S. K., Sarkar, S. & Fox, D. A. Th17 cells in human disease. Immunol. Rev. 223, 87–113 (2008).
Ye, J., Livergood, R. S. & Peng, G. The role and regulation of human Th17 cells in tumor immunity. Am. J. Pathol. 182, 10–20 (2013).
Gameiro, S. F. et al. Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy. Oncoimmunology 7, e1498439 (2018).
Bhatt, K. H. et al. Profiling HPV-16-specific T cell responses reveals broad antigen reactivities in oropharyngeal cancer patients. J. Exp. Med. 217, e20200389 (2020).
Albers, A. et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer Res. 65, 11146–11155 (2005).
Welters, M. J. P. et al. Intratumoral HPV16-specific T cells constitute a type I-oriented tumor microenvironment to improve survival in HPV16-driven oropharyngeal cancer. Clin. Cancer Res. 24, 634–647 (2018).
Kesselring, R., Thiel, A., Pries, R., Trenkle, T. & Wollenberg, B. Human Th17 cells can be induced through head and neck cancer and have a functional impact on HNSCC development. Br. J. Cancer 103, 1245–1254 (2010).
Li, C., Zhao, Y., Zhang, W. & Zhang, W. Increased prevalence of T(H)17 cells in the peripheral blood of patients with head and neck squamous cell carcinoma. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 112, 81–89 (2011).
Yang, M. et al. CXCL13 shapes immunoactive tumor microenvironment and enhances the efficacy of PD-1 checkpoint blockade in high-grade serous ovarian cancer. J. Immunother. Cancer 9, e001136 (2021).
Niogret, J. et al. Follicular helper-T cells restore CD8+-dependent antitumor immunity and anti-PD-L1/PD-1 efficacy. J. Immunother. Cancer 9, e002157 (2021).
Kim, S. T. et al. Human extrafollicular CD4+ Th cells help memory B cells produce Igs. J. Immunol. 201, 1359–1372 (2018).
Tahiliani, V., Hutchinson, T. E., Abboud, G., Croft, M. & Salek-Ardakani, S. OX40 Cooperates with ICOS to amplify follicular Th cell development and germinal center reactions during infection. J. Immunol. 198, 218–228 (2017).
Zotos, D. et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J. Exp. Med. 207, 365–378 (2010).
Dadey, R. E., Workman, C. J. & Vignali, D. A. A. Regulatory T cells in the tumor microenvironment. Adv. Exp. Med. Biol. 1273, 105–134 (2020).
Sawant, D. V. et al. Adaptive plasticity of IL-10+ and IL-35+ Treg cells cooperatively promotes tumor T cell exhaustion. Nat. Immunol. 20, 724–735 (2019).
Jie, H. B. et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br. J. Cancer 109, 2629–2635 (2013).
Chuckran, C. A. et al. Prevalence of intratumoral regulatory T cells expressing neuropilin-1 is associated with poorer outcomes in patients with cancer. Sci. Transl. Med. 13, eabf8495 (2021).
Strauss, L., Bergmann, C., Gooding, W., Johnson, J. T. & Whiteside, T. L. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res. 13, 6301–6311 (2007).
Jie, H.-B. et al. CTLA-4+ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 75, 2200–2210 (2015).
Oida, T., Xu, L., Weiner, H. L., Kitani, A. & Strober, W. TGF-beta-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J. Immunol. 177, 2331–2339 (2006).
Chuckran, C. A., Liu, C., Bruno, T. C., Workman, C. J. & Vignali, D. A. Neuropilin-1: a checkpoint target with unique implications for cancer immunology and immunotherapy. J. Immunother. Cancer 8, e000967 (2020).
Economopoulou, P., Kotsantis, I. & Psyrri, A. The promise of immunotherapy in head and neck squamous cell carcinoma: combinatorial immunotherapy approaches. ESMO Open 1, e000122 (2016).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Janjic, B. M., Kulkarni, A., Ferris, R. L., Vujanovic, L. & Vujanovic, N. L. Human B cells mediate innate anti-cancer cytotoxicity through concurrent engagement of multiple TNF superfamily ligands. Front. Immunol. 13, 837842 (2022).
Nelson, B. H. CD20+ B cells: the other tumor-infiltrating lymphocytes. J. Immunol. 185, 4977–4982 (2010).
Sharonov, G. V., Serebrovskaya, E. O., Yuzhakova, D. V., Britanova, O. V. & Chudakov, D. M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat. Rev. Immunol. 20, 294–307 (2020).
Yuen, G. J., Demissie, E. & Pillai, S. B lymphocytes and cancer: a love-hate relationship. Trends Cancer 2, 747–757 (2016).
Shen, P. & Fillatreau, S. Antibody-independent functions of B cells: a focus on cytokines. Nat. Rev. Immunol. 15, 441–451 (2015).
Germain, C., Gnjatic, S. & Dieu-Nosjean, M.-C. Tertiary lymphoid structure-associated B cells are key players in anti-tumor immunity. Front. Immunol. 6, 67 (2015).
Bruno, T. C. et al. Antigen-presenting intratumoral B cells affect CD4+ TIL phenotypes in non-small cell lung cancer patients. Cancer Immunol. Res. 5, 898–907 (2017).
Sautès-Fridman, C. et al. Tertiary lymphoid structures and B cells: clinical impact and therapeutic modulation in cancer. Semin. Immunol. 48, 101406 (2020).
Kim, S. S. et al. B cells improve overall survival in HPV-associated squamous cell carcinomas and are activated by radiation and PD-1 blockade. Clin. Cancer Res. 26, 3345–3359 (2020).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Lechner, A. et al. Tumor-associated B cells and humoral immune response in head and neck squamous cell carcinoma. Oncoimmunology 8, 1535293 (2019).
Russell, S. et al. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head. Neck Oncol. 5, 24 (2013).
Li, Q. et al. Prognostic value of tertiary lymphoid structure and tumour infiltrating lymphocytes in oral squamous cell carcinoma. Int. J. Oral. Sci. 12, 24 (2020).
Pretscher, D. et al. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer 9, 292 (2009).
Wieland, A. et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2021).
Jeske, S. S. et al. Adenosine-producing regulatory B cells in head and neck cancer. Cancer Immunol. Immunother. 69, 1205–1216 (2020).
Hladíková, K. et al. Tumor-infiltrating B cells affect the progression of oropharyngeal squamous cell carcinoma via cell-to-cell interactions with CD8+ T cells. J. Immunother. Cancer 7, 261 (2019).
van Erp, E. A., Luytjes, W., Ferwerda, G. & van Kasteren, P. B. Fc-Mediated antibody effector functions during respiratory syncytial virus infection and disease. Front. Immunol. 10, 548 (2019).
Gül, N. & van Egmond, M. Antibody-dependent phagocytosis of tumor cells by macrophages: a potent effector mechanism of monoclonal antibody therapy of cancer. Cancer Res. 75, 5008–5013 (2015).
Bald, T., Krummel, M. F., Smyth, M. J. & Barry, K. C. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat. Immunol. 21, 835–847 (2020).
Concha-Benavente, F. et al. PD-L1 mediates dysfunction in activated PD-1+ NK cells in head and neck cancer patients. Cancer Immunol. Res. 6, 1548–1560 (2018).
Vujanovic, L., Ballard, W., Thorne, S. H., Vujanovic, N. L. & Butterfield, L. H. Adenovirus-engineered human dendritic cells induce natural killer cell chemotaxis via CXCL8/IL-8 and CXCL10/IP-10. Oncoimmunology 1, 448–457 (2012).
Vujanovic, L. et al. CD56dim CD16- natural killer cell profiling in melanoma patients receiving a cancer vaccine and interferon-α. Front. Immunol. 10, 14 (2019).
Stabile, H. et al. Reconstitution of multifunctional CD56lowCD16low natural killer cell subset in children with acute leukemia given α/β T cell-depleted HLA-haploidentical haematopoietic stem cell transplantation. Oncoimmunology 6, e1342024 (2017).
Wagner, S. et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int. J. Cancer 138, 2263–2273 (2016).
Lisco, A. et al. Treatment of relapsing HPV diseases by restored function of natural killer cells. N. Engl. J. Med. 385, 921–929 (2021).
Srivastava, R. M. et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin. Cancer Res. 19, 1858–1872 (2013).
Kurai, J. et al. Antibody-dependent cellular cytotoxicity mediated by cetuximab against lung cancer cell lines. Clin. Cancer Res. 13, 1552–1561 (2007).
López-Albaitero, A. et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol. Immunother. 58, 1853–1864 (2009).
Ma, Y., Shurin, G. V., Peiyuan, Z. & Shurin, M. R. Dendritic cells in the cancer microenvironment. J. Cancer 4, 36–44 (2013).
Gerosa, F. et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195, 327–333 (2002).
Jardim, J. F., Gondak, R., Galvis, M. M., Pinto, C. A. L. & Kowalski, L. P. A decreased peritumoral CD1a+ cell number predicts a worse prognosis in oral squamous cell carcinoma. Histopathology 72, 905–913 (2018).
Reichert, T. E., Scheuer, C., Day, R., Wagner, W. & Whiteside, T. L. The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer 91, 2136–2147 (2001).
Partlová, S. et al. Distinct patterns of intratumoral immune cell infiltrates in patients with HPV-associated compared to non-virally induced head and neck squamous cell carcinoma. Oncoimmunology 4, e965570 (2015).
Kindt, N. et al. Langerhans cell number is a strong and independent prognostic factor for head and neck squamous cell carcinomas. Oral. Oncol. 62, 1–10 (2016).
Guess, J. C. & McCance, D. J. Decreased migration of Langerhans precursor-like cells in response to human keratinocytes expressing human papillomavirus type 16 E6/E7 is related to reduced macrophage inflammatory protein-3alpha production. J. Virol. 79, 14852–14862 (2005).
Vujanovic, L. et al. Virally infected and matured human dendritic cells activate natural killer cells via cooperative activity of plasma membrane-bound TNF and IL-15. Blood 116, 575–583 (2010).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2020).
Utispan, K. & Koontongkaew, S. Fibroblasts and macrophages: key players in the head and neck cancer microenvironment. J. Oral. Biosci. 59, 23–30 (2017).
Hu, Y. et al. Tumor-associated macrophages correlate with the clinicopathological features and poor outcomes via inducing epithelial to mesenchymal transition in oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 35, 12 (2016).
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L. & Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 14, 399–416 (2017).
Marcus, B. et al. Prognostic factors in oral cavity and oropharyngeal squamous cell carcinoma. Cancer 101, 2779–2787 (2004).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Kwak, T. et al. Distinct populations of immune-suppressive macrophages differentiate from monocytic myeloid-derived suppressor cells in cancer. Cell Rep. 33, 108571 (2020).
Cai, H., Zhang, Y., Wang, J. & Gu, J. Defects in macrophage reprogramming in cancer therapy: the negative impact of PD-L1/PD-1. Front. Immunol. 12, 690869 (2021).
Sinha, P., Clements, V. K., Bunt, S. K., Albelda, S. M. & Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 179, 977–983 (2007).
Mantovani, A. et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25, 677–686 (2004).
Fu, E. et al. M2 macrophages reduce the radiosensitivity of head and neck cancer by releasing HB-EGF. Oncol. Rep. 44, 698–710 (2020).
Krneta, T. et al. M2-polarized and tumor-associated macrophages alter NK cell phenotype and function in a contact-dependent manner. J. Leukoc. Biol. 101, 285–295 (2017).
Pan, Y., Yu, Y., Wang, X. & Zhang, T. Tumor-associated macrophages in tumor immunity. Front. Immunol. 11, 583084 (2020).
Ugel, S., De Sanctis, F., Mandruzzato, S. & Bronte, V. Tumor-induced myeloid deviation: when myeloid-derived suppressor cells meet tumor-associated macrophages. J. Clin. Invest. 125, 3365–3376 (2015).
Balermpas, P. et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br. J. Cancer 111, 1509–1518 (2014).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Veglia, F., Sanseviero, E. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 21, 485–498 (2021).
Gong, L. et al. Comprehensive single-cell sequencing reveals the stromal dynamics and tumor-specific characteristics in the microenvironment of nasopharyngeal carcinoma. Nat. Commun. 12, 1540 (2021).
Ma, X. et al. LncRNAs as an intermediate in HPV16 promoting myeloid-derived suppressor cell recruitment of head and neck squamous cell carcinoma. Oncotarget 8, 42061–42075 (2017).
Meyer, C. et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol. Immunother. 63, 247–257 (2014).
Mao, L. et al. TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells. Oral. Oncol. 121, 105472 (2021).
Highfill, S. L. et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 6, 237ra67 (2014).
Kumar, V. et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32, 654–668.e5 (2017).
Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 188, 21–28 (2012).
Marvel, D. & Gabrilovich, D. I. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J. Clin. Invest. 125, 3356–3364 (2015).
Davis, R. J. et al. Anti-PD-L1 efficacy can be enhanced by inhibition of myeloid-derived suppressor cells with a selective inhibitor of PI3Kδ/γ. Cancer Res. 77, 2607–2619 (2017).
Sobo-Vujanovic, A. et al. Inhibition of soluble tumor necrosis factor prevents chemically induced carcinogenesis in mice. Cancer Immunol. Res. 4, 441–451 (2016).
Knops, A. M. et al. Cancer-associated fibroblast density, prognostic characteristics, and recurrence in head and neck squamous cell carcinoma: a meta-analysis. Front. Oncol. 10, 565306 (2020).
Zhou, B. et al. A role for cancer-associated fibroblasts in inducing the epithelial-to-mesenchymal transition in human tongue squamous cell carcinoma. J. Oral. Pathol. Med. 43, 585–592 (2014).
Costea, D. E. et al. Identification of two distinct carcinoma-associated fibroblast subtypes with differential tumor-promoting abilities in oral squamous cell carcinoma. Cancer Res. 73, 3888–3901 (2013).
Bienkowska, K. J., Hanley, C. J. & Thomas, G. J. Cancer-associated fibroblasts in oral cancer: a current perspective on function and potential for therapeutic targeting. Front. Oral. Health 2, 686337 (2021).
Valkenburg, K. C., de Groot, A. E. & Pienta, K. J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 15, 366–381 (2018).
Kumar, D. et al. Cancer-associated fibroblasts drive glycolysis in a targetable signaling loop implicated in head and neck squamous cell carcinoma progression. Cancer Res. 78, 3769–3782 (2018).
Kang, S. H. et al. Cancer-associated fibroblast subgroups showing differential promoting effect on HNSCC progression. Cancers 13, 654 (2021).
Wheeler, S. E. et al. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head. Neck 36, 385–392 (2014).
Wu, X. et al. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 11, 345 (2020).
Kansy, B. A. et al. The bidirectional tumor–mesenchymal stromal cell interaction promotes the progression of head and neck cancer. Stem Cell Res. Ther. 5, 95 (2014).
Liu, C. et al. Bone marrow mesenchymal stem cells interact with head and neck squamous cell carcinoma cells to promote cancer progression and drug resistance. Neoplasia 23, 118–128 (2021).
Liotta, F. et al. Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br. J. Cancer 112, 745–754 (2015).
Mazzoni, A. et al. Human T cells interacting with HNSCC-derived mesenchymal stromal cells acquire tissue-resident memory like properties. Eur. J. Immunol. 50, 1571–1579 (2020).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010).
O’Sullivan, B. et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 17, 440–451 (2016).
Lee, N. Y. et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 22, 450–462 (2021).
Fakhry, C. et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J. Clin. Oncol. 32, 3365–3373 (2014).
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Sacco, A. G. et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 22, 883–892 (2021).
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394, 1915–1928 (2019).
Argiris, A. et al. LBA36 Nivolumab (N) + ipilimumab (I) vs EXTREME as first-line (1L) treatment (tx) for recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): final results of CheckMate 651. Ann. Oncol. https://doi.org/10.1016/j.annonc.2021.08.2113 (2021).
Astrazeneca. Update on KESTREL Phase III trial of Imfinzi with or without Tremelimumab in the 1st-line Treatment of Recurrent or Metastatic Head and Neck Cancer https://www.astrazeneca.com/content/astraz/media-centre/press-releases/2021/update-on-kestrel-phase-iii-trial-for-imfinzi.html (2021).
GSK. GSK Provides Update on Feladilimab, An Investigational Inducible T Cell Co-stimulatory (ICOS) Agonist https://www.gsk.com/en-gb/media/press-releases/gsk-provides-update-on-feladilimab-an-investigational-inducible-t-cell-co-stimulatory-icos-agonist/ (2021).
Cohen, R. B. et al. Combination of monalizumab and cetuximab in recurrent or metastatic head and neck cancer patients previously treated with platinum-based chemotherapy and PD-(L)1 inhibitors. J. Clin. Oncol. 38, 6516–6516 (2020).
Massarelli, E. et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 5, 67–73 (2019).
Doran, S. L. et al. T-cell receptor gene therapy for human papillomavirus-associated epithelial cancers: a first-in-human, phase I/II Study. J. Clin. Oncol. 37, 2759–2768 (2019).
Nagarsheth, N. B. et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 27, 419–425 (2021).
Seiwert, T. Y. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17, 956–965 (2016).
Ferris, R. L. et al. Abstract CT021: Tumor-associated immune cell PD-L1 expression and peripheral immune profiling: Analyses from CheckMate 141. In Clinical Trials CT021-CT021 (American Association for Cancer Research, 2017).
Chow, L. Q. M. et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J. Clin. Oncol. 34, 3838–3845 (2016).
Zandberg, D. P. et al. Tumor hypoxia is associated with resistance to PD-1 blockade in squamous cell carcinoma of the head and neck. J. Immunother. Cancer 9, e002088 (2021).
Ferris, R. L. et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral. Oncol. 81, 45–51 (2018).
Watermann, C. et al. Recurrent HNSCC harbor an immunosuppressive tumor immune microenvironment suggesting successful tumor immune evasion. Clin. Cancer Res. 27, 632–644 (2021).
Lu, N. et al. Human Semaphorin-4A drives Th2 responses by binding to receptor ILT-4. Nat. Commun. 9, 742 (2018).
Böttcher, J. P. et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell 172, 1022–1037.e14 (2018).
Park, M. H., Lee, J. S. & Yoon, J. H. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J. Surg. Oncol. 106, 386–392 (2012).
Schumacher, T. N. & Thommen, D. S. Tertiary lymphoid structures in cancer. Science https://doi.org/10.1126/science.abf9419 (2022).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and wrote the article. R.L.F, T.B. and D.Z. contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
L.V. is a co-inventor of a methodology licensed to INmune Bio, Inc., where soluble TNF sequestration using DN-TNF can be used to prevent or treat malignancies. D.P.Z. declares competing interests with Blueprint Medicines (advisory board), Macrogenics (consulting), Prelude Therapeutics (advisory board), and Merck (advisory board) and research support (institutional) from Merck, BMS, AstraZeneca, GlaxoSmithKline, Aduro, Astellas, Macrogenics, Lilly, Bicara, Checkmate Pharma, and Novasenta. R.L.F. declares competing interests with Aduro Biotech, Inc. (consulting), AstraZeneca/MedImmune (clinical trial, research funding), Bristol-Myers Squibb (advisory board, clinical trial, research funding), EMD Serono (advisory board), MacroGenics Inc. (advisory board), Merck (advisory board, clinical trial), Novasenta (consulting, stock, research funding), Numab Therapeutics AG (advisory board), Pfizer (advisory board), Sanofi (consultant), Tesaro (research funding) and Zymeworks Inc. (consultant). T.C.B. declares competing interests with Walking Fish Therapeutics (Scientific Advisory Board). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Zhijun Sun, Rieneke van de Ven and Moshe Elkabets for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ruffin, A.T., Li, H., Vujanovic, L. et al. Improving head and neck cancer therapies by immunomodulation of the tumour microenvironment. Nat Rev Cancer 23, 173–188 (2023). https://doi.org/10.1038/s41568-022-00531-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-022-00531-9
This article is cited by
-
Polycomb repressive complex 2 and its core component EZH2: potential targeted therapeutic strategies for head and neck squamous cell carcinoma
Clinical Epigenetics (2024)
-
Tumor-infiltrating immune cells and survival in head and neck squamous cell carcinoma: a retrospective computational study
Scientific Reports (2024)
-
Prognostic significance and immune escape implication of tumor-infiltrating neutrophil plasticity in human head and neck squamous cell carcinoma
Human Cell (2024)
-
The heterogeneity of tumour immune microenvironment revealing the CRABP2/CD69 signature discriminates distinct clinical outcomes in breast cancer
British Journal of Cancer (2023)
-
Serine and one-carbon metabolism sustain non-melanoma skin cancer progression
Cell Death Discovery (2023)