Abstract
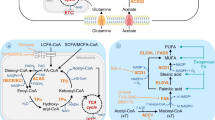
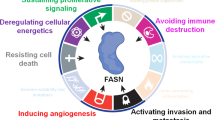
Fatty acid metabolism is known to support tumorigenesis and disease progression as well as treatment resistance through enhanced lipid synthesis, storage and catabolism. More recently, the role of membrane fatty acid composition, for example, ratios of saturated, monounsaturated and polyunsaturated fatty acids, in promoting cell survival while limiting lipotoxicity and ferroptosis has been increasingly appreciated. Alongside these insights, it has become clear that tumour cells exhibit plasticity with respect to fatty acid metabolism, responding to extratumoural and systemic metabolic signals, such as obesity and cancer therapeutics, to promote the development of aggressive, treatment-resistant disease. Here, we describe cellular fatty acid metabolic changes that are connected to therapy resistance and contextualize obesity-associated changes in host fatty acid metabolism that likely influence the local tumour microenvironment to further modify cancer cell behaviour while simultaneously creating potential new vulnerabilities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Faubert, B., Solmonson, A. & DeBerardinis, R. J. Metabolic reprogramming and cancer progression. Science 368, eaaw5473 (2020).
Balaban, S. et al. Heterogeneity of fatty acid metabolism in breast cancer cells underlies differential sensitivity to palmitate-induced apoptosis. Mol. Oncol. 12, 1623–1638 (2018).
Balaban, S. et al. Extracellular fatty acids are the major contributor to lipid synthesis in prostate cancer. Mol. Cancer Res. 17, 949–962 (2019).
Balaban, S. et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 5, 1 (2017).
Li, H. et al. The landscape of cancer cell line metabolism. Nat. Med. 25, 850–860 (2019). This study reported the metabolome of 928 cell lines from more than 20 cancer types in the Cancer Cell Line Encyclopedia and the heterogeneity of this panel of cancer cells.
Chen, P. H. et al. Metabolic diversity in human non-small cell lung cancer cells. Mol. Cell 76, 838–851.e5 (2019).
Hakimi, A. A. et al. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell 29, 104–116 (2016).
Garg, G. et al. Targeted metabolomic profiling of low and high grade serous epithelial ovarian cancer tissues: a pilot study. Metabolomics 14, 154 (2018).
Lin, H. M. et al. A distinct plasma lipid signature associated with poor prognosis in castration-resistant prostate cancer. Int. J. Cancer 141, 2112–2120 (2017).
Christen, S. et al. Breast cancer-derived lung metastases show increased pyruvate carboxylase-dependent anaplerosis. Cell Rep. 17, 837–848 (2016).
Elia, I., Doglioni, G. & Fendt, S. M. Metabolic hallmarks of metastasis formation. Trends Cell Biol. 28, 673–684 (2018).
Fendt, S. M., Frezza, C. & Erez, A. Targeting metabolic plasticity and flexibility dynamics for cancer therapy. Cancer Discov. 10, 1797–1807 (2020).
Zhu, J. & Thompson, C. B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 20, 436–450 (2019).
Butler, L. et al. Lipids and cancer: emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 159, 245–293 (2020).
Nagarajan, S. R., Butler, L. M. & Hoy, A. J. The diversity and breadth of cancer cell fatty acid metabolism. Cancer Metab. 9, 2 (2021).
Harayama, T. & Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 19, 281–296 (2018).
Rysman, E. et al. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 70, 8117–8126 (2010).
Peetla, C. et al. Drug resistance in breast cancer cells: biophysical characterization of and doxorubicin interactions with membrane lipids. Mol. Pharm. 7, 2334–2348 (2010).
Callaghan, R., van Gorkom, L. C. & Epand, R. M. A comparison of membrane properties and composition between cell lines selected and transfected for multi-drug resistance. Br. J. Cancer 66, 781–786 (1992).
Zong, L. et al. Liquid extraction surface analysis nanospray electrospray ionization based lipidomics for in situ analysis of tumor cells with multidrug resistance. Rapid Commun. Mass. Spectrom. 32, 1683–1692 (2018).
Roy, J., Dibaeinia, P., Fan, T. M., Sinha, S. & Das, A. Global analysis of osteosarcoma lipidomes reveal altered lipid profiles in metastatic versus nonmetastatic cells. J. Lipid Res. 60, 375–387 (2019).
Kim, H. Y. et al. Discovery of potential biomarkers in human melanoma cells with different metastatic potential by metabolic and lipidomic profiling. Sci. Rep. 7, 8864 (2017).
Hoxhaj, G. & Manning, B. D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020).
Hopkins, B. D., Goncalves, M. D. & Cantley, L. C. Insulin-PI3K signalling: an evolutionarily insulated metabolic driver of cancer. Nat. Rev. Endocrinol. 16, 276–283 (2020).
Marien, E. et al. Phospholipid profiling identifies acyl chain elongation as a ubiquitous trait and potential target for the treatment of lung squamous cell carcinoma. Oncotarget 7, 12582–12597 (2016). By profiling phospholipids in a large clinical cohort of lung cancers, this study revealed fatty acid elongation as a cancer-related lipidomic feature that could be targeted via the elongase ELOVL6.
Park, J., Morley, T. S., Kim, M., Clegg, D. J. & Scherer, P. E. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 10, 455–465 (2014).
Balaban, S., Lee, L. S., Schreuder, M. & Hoy, A. J. Obesity and cancer progression: is there a role of fatty acid metabolism? Biomed. Res. Int. 2015, 274585 (2015).
Avery, C. L., Howard, A. G. & Nichols, H. B. Comparison of 20-year obesity-associated cancer mortality trends with heart disease mortality trends in the US. JAMA Netw. Open 4, e218356 (2021).
Veldman, R. J. et al. Altered sphingolipid metabolism in multidrug-resistant ovarian cancer cells is due to uncoupling of glycolipid biosynthesis in the Golgi apparatus. FASEB J. 16, 1111–1113 (2002).
Mannechez, A., Reungpatthanaphong, P., de Certaines, J. D., Leray, G. & Le Moyec, L. Proton NMR visible mobile lipid signals in sensitive and multidrug-resistant K562 cells are modulated by rafts. Cancer Cell Int. 5, 2 (2005).
Kopecka, J. et al. Phospholipids and cholesterol: inducers of cancer multidrug resistance and therapeutic targets. Drug Resist. Updat. 49, 100670 (2019).
Hajjaji, N. & Bougnoux, P. Selective sensitization of tumors to chemotherapy by marine-derived lipids: a review. Cancer Treat. Rev. 39, 473–488 (2013).
Yang, W. H. et al. A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol. Cancer Res. 18, 79–90 (2020).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Hangauer, M. J. et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250 (2017). Viswanathan et al.34 and Hangauer et al.35 reported a mechanism of therapy resistance in cancer cells that arises from a dependency on the antioxidant enzyme glutathione peroxidase, which protects cells from a lipid peroxidation-induced form of cell death called ferroptosis.
Alexa-Stratulat, T., Pesic, M., Gasparovic, A. C., Trougakos, I. P. & Riganti, C. What sustains the multidrug resistance phenotype beyond ABC efflux transporters? Looking beyond the tip of the iceberg. Drug Resist. Updat. 46, 100643 (2019).
Bauerschlag, D. O. et al. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. J. Transl. Med. 13, 146 (2015).
Wu, X. et al. FASN regulates cellular response to genotoxic treatments by increasing PARP-1 expression and DNA repair activity via NF-kappaB and SP1. Proc. Natl Acad. Sci. USA 113, E6965–E6973 (2016).
Kant, S., Kumar, A. & Singh, S. M. Tumor growth retardation and chemosensitizing action of fatty acid synthase inhibitor orlistat on T cell lymphoma: implication of reconstituted tumor microenvironment and multidrug resistance phenotype. Biochim. Biophys. Acta 1840, 294–302 (2014).
Papaevangelou, E., Almeida, G. S., Box, C., deSouza, N. M. & Chung, Y. L. The effect of FASN inhibition on the growth and metabolism of a cisplatin-resistant ovarian carcinoma model. Int. J. Cancer 143, 992–1002 (2018).
Wang, T. et al. JAK/STAT3-regulated fatty acid beta-oxidation is critical for breast cancer stem cell self-renewal and chemoresistance. Cell Metab. 27, 136–150 e135 (2018).
He, W. et al. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene 38, 4637–4654 (2019).
Luo, J. et al. An indispensable role of CPT-1a to survive cancer cells during energy stress through rewiring cancer metabolism. Tumour Biol. 37, 15795–15804 (2016).
Sirois, I. et al. A unique morphological phenotype in chemoresistant triple-negative breast cancer reveals metabolic reprogramming and PLIN4 expression as a molecular vulnerability. Mol. Cancer Res. 17, 2492–2507 (2019).
Hultsch, S. et al. Association of tamoxifen resistance and lipid reprogramming in breast cancer. BMC Cancer 18, 850 (2018).
Cotte, A. K. et al. Lysophosphatidylcholine acyltransferase 2-mediated lipid droplet production supports colorectal cancer chemoresistance. Nat. Commun. 9, 322 (2018). This study provided new mechanistic insights into the role of lipid droplet accumulation, driven by LPCAT2, in cancer cell chemoresistance and reduced immunogenicity.
Dubey, R. et al. Lipid droplets can promote drug accumulation and activation. Nat. Chem. Biol. 16, 206–213 (2020).
Tan, Z. et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics 8, 2329–2347 (2018).
Han, S. et al. CPT1A/2-mediated FAO enhancement-a metabolic target in radioresistant breast cancer. Front. Oncol. 9, 1201 (2019).
Du, Q. et al. PGC1alpha/CEBPB/CPT1A axis promotes radiation resistance of nasopharyngeal carcinoma through activating fatty acid oxidation. Cancer Sci. 110, 2050–2062 (2019).
Wan, H., Xu, B., Zhu, N. & Ren, B. PGC-1alpha activator-induced fatty acid oxidation in tumor-infiltrating CTLs enhances effects of PD-1 blockade therapy in lung cancer. Tumori 106, 55–63 (2019).
Dheeraj, A. et al. A novel approach to target hypoxic cancer cells via combining beta-oxidation inhibitor etomoxir with radiation. Hypoxia 6, 23–33 (2018).
Mims, J. et al. Energy metabolism in a matched model of radiation resistance for head and neck squamous cell cancer. Radiat. Res. 183, 291–304 (2015).
Chuang, H. Y., Lee, Y. P., Lin, W. C., Lin, Y. H. & Hwang, J. J. Fatty acid inhibition sensitizes androgen-dependent and -independent prostate cancer to radiotherapy via FASN/NF-kappaB pathway. Sci. Rep. 9, 13284 (2019).
Zhan, N., Li, B., Xu, X., Xu, J. & Hu, S. Inhibition of FASN expression enhances radiosensitivity in human non-small cell lung cancer. Oncol. Lett. 15, 4578–4584 (2018).
Li, N. et al. Fatty acid synthase regulates proliferation and migration of colorectal cancer cells via HER2-PI3K/Akt signaling pathway. Nutr. Cancer 64, 864–870 (2012).
Jin, Q. et al. Fatty acid synthase phosphorylation: a novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res. 12, R96 (2010).
Long, X. H. et al. Lapatinib alters the malignant phenotype of osteosarcoma cells via downregulation of the activity of the HER2-PI3K/AKT-FASN axis in vitro. Oncol. Rep. 31, 328–334 (2014).
Grunt, T. W. et al. Interaction between fatty acid synthase- and ErbB-systems in ovarian cancer cells. Biochem. Biophys. Res. Commun. 385, 454–459 (2009).
Vazquez-Martin, A., Colomer, R., Brunet, J. & Menendez, J. A. Pharmacological blockade of fatty acid synthase (FASN) reverses acquired autoresistance to trastuzumab (Herceptin by transcriptionally inhibiting ‘HER2 super-expression’ occurring in high-dose trastuzumab-conditioned SKBR3/Tzb100 breast cancer cells. Int. J. Oncol. 31, 769–776 (2007).
Blancafort, A. et al. Dual fatty acid synthase and HER2 signaling blockade shows marked antitumor activity against breast cancer models resistant to anti-HER2 drugs. PLoS ONE 10, e0131241 (2015).
Puig, T. et al. A novel inhibitor of fatty acid synthase shows activity against HER2+ breast cancer xenografts and is active in anti-HER2 drug-resistant cell lines. Breast Cancer Res. 13, R131 (2011).
Li, C. F. et al. Overexpressed fatty acid synthase in gastrointestinal stromal tumors: targeting a progression-associated metabolic driver enhances the antitumor effect of imatinib. Clin. Cancer Res. 23, 4908–4918 (2017).
Lue, H. W. et al. Metabolic reprogramming ensures cancer cell survival despite oncogenic signaling blockade. Genes Dev. 31, 2067–2084 (2017).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).
Feng, W. W. et al. CD36-mediated metabolic rewiring of breast cancer cells promotes resistance to HER2-targeted therapies. Cell Rep. 29, 3405–3420.e5 (2019).
Nassar, Z. D. et al. Fatty acid oxidation is an adaptive survival pathway induced in prostate tumors by heat shock protein 90 inhibition. Mol. Cancer Res. 18, 1500–1511 (2020).
Butler, L. M., Centenera, M. M. & Swinnen, J. V. Androgen control of lipid metabolism in prostate cancer: novel insights and future applications. Endocr. Relat. Cancer 23, R219–R227 (2016).
Harrelson, J. P. & Lee, M. W. Expanding the view of breast cancer metabolism: promising molecular targets and therapeutic opportunities. Pharmacol. Ther. 167, 60–73 (2016).
Latonen, L. et al. Integrative proteomics in prostate cancer uncovers robustness against genomic and transcriptomic aberrations during disease progression. Nat. Commun. 9, 1176 (2018).
Iglesias-Gato, D. et al. The proteome of prostate cancer bone metastasis reveals heterogeneity with prognostic implications. Clin. Cancer Res. 24, 5433–5444 (2018).
Sharma, N. L. et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 23, 35–47 (2013).
Nguyen, V. T. et al. Differential epigenetic reprogramming in response to specific endocrine therapies promotes cholesterol biosynthesis and cellular invasion. Nat. Commun. 6, 10044 (2015).
Han, W. et al. Reactivation of androgen receptor-regulated lipid biosynthesis drives the progression of castration-resistant prostate cancer. Oncogene 37, 710–721 (2018).
Du, T. et al. Key regulators of lipid metabolism drive endocrine resistance in invasive lobular breast cancer. Breast Cancer Res. 20, 106 (2018).
Karantanos, T. et al. Understanding the mechanisms of androgen deprivation resistance in prostate cancer at the molecular level. Eur. Urol. 67, 470–479 (2015).
Swinnen, J. V., Ulrix, W., Heyns, W. & Verhoeven, G. Coordinate regulation of lipogenic gene expression by androgens: evidence for a cascade mechanism involving sterol regulatory element binding proteins. Proc. Natl Acad. Sci. USA 94, 12975–12980 (1997).
Heemers, H. V., Verhoeven, G. & Swinnen, J. V. Androgen activation of the sterol regulatory element-binding protein pathway: Current insights. Mol. Endocrinol. 20, 2265–2277 (2006).
Massie, C. E. et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 30, 2719–2733 (2011).
Tennakoon, J. B. et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1alpha-mediated metabolic switch. Oncogene 33, 5251–5261 (2014).
Tousignant, K. D. et al. Lipid uptake is an androgen-enhanced lipid supply pathway associated with prostate cancer disease progression and bone metastasis. Mol. Cancer Res. 17, 1166–1179 (2019).
Shafi, A. A. et al. Differential regulation of metabolic pathways by androgen receptor (AR) and its constitutively active splice variant, AR-V7, in prostate cancer cells. Oncotarget 6, 31997–32012 (2015).
Zadra, G. & Loda, M. Metabolic vulnerabilities of prostate cancer: diagnostic and therapeutic opportunities. Cold Spring Harb. Perspect. Med. 8, a030569 (2018).
Schlaepfer, I. R. et al. Lipid catabolism via CPT1 as a therapeutic target for prostate cancer. Mol. Cancer Ther. 13, 2361–2371 (2014).
Zadra, G. et al. A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Mol. Med. 6, 519–538 (2014).
Zadra, G. et al. Inhibition of de novo lipogenesis targets androgen receptor signaling in castration-resistant prostate cancer. Proc. Natl Acad. Sci. USA 116, 631–640 (2019).
Itkonen, H. M. et al. Lipid degradation promotes prostate cancer cell survival. Oncotarget 8, 38264–38275 (2017).
Kong, Y. et al. Inhibition of cholesterol biosynthesis overcomes enzalutamide resistance in castration-resistant prostate cancer (CRPC). J. Biol. Chem. 293, 14328–14341 (2018).
Tousignant, K. D. et al. Therapy-induced lipid uptake and remodeling underpin ferroptosis hypersensitivity in prostate cancer. Cancer Metab. 8, 11 (2020).
Blomme, A. et al. 2,4-dienoyl-CoA reductase regulates lipid homeostasis in treatment-resistant prostate cancer. Nat. Commun. 11, 2508 (2020).
Nelson, E. R. et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 342, 1094–1098 (2013).
Perone, Y. & Magnani, L. Going off the grid: ERα breast cancer beyond estradiol. J. Mol. Endocrinol. 57, F1–F5 (2016).
Hopkins, B. D., Goncalves, M. D. & Cantley, L. C. Obesity and cancer mechanisms: cancer metabolism. J. Clin. Oncol. 34, 4277–4283 (2016).
Labbe, D. P. et al. High-fat diet fuels prostate cancer progression by rewiring the metabolome and amplifying the MYC program. Nat. Commun. 10, 4358 (2019).
Chang, H. H. et al. Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. PLoS ONE 12, e0184455 (2017).
Ringel, A. E. et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 183, 1848–1866.e26 (2020). This study reported that tumours and local immune cells compete for fatty acids in high-fat diet-fed animal models and that blocking tumour fatty acid oxidation increased fatty acid uptake by T cells, which led to their activation.
Broadfield, L. A. et al. Fat induces glucose metabolism in non-transformed liver cells and promotes liver tumorigenesis. Cancer Res. 81, 1988–2001 (2021).
Charles, M. A. et al. High plasma nonesterified fatty acids are predictive of cancer mortality but not of coronary heart disease mortality: results from the Paris Prospective Study. Am. J. Epidemiol. 153, 292–298 (2001).
Brennan, S. F., Woodside, J. V., Lunny, P. M., Cardwell, C. R. & Cantwell, M. M. Dietary fat and breast cancer mortality: a systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 57, 1999–2008 (2017).
Van Blarigan, E. L. et al. Dietary fat intake after colon cancer diagnosis in relation to cancer recurrence and survival: CALGB 89803 (Alliance). Cancer Epidemiol. Biomarkers Prev. 27, 1227–1230 (2018).
Yammine, S. et al. Dietary and circulating fatty acids and ovarian cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol. Biomarkers Prev. 29, 1739–1749 (2020).
McQuaid, S. E. et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes 60, 47–55 (2011).
Jocken, J. W. et al. Effect of beta-adrenergic stimulation on whole-body and abdominal subcutaneous adipose tissue lipolysis in lean and obese men. Diabetologia 51, 320–327 (2008).
Horowitz, J. F. & Klein, S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am. J. Physiol. Endocrinol. Metab. 278, E1144–E1152 (2000).
Ahima, R. S. & Lazar, M. A. Physiology. The health risk of obesity–better metrics imperative. Science 341, 856–858 (2013).
Stefan, N., Schick, F. & Haring, H. U. Causes, characteristics, and consequences of metabolically unhealthy normal weight in humans. Cell Metab. 26, 292–300 (2017).
Iacobini, C., Pugliese, G., Blasetti Fantauzzi, C., Federici, M. & Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 92, 51–60 (2019).
Moore, L. L., Chadid, S., Singer, M. R., Kreger, B. E. & Denis, G. V. Metabolic health reduces risk of obesity-related cancer in framingham study adults. Cancer Epidemiol. Biomarkers Prev. 23, 2057–2065 (2014).
Hoy, A. J., Balaban, S. & Saunders, D. N. Adipocyte-tumor cell metabolic crosstalk in breast cancer. Trends Mol. Med. 23, 381–392 (2017).
Kapoor, J. et al. Extraprostatic extension into periprostatic fat is a more important determinant of prostate cancer recurrence than an invasive phenotype. J. Urol. 190, 2061–2066 (2013).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011). This study demonstrated metabolic symbiosis between a range of cancer cells and adipocytes.
Cai, Z. et al. Cancer-associated adipocytes exhibit distinct phenotypes and facilitate tumor progression in pancreatic cancer. Oncol. Rep. 42, 2537–2549 (2019).
Cha, Y. J. & Koo, J. S. Roles of omental and bone marrow adipocytes in tumor biology. Adipocyte 8, 304–317 (2019).
Xiang, F. et al. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int. J. Biochem. Cell Biol. 84, 14–21 (2017).
Laurent, V. et al. Periprostatic adipose tissue favors prostate cancer cell invasion in an obesity-dependent manner: role of oxidative stress. Mol. Cancer Res. 17, 821–835 (2019). This study reported that adipocytes from obese donors further promotes cancer cell progression above that of adipocytes from lean donors.
Tan, Y. et al. Adipocytes fuel gastric cancer omental metastasis via PITPNC1-mediated fatty acid metabolic reprogramming. Theranostics 8, 5452–5468 (2018).
Yang, D. et al. Utilization of adipocyte-derived lipids and enhanced intracellular trafficking of fatty acids contribute to breast cancer progression. Cell Commun. Signal. 16, 32 (2018).
Zhang, M. et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 8, 1006–1025 (2018).
Dirat, B. et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 71, 2455–2465 (2011).
Miladinovic, D. et al. Assessment of periprostatic and subcutaneous adipose tissue lipolysis and adipocyte size from men with localized prostate cancer. Cancers 12, 1385 (2020).
Wang, Y. Y. et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2, e87489 (2017).
Laurent, V. et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 7, 10230 (2016).
Lazar, I. et al. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. 76, 4051–4057 (2016).
Attane, C. & Muller, C. Drilling for oil: tumor-surrounding adipocytes fueling cancer. Trends Cancer 6, 593–604 (2020).
Nassar, Z. D. et al. Peri-prostatic adipose tissue: the metabolic microenvironment of prostate cancer. BJU Int. 121(Suppl. 3), 9–21 (2018).
Nieman, K. M., Romero, I. L., Van Houten, B. & Lengyel, E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta 1831, 1533–1541 (2013).
Quail, D. F. & Dannenberg, A. J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15, 139–154 (2019).
Clement, E. et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 39, e102525 (2020).
Nimri, L., Peri, I., Yehuda-Shnaidman, E. & Schwartz, B. Adipocytes isolated from visceral and subcutaneous depots of donors differing in BMI crosstalk with colon cancer cells and modulate their invasive phenotype. Transl. Oncol. 12, 1404–1415 (2019).
Theriau, C. F., Sauve, O. S., Beaudoin, M. S., Wright, D. C. & Connor, M. K. Proliferative endocrine effects of adipose tissue from obese animals on MCF7 cells are ameliorated by resveratrol supplementation. PLoS ONE 12, e0183897 (2017).
Incio, J. et al. Obesity-induced inflammation and desmoplasia promote pancreatic cancer progression and resistance to chemotherapy. Cancer Discov. 6, 852–869 (2016). This study implicated aggravation of desmoplasia as a key mechanism of obesity-promoted pancreatic cancer progression, indicating that clinically available antifibrotic/inflammatory agents could improve the treatment response of pancreatic cancer in obese hosts.
Iwase, T. et al. Quality and quantity of visceral fat tissue are associated with insulin resistance and survival outcomes after chemotherapy in patients with breast cancer. Breast Cancer Res. Treat. 179,435–443 (2020).
Guiu, B. et al. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut 59, 341–347 (2010).
Osman, M. A. & Hennessy, B. T. Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clin. Med. Insights Oncol. 9, 105–112 (2015).
Horowitz, N. S. & Wright, A. A. Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol. Oncol. 138, 201–206 (2015).
Sheng, X. et al. Adipocytes sequester and metabolize the chemotherapeutic daunorubicin. Mol. Cancer Res. 15, 1704–1713 (2017). This study reported a mechanism of chemoresistance in which adipocytes metabolize and inactivate daunorubicin and thereby clear it from the tumour microenvironment.
Behan, J. W. et al. Adipocytes impair leukemia treatment in mice. Cancer Res. 69, 7867–7874 (2009).
Engin, A. Adipose tissue hypoxia in obesity and its impact on preadipocytes and macrophages: hypoxia hypothesis. Adv. Exp. Med. Biol. 960, 305–326 (2017).
Su, F., Ahn, S., Saha, A., DiGiovanni, J. & Kolonin, M. G. Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance. Oncogene 38, 1979–1988 (2019).
Herroon, M. K. et al. Prostate tumor cell-derived IL1beta induces an inflammatory phenotype in bone marrow adipocytes and reduces sensitivity to docetaxel via lipolysis-dependent mechanisms. Mol. Cancer Res. 17, 2508–2521 (2019).
Liu, Z. et al. Mature adipocytes in bone marrow protect myeloma cells against chemotherapy through autophagy activation. Oncotarget 6, 34329–34341 (2015).
Lehuede, C. et al. Adipocytes promote breast cancer resistance to chemotherapy, a process amplified by obesity: role of the major vault protein (MVP). Breast Cancer Res. 21, 7 (2019).
Mentoor, I., Engelbrecht, A. M., van Jaarsveld, P. J. & Nell, T. Chemoresistance: intricate interplay between breast tumor cells and adipocytes in the tumor microenvironment. Front. Endocrinol. 9, 758 (2018).
Cao, Y. Adipocyte and lipid metabolism in cancer drug resistance. J. Clin. Invest. 129, 3006–3017 (2019).
Bougaret, L. et al. Adipocyte/breast cancer cell crosstalk in obesity interferes with the anti-proliferative efficacy of tamoxifen. PLoS ONE 13, e0191571 (2018).
Mentoor, I. et al. Decreased efficacy of doxorubicin corresponds with modifications in lipid metabolism markers and fatty acid profiles in breast tumors from obese vs. Lean mice. Front. Oncol. 10, 306 (2020).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Zou, Y. et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat. Commun. 10, 1617 (2019).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
Bersuker, K. et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature 575, 688–692 (2019).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA 113, E4966–E4975 (2016).
Magtanong, L. et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem. Biol. 26, 420–432.e9 (2019). This study demonstrated the influence of MUFA abundance on the sensitivity of cancer cells to ferroptotic cell death.
Lee, J. Y. et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl Acad. Sci. USA 117, 32433–32442 (2020).
Dixon, S. J. et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015).
Nassar, Z. D. et al. Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis. eLife 9, e54166 (2020).
Luiken, J. J. et al. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am. J. Physiol. Endocrinol. Metab. 282, E491–E495 (2002).
Coleman, R. A. & Mashek, D. G. Mammalian triacylglycerol metabolism: synthesis, lipolysis, and signaling. Chem. Rev. 111, 6359–6386 (2011).
Zhang, J. et al. EGFR modulates monounsaturated fatty acid synthesis through phosphorylation of SCD1 in lung cancer. Mol. Cancer 16, 127 (2017).
Gallagher, E. J. & LeRoith, D. Hyperinsulinaemia in cancer. Nat. Rev. Cancer 20, 629–644 (2020).
Acknowledgements
The authors apologise to colleagues whose work they were unable to discuss due to space constraints. Research in the Hoy lab related to the subject of this review is supported by a Robinson Fellowship and funding from the University of Sydney and Sydney Catalyst. L.M.B. is supported by a Principal Cancer Research Fellowship produced with the financial and other support of Cancer Council SA’s Beat Cancer Project on behalf of its donors and the State Government of South Australia through the Department of Health, and the US Department of Defense. The Hoy and Butler labs are supported by a Movember Revolutionary Team Award from the Movember Foundation and the Prostate Cancer Foundation of Australia (MRTA3).
Author information
Authors and Affiliations
Contributions
A.J.H., S.R.N. and L.M.B. researched and discussed the relevant research literature, wrote the manuscript, and drafted the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Peer review information
Nature Reviews Cancer thanks R. Perry, A. Schulze and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Data by the World Health Organization: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
Glossary
- Tumour lipidome
-
The full lipid complement of the tumour that captures the levels of lipid classes and species.
- Macropinocytosis
-
An endocytic process that involves the engulfment of extracellular content, including soluble molecules, nutrients and antigens, in vesicles known as macropinosomes.
- Type 2 diabetes
-
A progressive metabolic condition in which the body becomes resistant to the normal effects of insulin and/or gradually loses the capacity to produce enough insulin in the pancreas.
- Metabolic syndrome
-
A cluster of conditions, including abdominal obesity, high blood pressure, high blood glucose, high serum triglycerides and low serum high-density lipoprotein, that occur together and increase the risk of heart disease, stroke and type 2 diabetes.
- Lipophagy
-
The autophagic degradation of intracellular lipid droplets.
- Resveratrol
-
A phenolic compound of the stilbene family present in wines and various parts of the grape that exhibits antioxidant and antiproliferative activities.
Rights and permissions
About this article
Cite this article
Hoy, A.J., Nagarajan, S.R. & Butler, L.M. Tumour fatty acid metabolism in the context of therapy resistance and obesity. Nat Rev Cancer 21, 753–766 (2021). https://doi.org/10.1038/s41568-021-00388-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-021-00388-4
This article is cited by
-
Identification of fatty acids synthesis and metabolism-related gene signature and prediction of prognostic model in hepatocellular carcinoma
Cancer Cell International (2024)
-
Reprogramming of lipid metabolism in the tumor microenvironment: a strategy for tumor immunotherapy
Lipids in Health and Disease (2024)
-
RBM45 reprograms lipid metabolism promoting hepatocellular carcinoma via Rictor and ACSL1/ACSL4
Oncogene (2024)
-
Hsa_circ_0021205 enhances lipolysis via regulating miR-195-5p/HSL axis and drives malignant progression of glioblastoma
Cell Death Discovery (2024)
-
Leukemia inhibitory factor suppresses hepatic de novo lipogenesis and induces cachexia in mice
Nature Communications (2024)