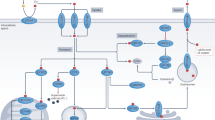
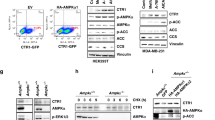
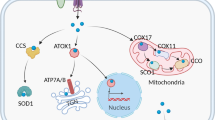
Abstract
Copper is an essential nutrient whose redox properties make it both beneficial and toxic to the cell. Recent progress in studying transition metal signalling has forged new links between researchers of different disciplines that can help translate basic research in the chemistry and biology of copper into clinical therapies and diagnostics to exploit copper-dependent disease vulnerabilities. This concept is particularly relevant in cancer, as tumour growth and metastasis have a heightened requirement for this metal nutrient. Indeed, the traditional view of copper as solely an active site metabolic cofactor has been challenged by emerging evidence that copper is also a dynamic signalling metal and metalloallosteric regulator, such as for copper-dependent phosphodiesterase 3B (PDE3B) in lipolysis, mitogen-activated protein kinase kinase 1 (MEK1) and MEK2 in cell growth and proliferation and the kinases ULK1 and ULK2 in autophagy. In this Perspective, we summarize our current understanding of the connection between copper and cancer and explore how challenges in the field could be addressed by using the framework of cuproplasia, which is defined as regulated copper-dependent cell proliferation and is a representative example of a broad range of metalloplasias. Cuproplasia is linked to a diverse array of cellular processes, including mitochondrial respiration, antioxidant defence, redox signalling, kinase signalling, autophagy and protein quality control. Identifying and characterizing new modes of copper-dependent signalling offers translational opportunities that leverage disease vulnerabilities to this metal nutrient.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Lippard S. J., Berg J. M. Principles of bioinorganic chemistry. vol. xvii, 411 p (University Science Books; 1994).
Solomon, E. I., Sundaram, U. M. & Machonkin, T. E. Multicopper oxidases and oxygenases. Chem. Rev. 96, 2563–2606 (1996).
Que, E. L., Domaille, D. W. & Chang, C. J. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem. Rev. 108, 1517–1549 (2008).
Brady, D. C. et al. Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 509, 492–496 (2014).
Chang, C. J. Searching for harmony in transition-metal signaling. Nat. Chem. Biol. 11, 744–747 (2015).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Ackerman, C. M., Lee, S. & Chang, C. J. Analytical methods for imaging metals in biology: from transition metal metabolism to transition metal signaling. Anal. Chem. 89, 22–41 (2017).
Hare, D. J., New, E. J., de Jonge, M. D. & McColl, G. Imaging metals in biology: balancing sensitivity, selectivity and spatial resolution. Chem. Soc. Rev. 44, 5941–5958 (2015).
Rosenzweig, A. Structure and chemistry of the copper chaperone proteins. Curr. Opin. Chem. Biol. 4, 140–147 (2000).
Thiele, D. J. & Gitlin, J. D. Assembling the pieces. Nat. Chem. Biol. 4, 145–147 (2008).
Cobine, P. A., Moore, S. A. & Leary, S. C. Getting out what you put in: copper in mitochondria and its impacts on human disease. Biochim. Biophys. Acta Mol. Cell Res 1868, 118867 (2021).
Gudekar, N. et al. Metallothioneins regulate ATP7A trafficking and control cell viability during copper deficiency and excess. Sci. Rep. 10, 7856 (2020).
Gupta, A. & Lutsenko, S. Evolution of copper transporting ATPases in eukaryotic organisms. Curr. Genomics 13, 124–133 (2012).
Kaler, S. G. ATP7A-related copper transport diseases-emerging concepts and future trends. Nat. Rev. Neurol. 7, 15–29 (2011).
Ackerman, C. M. & Chang, C. J. Copper signaling in the brain and beyond. J. Biol. Chem. 293, 4628–4635 (2018).
Prohaska J. R. Copper. In: Present Knowledge in Nutrition 10 (eds. Erdman J. W. MIA, Zeisel S. H.) p. 540–553 (Wiley-Blackwell; 2012).
Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (National Academies Press, 2001).
Wang, Y. et al. Maternofetal and neonatal copper requirements revealed by enterocyte-specific deletion of the Menkes disease protein. Am. J. Physiol. Gastrointest. Liver Physiol 303, G1236–G1244 (2012).
Roelofsen, H. et al. Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119, 782–793 (2000).
Polishchuk, E. V. et al. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev. Cell 29, 686–700 (2014).
Saroli Palumbo, C. & Schilsky, M. L. Clinical practice guidelines in Wilson disease. Ann. Transl. Med. 7, S65 (2019).
Harvey, L. J., Ashton, K., Hooper, L., Casgrain, A. & Fairweather-Tait, S. J. Methods of assessment of copper status in humans: a systematic review. Am. J. Clin. Nutr. 89, 2009S–2024S (2009).
Linder, M. C. Ceruloplasmin and other copper binding components of blood plasma and their functions: an update. Metallomics 8, 887–905 (2016).
Meyer, L. A., Durley, A. P., Prohaska, J. R. & Harris, Z. L. Copper transport and metabolism are normal in aceruloplasminemic mice. J. Biol. Chem. 276, 36857–36861 (2001).
Gray, L. W. et al. Urinary copper elevation in a mouse model of Wilson’s disease is a regulated process to specifically decrease the hepatic copper load. PLoS ONE 7, e38327 (2012).
Lee, J., Pena, M. M., Nose, Y. & Thiele, D. J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 277, 4380–4387 (2002).
Ren, F. et al. X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun. 10, 1386 (2019).
Ohgami, R. S., Campagna, D. R., McDonald, A. & Fleming, M. D. The Steap proteins are metalloreductases. Blood. 108, 1388–1394 (2006).
Kaler S. G., DiStasio A. T. ATP7A-related copper transport disorders. In: (eds Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Mirzaa G. et al.) (GeneReviews, 1993).
Czlonkowska, A. et al. Wilson disease. Nat. Rev. Dis. Prim. 4, 21 (2018).
Gunjan, D. et al. Hepatocellular carcinoma: an unusual complication of longstanding Wilson disease. J. Clin. Exp. Hepatol. 7, 152–154 (2017).
Blockhuys, S. et al. Defining the human copper proteome and analysis of its expression variation in cancers. Metallomics 9, 112–123 (2017).
Turnlund, J. R., Keyes, W. R., Anderson, H. L. & Acord, L. L. Copper absorption and retention in young men at three levels of dietary copper by use of the stable isotope 65Cu. Am. J. Clin. Nutr. 49, 870–878 (1989).
Prohaska, J. R. Impact of copper deficiency in humans. Ann. N. Y. Acad. Sci. 1314, 1–5 (2014).
Gambling, L., Kennedy, C. & McArdle, H. J. Iron and copper in fetal development. Semin. Cell Dev. Biol. 22, 637–44 (2011).
Lopez, J., Ramchandani, D. & Vahdat, L. Copper depletion as a therapeutic strategy in cancer. Met. Ions Life Sci. https://doi.org/10.1515/9783110527872-018 (2019).
Ding, X. et al. Analysis of serum levels of 15 trace elements in breast cancer patients in Shandong, China. Env. Sci. Pollut. Res. Int. 22, 7930–7935 (2015).
Adeoti, M. L., Oguntola, A. S., Akanni, E. O., Agodirin, O. S. & Oyeyemi, G. M. Trace elements; copper, zinc and selenium, in breast cancer afflicted female patients in LAUTECH Osogbo, Nigeria. Indian J. Cancer 52, 106–109 (2015).
Kuo, H. W., Chen, S. F., Wu, C. C., Chen, D. R. & Lee, J. H. Serum and tissue trace elements in patients with breast cancer in Taiwan. Biol. Trace Elem. Res. 89, 1–11 (2002).
Pavithra, V. et al. Serum levels of metal ions in female patients with breast cancer. J. Clin. Diagn. Res. 9, BC25–BC27 (2015).
Feng, J. F. et al. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int. J. Clin. Oncol. 17, 575–583 (2012).
Zowczak, M., Iskra, M., Torlinski, L. & Cofta, S. Analysis of serum copper and zinc concentrations in cancer patients. Biol. Trace Elem. Res. 82, 1–8 (2001).
Diez, M., Cerdan, F. J., Arroyo, M. & Balibrea, J. L. Use of the copper/zinc ratio in the diagnosis of lung cancer. Cancer 63, 726–730 (1989).
Jin, Y. et al. Combined effects of serum trace metals and polymorphisms of CYP1A1 or GSTM1 on non-small cell lung cancer: a hospital based case-control study in China. Cancer Epidemiol. 35, 182–187 (2011).
Oyama, T. et al. Efficiency of serum copper/zinc ratio for differential diagnosis of patients with and without lung cancer. Biol. Trace Elem. Res. 42, 115–127 (1994).
Stepien, M. et al. Pre-diagnostic copper and zinc biomarkers and colorectal cancer risk in the European prospective investigation into cancer and nutrition cohort. Carcinogenesis 38, 699–707 (2017).
Sohrabi, M. et al. Trace element and heavy metal levels in colorectal cancer: comparison between cancerous and non-cancerous tissues. Biol. Trace Elem. Res. 183, 1–8 (2018).
Ribeiro, S. M. et al. Copper-Zinc ratio and nutritional status in colorectal cancer patients during the perioperative period. Acta Cir. Bras. 31, 24–28 (2016).
Nayak, S. B., Bhat, V. R., Upadhyay, D. & Udupa, S. L. Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian. J. Physiol. Pharmacol. 47, 108–110 (2003).
Margalioth, E. J., Schenker, J. G. & Chevion, M. Copper and zinc levels in normal and malignant tissues. Cancer 52, 868–872 (1983).
Yaman, M., Kaya, G. & Yekeler, H. Distribution of trace metal concentrations in paired cancerous and non-cancerous human stomach tissues. World J. Gastroenterol. 13, 612–618 (2007).
Khanna, S. S. & Karjodkar, F. R. Circulating immune complexes and trace elements (Copper, Iron and Selenium) as markers in oral precancer and cancer: a randomised, controlled clinical trial. Head Face Med. 2, 33 (2006).
Baltaci, A. K., Dundar, T. K., Aksoy, F. & Mogulkoc, R. Changes in the serum levels of trace elements before and after the operation in thyroid cancer patients. Biol. Trace Elem. Res. 175, 57–64 (2017).
Basu, S. et al. Heavy and trace metals in carcinoma of the gallbladder. World J. Surg. 37, 2641–2646 (2013).
Saleh, S. A. K., Adly, H. M., Abdelkhaliq, A. A. & Nassir, A. M. Serum levels of selenium, zinc, copper, manganese, and iron in prostate cancer patients. Curr. Urol. 14, 44–49 (2020).
Ishida, S., Andreux, P., Poitry-Yamate, C., Auwerx, J. & Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl Acad. Sci. USA 110, 19507–19512 (2013).
Wooton-Kee, C. R. et al. Metabolic dysregulation in the Atp7b (-/-) Wilson’s disease mouse model. Proc. Natl Acad. Sci. USA 117, 2076–2083 (2020).
Yang, H. et al. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLoS Biol. 16, e2006519 (2018).
Shanbhag, V. et al. ATP7A delivers copper to the lysyl oxidase family of enzymes and promotes tumorigenesis and metastasis. Proc. Natl Acad. Sci. USA 116, 6836–41 (2019).
Tsang, T. et al. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Nat. Cell Biol. 22, 412–24 (2020).
Opazo, C. M. et al. Copper signaling promotes proteostasis and animal development via allosteric activation of ubiquitin E2D conjugases. Preprint at bioRxiv https://doi.org/10.1101/2021.02.15.431211 (2021).
Dodani, S. C. et al. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc. Natl Acad. Sci. USA 108, 5980–5985 (2011).
Dodani, S. C. et al. Copper is an endogenous modulator of neural circuit spontaneous activity. Proc. Natl Acad. Sci. USA 111, 16280–16285 (2014).
Hong-Hermesdorf, A. et al. Subcellular metal imaging identifies dynamic sites of Cu accumulation in Chlamydomonas. Nat. Chem. Biol 10, 1034–1042 (2014).
Krishnamoorthy, L. et al. Copper regulates cyclic-AMP-dependent lipolysis. Nat. Chem. Biol. 12, 586–592 (2016).
Morgan, M. T. et al. Ratiometric two-photon microscopy reveals attomolar copper buffering in normal and Menkes mutant cells. Proc. Natl Acad. Sci. USA 116, 12167–12172 (2019).
Chung, C. Y. et al. Activity-based ratiometric FRET probe reveals oncogene-driven changes in labile copper pools induced by altered glutathione metabolism. Proc. Natl Acad. Sci. USA 116, 18285–18294 (2019).
Bruemmer, K. J., Crossley, S. W. M. & Chang, C. J. Activity-based sensing: a synthetic methods approach for selective molecular imaging and beyond. Angew. Chem. Int. Ed. 59, 13734–13762 (2020).
Lee, S. et al. Activity-based sensing with a metal-directed acyl imidazole strategy reveals cell type-dependent pools of labile brain copper. J. Am. Chem. Soc. 142, 14993–15003 (2020).
Cotruvo, J. A. Jr., Aron, A. T., Ramos-Torres, K. M. & Chang, C. J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 44, 4400–4414 (2015).
Aron, A. T., Ramos-Torres, K. M., Cotruvo, J. A. Jr. & Chang, C. J. Recognition- and reactivity-based fluorescent probes for studying transition metal signaling in living systems. Acc. Chem. Res. 48, 2434–2442 (2015).
Hwang, J. J., Park, M. H. & Koh, J. Y. Copper activates TrkB in cortical neurons in a metalloproteinase-dependent manner. J. Neurosci. Res. 85, 2160–2166 (2007).
Michniewicz, F. et al. Copper: an intracellular Achilles’ heel allowing the targeting of epigenetics, kinase pathways, and cell metabolism in cancer therapeutics. ChemMedChem 16, 2315–2329 (2021).
He, F. et al. Copper (II) ions activate ligand-independent receptor tyrosine kinase (RTK) signaling pathway. Biomed. Res. Int. 2019, 4158415 (2019).
Turski, M. L. et al. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol. Cell. Biol. 32, 1284–1295 (2012).
Aubert, L. et al. Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat. Commun. 11, 3701 (2020).
Polishchuk, E. V. et al. Activation of autophagy, observed in liver tissues from patients with Wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology 156, 1173–89 e5 (2019).
Guo, J. et al. Copper promotes tumorigenesis by activating the PDK1-AKT oncogenic pathway in a copper transporter 1 dependent manner. Adv. Sci. 8, e2004303 (2021).
Kang, J., Lin, C., Chen, J. & Liu, Q. Copper induces histone hypoacetylation through directly inhibiting histone acetyltransferase activity. Chem. Biol. Interact. 148, 115–123 (2004).
Kieffer, D. A. & Medici, V. Wilson disease: at the crossroads between genetics and epigenetics-a review of the evidence. Liver Res. 1, 121–130 (2017).
Itoh, S. et al. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J. Biol. Chem. 283, 9157–9167 (2008).
Walshe, J. M. Treatment of Wilson’s disease with trientine (triethylene tetramine) dihydrochloride. Lancet 319, 643–647 (1982).
Ishida, S., McCormick, F., Smith-McCune, K. & Hanahan, D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 17, 574–583 (2010).
University D. A Pilot Study of Trientine with Vemurafenib for the Treatment BRAF Mutated Metastatic Melanoma https://ClinicalTrials.gov/show/NCT02068079 (2014).
Brewer, G. J. et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: phase I study. Clin. Cancer Res. 6, 1–10 (2000).
Center MSKC, University WMCoC. Phase II Study of Tetrathiomolybdate (TM) in Patients With Breast Cancer https://ClinicalTrials.gov/show/NCT00195091 (2003).
UK CR, Institute NC. Exemestane With or Without ATN-224 in Treating Postmenopausal Women With Recurrent or Advanced Breast Cancer https://ClinicalTrials.gov/show/NCT00674557 (2008).
Folkman, J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann. Surg. 175, 409–416 (1972).
Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1, 27–31 (1995).
Parke, A., Bhattacherjee, P., Palmer, R. M. & Lazarus, N. R. Characterization and quantification of copper sulfate-induced vascularization of the rabbit cornea. Am. J. Pathol. 130, 173–8 (1988).
Raju, K. S., Alessandri, G., Ziche, M. & Gullino, P. M. Ceruloplasmin, copper ions, and angiogenesis2. J. Natl Cancer Inst 69, 1183–1188 (1982).
Sen, C. K. et al. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol 282, H1821–H1827 (2002).
Chan, N. et al. Influencing the tumor microenvironment: a phase II study of copper depletion using tetrathiomolybdate in patients with breast cancer at high risk for recurrence and in preclinical models of lung metastases. Clin. Cancer Res. 23, 666–676 (2017).
Jiao, Y., Hannafon, B. N. & Ding, W. Q. Disulfiram’s anticancer activity: evidence and mechanisms. Anticancer. Agents Med. Chem. 16, 1378–1384 (2016).
Huang, J. et al. A phase I study to repurpose disulfiram in combination with temozolomide to treat newly diagnosed glioblastoma after chemoradiotherapy. J. Neurooncol 128, 259–66 (2016).
O’Day, S. et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J. Clin. Oncol. 27, 5452–5458 (2009).
O’Day, S. J. et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J. Clin. Oncol. 31, 1211–1218 (2013).
Sahlgrenska University Hospital S, Hospital SO, Hospital LU, Hospital KU, University Hospital L, County RÖ, et al. Disulfiram in Recurrent Glioblastoma https://ClinicalTrials.gov/show/NCT02678975 (2017).
Gohil, V. M. Repurposing elesclomol, an investigational drug for the treatment of copper metabolism disorders. Expert. Opin. Investig. Drugs 30, 1–4 (2021).
Ascierto, P. A. et al. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 10, 85 (2012).
Brady, D. C., Crowe, M. S., Greenberg, D. N. & Counter, C. M. Copper chelation inhibits BRAF(V600E)-driven melanomagenesis and counters resistance to BRAF(V600E) and MEK1/2 inhibitors. Cancer Res. 77, 6240–6252 (2017).
Amaravadi, R., Kimmelman, A. C. & White, E. Recent insights into the function of autophagy in cancer. Genes. Dev. 30, 1913–1930 (2016).
Piffoux, M., Eriau, E. & Cassier, P. A. Autophagy as a therapeutic target in pancreatic cancer. Br. J. Cancer 124, 333–344 (2021).
Yang, H., Liang, S. Q., Schmid, R. A. & Peng, R. W. New horizons in KRAS-mutant lung cancer: dawn after darkness. Front. Oncol. 9, 953 (2019).
Bryant, K. L. et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 25, 628–640 (2019).
Krishnan, N., Felice, C., Rivera, K., Pappin, D. J. & Tonks, N. K. DPM-1001 decreased copper levels and ameliorated deficits in a mouse model of Wilson’s disease. Genes. Dev. 32, 944–952 (2018).
Krishnan, N., Konidaris, K. F., Gasser, G. & Tonks, N. K. A potent, selective, and orally bioavailable inhibitor of the protein-tyrosine phosphatase PTP1B improves insulin and leptin signaling in animal models. J. Biol. Chem. 293, 1517–1525 (2018).
Pan, Q. et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 62, 4854–4859 (2002).
Pan, Q., Rosenthal, D. T., Bao, L., Kleer, C. G. & Merajver, S. D. Antiangiogenic tetrathiomolybdate protects against Her2/neu-induced breast carcinoma by hypoplastic remodeling of the mammary gland. Clin. Cancer Res. 15, 7441–7446 (2009).
Lee, K. et al. The copper chelator ATN-224 induces peroxynitrite-dependent cell death in hematological malignancies. Free. Radic. Biol. Med. 60, 157–167 (2013).
Glasauer, A., Sena, L. A., Diebold, L. P., Mazar, A. P. & Chandel, N. S. Targeting SOD1 reduces experimental non-small-cell lung cancer. J. Clin. Invest. 124, 117–128 (2014).
Juarez, J. C. et al. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase 1. Clin. Cancer Res. 12, 4974–4982 (2006).
Tsang, T. et al. Copper is an essential regulator of the autophagic kinases ULK1/2 to drive lung adenocarcinoma. Preprint at bioRxiv 22, 412–424 (2020).
Lin, N. U. et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 113, 2638–2645 (2008).
Kennecke, H. et al. Metastatic behavior of the breast cancer subtypes. J. Clin. Oncol. 28, 3271–3277 (2010).
Voli, F. et al. Intratumoral copper modulates PD-L1 expression and influences tumor immune evasion. Cancer Res. 80, 4129–4144 (2020).
Zhao, Z., Ukidve, A., Kim, J. & Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 181, 151–167 (2020).
Halliwell, B. & Gutteridge, J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219, 1–14 (1984).
Trachootham, D., Alexandre, J. & Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug. Discov. 8, 579–591 (2009).
Schumacker, P. T. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10, 175–176 (2006).
Cabello, C. M., Bair, W. B. 3rd & Wondrak, G. T. Experimental therapeutics: targeting the redox Achilles heel of cancer. Curr. Opin. Investig. Drugs 8, 1022–1037 (2007).
Fruehauf, J. P. & Meyskens, F. L. Jr. Reactive oxygen species: a breath of life or death? Clin. Cancer Res. 13, 789–794 (2007).
Kirshner, J. R. et al. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol. Cancer Ther. 7, 2319–2327 (2008).
Tsvetkov, P. et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat. Chem. Biol. 15, 681–689 (2019).
Viola-Rhenals, M. et al. Recent advances in Antabuse (disulfiram): the importance of its metal-binding ability to its anticancer activity. Curr. Med. Chem. 25, 506–524 (2018).
Wattenberg, L. W. Inhibition of dimethylhydrazine-induced neoplasia of the large intestine by disulfiram. J. Natl Cancer Inst. 54, 1005–1006 (1975).
Sunderman, F. W. Efficacy of sodium diethyldithiocarbamate (dithiocarb) in acute nickel carbonyl poisoning. Ann. Clin. Lab. Sci. 9, 1–10 (1979).
Victoriano, L. I. The reactivity of metal species towards thiuram sulfides: an alternative route to the syntheses of metal dithiocarbamates. Coord. Chem. Rev. 196, 383–398 (2000).
Lewis, D. J., Deshmukh, P., Tedstone, A. A., Tuna, F. & O’Brien, P. On the interaction of copper(II) with disulfiram. Chem. Commun. 50, 13334–13337 (2014).
Cvek, B., Milacic, V., Taraba, J. & Dou, Q. P. Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J. Med. Chem. 51, 6256–6258 (2008).
Tawari, P. E. et al. The cytotoxic mechanisms of disulfiram and copper(II) in cancer cells. Toxicol. Res. 4, 1439–1442 (2015).
Chen, D., Cui, Q. C., Yang, H. & Dou, Q. P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 66, 10425–10433 (2006).
Chen, X. et al. Metal-based proteasomal deubiquitinase inhibitors as potential anticancer agents. Cancer Metastasis Rev. 36, 655–668 (2017).
Skrott, Z. et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 552, 194–199 (2017).
Yang, H. et al. Repurposing old drugs as new inhibitors of the ubiquitin-proteasome pathway for cancer treatment. Semin. Cancer Biol. 68, 105–122 (2021).
Liu, P. et al. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br. J. Cancer 109, 1876–1885 (2013).
Wang, Y. et al. Blocking the formation of radiation-induced breast cancer stem cells. Oncotarget. 5, 3743–3755 (2014).
Wu, L. et al. Disulfiram and BKM120 in combination with chemotherapy impede tumor progression and delay tumor recurrence in tumor initiating cell-rich TNBC. Sci. Rep. 9, 236 (2019).
Gehrmann, M. Drug evaluation: STA-4783–enhancing taxane efficacy by induction of Hsp70. Curr. Opin. Investig. Drugs 7, 574–580 (2006).
Yadav, A. A., Patel, D., Wu, X. & Hasinoff, B. B. Molecular mechanisms of the biological activity of the anticancer drug elesclomol and its complexes with Cu(II), Ni(II) and Pt(II). J. Inorg. Biochem. 126, 1–6 (2013).
Nagai, M. et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free. Radic. Biol. Med. 52, 2142–2150 (2012).
Berkenblit, A. et al. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clin. Cancer Res. 13, 584–590 (2007).
Chakravarty, R., Chakraborty, S. & Dash, A. 64Cu2+ ions as PET Probe: an emerging paradigm in molecular imaging of cancer. Mol. Pharm. 13, 3601–3612 (2016).
Qin, C. et al. Theranostics of malignant melanoma with 64CuCl2. J. Nucl. Med. 55, 812–817 (2014).
Peng, F., Lu, X., Janisse, J., Muzik, O. & Shields, A. F. PET of human prostate cancer xenografts in mice with increased uptake of 64CuCl2. J. Nucl. Med. 47, 1649–1652 (2006).
Kim, K. I. et al. Detection of increased 64Cu uptake by human copper transporter 1 gene overexpression using PET with 64CuCl2 in human breast cancer xenograft model. J. Nucl. Med. 55, 1692–1698 (2014).
Jorgensen, J. T., Persson, M., Madsen, J. & Kjaer, A. High tumor uptake of 64Cu: implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl. Med. Biol. 40, 345–350 (2013).
Ferrari, C. et al. Copper-64 dichloride as theranostic agent for glioblastoma multiforme: a preclinical study. Biomed. Res. Int. 2015, 129764 (2015).
Cai, H. et al. Reduced 64Cu uptake and tumor growth inhibition by knockdown of human copper transporter 1 in xenograft mouse model of prostate cancer. J. Nucl. Med. 55, 622–628 (2014).
Capasso, E. et al. Role of 64CuCl2 PET/CT in staging of prostate cancer. Ann. Nucl. Med. 29, 482–8 (2015).
Piccardo, A. et al. 64CuCl2 PET/CT in prostate cancer relapse. J. Nucl. Med. 59, 444–451 (2018).
Panichelli, P. et al. Imaging of brain tumors with copper-64 chloride: early experience and results. Cancer Biother Radiopharm. 31, 159–167 (2016).
Santini, C. et al. Advances in copper complexes as anticancer agents. Chem. Rev. 114, 815–862 (2014).
De Luca, A., Barile, A., Arciello, M. & Rossi, L. Copper homeostasis as target of both consolidated and innovative strategies of anti-tumor therapy J. Trace Elem. Med. Biol. 55, 204–13 (2019).
Kellett A., Molphy Z., McKee V., Slator C. Recent advances in anticancer copper compounds. in Metal-based Anticancer Agents. p. 91–119 (Royal Society of Chemistry, 2019).
Gandin, V. et al. In vitro and in vivo anticancer activity of copper(I) complexes with homoscorpionate tridentate tris(pyrazolyl)borate and auxiliary monodentate phosphine ligands. J. Med. Chem. 57, 4745–4760 (2014).
Marzano C. et al. [CU(thp)4]n[X]-n Compounds for the Treatment of a Broad Range of Human Solid Tumors, Including Refractory Tumors. US Patent 9114149, Abstract (2015). Inventors; Universita degli Studi di Camerino, Universita degli Studi di Padova, assignee.
Trejo-Solis, C. et al. Copper compound induces autophagy and apoptosis of glioma cells by reactive oxygen species and JNK activation. BMC Cancer 12, 156 (2012).
Serment-Guerrero, J. et al. Genotoxicity of the copper antineoplastic coordination complexes casiopeinas. Toxicol. In Vitro 25, 1376–1384 (2011).
Hirayama, T., Van de Bittner, G. C., Gray, L. W., Lutsenko, S. & Chang, C. J. Near-infrared fluorescent sensor for in vivo copper imaging in a murine Wilson disease model. Proc. Natl Acad. Sci. USA. 109, 2228–2233 (2012).
Heffern, M. C. et al. In vivo bioluminescence imaging reveals copper deficiency in a murine model of nonalcoholic fatty liver disease. Proc. Natl Acad. Sci. USA. 113, 14219–14224 (2016).
Cui, L. et al. Mitochondrial copper depletion suppresses triple-negative breast cancer in mice. Nat. Biotechnol. 39, 357–367 (2021).
Iovan, D. A., Jia, S. & Chang, C. J. Inorganic chemistry approaches to activity-based sensing: from metal sensors to bioorthogonal metal chemistry. Inorg. Chem. 58, 13546–13560 (2019).
Ramos-Torres, K. M., Kolemen, S. & Chang, C. J. Thioether coordination chemistry for molecular imaging of copper in biological systems. Isr. J. Chem. 56, 724–737 (2016).
Morgan, M. T. et al. Stabilization of aliphatic phosphines by auxiliary phosphine sulfides offers zeptomolar affinity and unprecedented selectivity for probing biological Cu(I). Angew. Chem. Int. Ed. 57, 9711–9715 (2018).
Wang, J. et al. Inhibition of human copper trafficking by a small molecule significantly attenuates cancer cell proliferation. Nat. Chem. 7, 968–979 (2015).
Karginova, O. et al. Inhibition of copper transport induces apoptosis in triple-negative breast cancer cells and suppresses tumor angiogenesis. Mol. Cancer Ther. 18, 873–885 (2019).
Haddad, M. R. et al. Cerebrospinal fluid-directed rAAV9-rsATP7A plus subcutaneous copper histidinate advance survival and outcomes in a Menkes disease mouse model. Mol. Ther. Methods Clin. Dev. 10, 165–178 (2018).
Su, T. A. et al. A modular ionophore platform for liver-directed copper supplementation in cells and animals. J. Am. Chem. Soc. 140, 13764–13774 (2018).
Pinho, J. O. et al. Therapeutic potential of a copper complex loaded in pH-sensitive long circulating liposomes for colon cancer management. Int. J. Pharm. 599, 120463 (2021).
Pinho, J. O. et al. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine 14, 835–850. (2019).
Hunsaker, E. W. & Franz, K. J. Emerging opportunities to manipulate metal trafficking for therapeutic benefit. Inorg. Chem. 58, 13528–13545 (2019).
Walshe, J. M. Penicillamine, a new oral therapy for Wilson’s disease. Am. J. Med. 21, 487–495 (1956).
Brewer, G. J. et al. Treatment of Wilson’s disease with ammonium tetrathiomolybdate. I. Initial therapy in 17 neurologically affected patients. Arch. Neurol. 51, 545–554 (1994).
NCRR, University of Michigan. Study of Tetrathiomolybdate in Patients With Wilson Disease https://ClinicalTrials.gov/show/NCT00004339 (1994).
Bell, R. G. & Smith, H. W. Preliminary report on clinical trials of Antabuse. Can. Med. Assoc. J. 60, 286–288 (1949).
Limited CMDP. Treatment Continuation Study for Patients With ALS/MND Who Completed Study CMD-2019-001 https://ClinicalTrials.gov/show/NCT04313166 (2020).
Torres, J. B. et al. PET Imaging of copper trafficking in a mouse model of Alzheimer disease. J. Nucl. Med. 57, 109–114 (2016).
Mao, X. & Schimmer, A. D. The toxicology of clioquinol. Toxicol. Lett. 182, 1–6 (2008).
Ltd CP. 64Cu-SAR-bisPSMA and 67Cu-SAR-bisPSMA for Identification and Treatment of PSMA-expressing Metastatic Castrate Resistant Prostate Cancer https://ClinicalTrials.gov/show/NCT04868604 (2021).
Acknowledgements
The authors thank the following sources for funding: NIH (GM79465 and GM139245 to C.J.C., GM124749 to D.C.B., R01-GM084176 to K.J.F., GM120211 to P.A.C., GM111672 to V.M.G., R21-GM129592 to M.R., R01-NS109307 to P.Y., CA190265 and DK116859 to M.J.P., Z01 HD008768 and Z01 HD008892 to S.G.K., CA53840 and DK124907 to N.K.T. and R01-GM101502, R01-DK117396 and R01-DK071865 to S.L.), the US National Cancer Institute (R21CA184788 to Q.P.D.), the Welch Foundation (A-1810 to V.M.G.), Pew Charitable Trusts (Pew Scholars Program in Biomedical Science award no. 50350 to D.C.B.)), AIRC Italy (IG 17118 to R.P.), the Florida Department of Health Bankhead-Coley Cancer Research Program (9BC07 to G.M.D.), the Breast Cancer Research Foundation, Susan G. Komen Greater New York City (L.T.V.), the US Department of Army (W81XH-20-1-0754 to L.V.A.), the CSHL Cancer Centre Support Grant (CA45508 to N.K.T.) and the V Foundation Scholar Award (3C59 8ABS 3424 3BDA to D.C.B.) V.M. was supported by the Center on the Physics of Cancer Metabolism through award number U54CA210184 from the National Cancer Institute, and also by National Cancer Institute award R01 CA257254-01A1 (V.M. and L.T.V.). S.G. received support from NIDDK R01-DK-071111 and NIDDK Center grants, P30-DK-41296 and P30-DK-020541 and NCI Center grant P30-CA-13330. A.I.B. is funded by the National Health and Medical Research Council of Australia. C.J.C. is a CIFAR Fellow.
Author information
Authors and Affiliations
Contributions
All authors contributed to discussion of the content. C.J.C. outlined the article. C.J.C., E.J.G., M.J.P., D.C.B., V.M.G., Q.P.D., L.T.V., S.G.K., A.I.B., A.C. and M.L.S. wrote portions of the article. E.J.G., D.C.B. and C.J.C. reviewed and edited the article before submission. C.J.C. and D.C.B. designed the figures.
Corresponding authors
Ethics declarations
Competing interests
N.K.T. is a member of the Scientific Advisory Board of DepYmed Inc. V.M.G. is listed as an inventor on the patent application PCT/US2019/041571 submitted by Texas A&M University entitled “Compositions for the treatment of copper deficiency and methods of use”. D.C.B. holds ownership in Merlon Inc. A.I.B. holds equity in Alterity Biotechnology Ltd, Cogstate Ltd, Mesoblast Ltd and Collaborative Medicinal Development LLC and is a paid consultant for Collaborative Medicinal Development Pty Ltd. L.T.V. is a consultant for Berg Pharma, Osmol Therapeutics and Sema4, serves on the advisory board of Seattle Genetics and Immunomedics/Gilead, and receives research funding from Genentech, Arvinas, and Oncotheraphy Sciences. E.J.G, A.C., P.A.C., J.R.C, G.M.D., Q.P.D., K.J.F., S.G., S.G.K., S.L., V.M., M.J.P., R.P., M.R., M.L.S., L.V.A., D.X., P.Y. and C.J.C. declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks James Camakaris, Victor Faundez, Žaklina Kovačević and Orazio Vittorio for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ge, E.J., Bush, A.I., Casini, A. et al. Connecting copper and cancer: from transition metal signalling to metalloplasia. Nat Rev Cancer 22, 102–113 (2022). https://doi.org/10.1038/s41568-021-00417-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00417-2
This article is cited by
-
Immunomodulation of cuproptosis and ferroptosis in liver cancer
Cancer Cell International (2024)
-
NIR-IIb fluorescence antiangiogenesis copper nano-reaper for enhanced synergistic cancer therapy
Journal of Nanobiotechnology (2024)
-
A cuproptosis score model and prognostic score model can evaluate clinical characteristics and immune microenvironment in NSCLC
Cancer Cell International (2024)
-
Metal-binding amino acid ligands commonly found in metalloproteins differentially fractionate copper isotopes
Scientific Reports (2024)
-
Targeting cuproplasia and cuproptosis in cancer
Nature Reviews Clinical Oncology (2024)