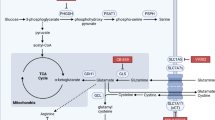
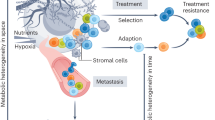
Abstract
Metastasis formation is the major cause of death in most patients with cancer. Despite extensive research, targeting metastatic seeding and colonization is still an unresolved challenge. Only recently, attention has been drawn to the fact that metastasizing cancer cells selectively and dynamically adapt their metabolism at every step during the metastatic cascade. Moreover, many metastases display different metabolic traits compared with the tumours from which they originate, enabling survival and growth in the new environment. Consequently, the stage-dependent metabolic traits may provide therapeutic windows for preventing or reducing metastasis, and targeting the new metabolic traits arising in established metastases may allow their eradication.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fares, J., Fares, M. Y., Khachfe, H. H., Salhab, H. A. & Fares, Y. Molecular principles of metastasis: a hallmark of cancer revisited. Sig. Transduct Target Ther. 5, 28 (2020).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Vanharanta, S. & Massagué, J. Origins of metastatic traits. Cancer Cell 24, 410–421 (2013).
Steeg, P. S. Targeting metastasis. Nat. Rev. Cancer 16, 201–218 (2016).
Turajlic, S. & Swanton, C. Metastasis as an evolutionary process. Science 352, 169–175 (2016).
Plaks, V., Kong, N. & Werb, Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16, 225–238 (2015).
Gonzalez, H., Hagerling, C. & Werb, Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 32, 1267–1284 (2018).
Fidler, I. J. & Kripke, M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science 197, 893 (1977).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Ewing, J. Neoplastic Diseases: A Treatise on Tumors (W. B. Saunders, 1928).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 133, 571–573 (1889).
Nguyen, D. X., Bos, P. D. & Massagué, J. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 (2009).
Pavlovic, M. et al. Enhanced MAF oncogene expression and breast cancer bone metastasis. J. Natl Cancer Inst. 107, djv256 (2015).
Ganesh, K. et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat. Cancer 1, 28–45 (2020).
Gao, Y. et al. Metastasis organotropism: redefining the congenial soil. Dev. Cell 49, 375–391 (2019).
Tian, X. et al. Organ-specific metastases obtained by culturing colorectal cancer cells on tissue-specific decellularized scaffolds. Nat. Biomed. Eng. 2, 443–452 (2018).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Doglioni, G., Parik, S. & Fendt, S.-M. Interactions in the (pre)metastatic niche support metastasis formation. Front. Oncol. 9, 219–219 (2019).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Olmeda, D. et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 546, 676–680 (2017).
Teoh Shao, T. & Lunt Sophia, Y. Metabolism in cancer metastasis: bioenergetics, biosynthesis, and beyond. Wiley Interdiscip. Rev. Syst. Biol. Med. 10, e1406 (2018).
Elia, I., Doglioni, G. & Fendt, S.-M. Metabolic hallmarks of metastasis formation. Trends Cell Biol. 28, 673–687 (2018).
Faubert, B., Solmonson, A. & DeBerardinis, R. J. Metabolic reprogramming and cancer progression. Science 368, eaaw5473 (2020).
Lorendeau, D., Christen, S., Rinaldi, G. & Fendt, S.-M. Metabolic control of signaling pathways and metabolic auto-regulation. Biol. Cell 107, 251–272 (2015).
Kim, J. & DeBerardinis, R. J. Mechanisms and implications of metabolic heterogeneity in cancer. Cell Metab. 30, 434–446 (2019).
Kelley, D. E. & Mandarino, L. J. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49, 677–683 (2000).
Randle, P. J., Garland, P. B., Hales, C. N. & Newsholme, E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789 (1963).
Phannasil, P. et al. Mass spectrometry analysis shows the biosynthetic pathways supported by pyruvate carboxylase in highly invasive breast cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 537–551 (2017).
Phannasil, P. et al. Pyruvate carboxylase is up-regulated in breast cancer and essential to support growth and invasion of MDA-MB-231 cells. PLoS ONE 10, e0129848 (2015).
Rios Garcia, M. et al. Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab. 26, 842–855.e5 (2017).
Gaude, E. & Frezza, C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat. Commun. 7, 13041 (2016).
Chuang, C-H. et al. Altered mitochondria functionality defines a metastatic cell state in lung cancer and creates an exploitable vulnerability. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-20-1865 (2020).
Xian, Z.-Y. et al. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumor Biol. 36, 8093–8100 (2015).
He, T.-L. et al. The c-Myc–LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer. Med. Oncol. 32, 187 (2015).
Zhao, J. et al. LDHA promotes tumor metastasis by facilitating epithelial–mesenchymal transition in renal cell carcinoma. Mol. Med. Rep. 16, 8335–8344 (2017).
Li, L. et al. miR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effect. Cancer Lett. 400, 89–98 (2017).
He, Y. et al. LDHA is a direct target of miR-30d-5p and contributes to aggressive progression of gallbladder carcinoma. Mol. Carcinog. 57, 772–783 (2018).
Jin, L. et al. Phosphorylation-mediated activation of LDHA promotes cancer cell invasion and tumour metastasis. Oncogene 36, 3797 (2017).
Pérez-Escuredo, J. et al. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta 1863, 2481–2497 (2016).
Cao, Y.-W. et al. Monocarboxylate transporters MCT1 and MCT4 are independent prognostic biomarkers for the survival of patients with clear cell renal cell carcinoma and those receiving therapy targeting angiogenesis. Urol. Oncol. 36, 311.e315–311.e25 (2018).
Zhang, G. et al. MCT1 regulates aggressive and metabolic phenotypes in bladder cancer. J. Cancer 9, 2492–2501 (2018).
De Saedeleer, C. J. et al. Glucose deprivation increases monocarboxylate transporter 1 (MCT1) expression and MCT1-dependent tumor cell migration. Oncogene 33, 4060 (2013).
Fan, Q. et al. Autophagy promotes metastasis and glycolysis by upregulating MCT1 expression and Wnt/β-catenin signaling pathway activation in hepatocellular carcinoma cells. J. Exp. Clin. Cancer Res. 37, 9 (2018).
Xiong, L., Edwards, C. K. 3rd & Zhou, L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int. J. Mol. Sci. 15, 17411–17441 (2014).
Payen, V. L. et al. Monocarboxylate transporter MCT1 promotes tumor metastasis independently of its activity as a lactate transporter. Cancer Res. 77, 5591 (2017).
Zhao, Z. et al. Downregulation of MCT1 inhibits tumor growth, metastasis and enhances chemotherapeutic efficacy in osteosarcoma through regulation of the NF-κB pathway. Cancer Lett. 342, 150–158 (2014).
Gupta, S. C., Singh, R., Pochampally, R., Watabe, K. & Mo, Y.-Y. Acidosis promotes invasiveness of breast cancer cells through ROS–AKT–NF-κB pathway. Oncotarget 5, 12070–12082 (2014).
Bonuccelli, G. et al. Ketones and lactate “fuel” tumor growth and metastasis. Cell Cycle 9, 3506–3514 (2010).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Piskounova, E. et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 12, 186–191 (2015). This article provides evidence that antioxidants increase the survival of melanoma cells in the circulation.
Le Gal, K. et al. Antioxidants can increase melanoma metastasis in mice. Sci. Transl Med. 7, 308re308 (2015). This article provides evidence that antioxidants and the glutathione synthesis can increase lymph node metastasis formation of melanoma.
Wiel, C. et al. BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell 178, 330–345.e22 (2019).
Guadamillas, M. C., Cerezo, A. & del Pozo, M. A. Overcoming anoikis — pathways to anchorage-independent growth in cancer. J. Cell Sci. 124, 3189 (2011).
Grossmann, J. Molecular mechanisms of “detachment-induced apoptosis — anoikis”. Apoptosis 7, 247–260 (2002).
Labuschagne, C. F., Cheung, E. C., Blagih, J., Domart, M.-C. & Vousden, K. H. Cell clustering promotes a metabolic switch that supports metastatic colonization. Cell Metab. 30, 720–734.e725 (2019). This work reveals hypoxia-induced mitophagy of clustering tumour cells in the circulation that limits mitochondrial ROS production, and promotes survival and metastasis formation.
Caneba, C. A., Bellance, N., Yang, L., Pabst, L. & Nagrath, D. Pyruvate uptake is increased in highly invasive ovarian cancer cells under anoikis conditions for anaplerosis, mitochondrial function, and migration. Am. J. Physiol. Endocrinol. Metab. 303, E1036–E1052 (2012).
Maneche, H. C. Blood pyruvate in malignant neoplastic disorders. Clin. Chem. 12, 158–164 (1966).
Jobard, E. et al. A serum nuclear magnetic resonance-based metabolomic signature of advanced metastatic human breast cancer. Cancer Lett. 343, 33–41 (2014).
Donnell-Tormey, J., Nathan, C. F., Lanks, K., DeBoer, C. J. & de la Harpe, J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J. Exp. Med. 165, 500 (1987).
Wei, Y. et al. Prognostic significance of serum lactic acid, lactate dehydrogenase, and albumin levels in patients with metastatic colorectal cancer. BioMed. Res. Int. 2018, 1804086 (2018).
Wilmanski, T. et al. Inhibition of pyruvate carboxylase by 1α,25-dihydroxyvitamin D promotes oxidative stress in early breast cancer progression. Cancer Lett. 411, 171–181 (2017).
Tasdogan, A. et al. Metabolic heterogeneity confers differences in melanoma metastatic potential. Nature 577, 115–120 (2020). This study shows that MCT1-dependent lactate uptake increases melanoma metastasis by elevating the survival of cancer cells in the circulation.
Vande Voorde, J. et al. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 5, eaau7314 (2019).
Zera, K. & Zastre, J. Stabilization of the hypoxia-inducible transcription factor-1α (HIF-1α) in thiamine deficiency is mediated by pyruvate accumulation. Toxicol. Appl. Pharmacol. 355, 180–188 (2018).
Saedeleer, C. J. et al. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PloS ONE 7, e46571 (2012).
Lee, D. C. et al. A lactate-induced response to hypoxia. Cell 161, 595–609 (2015).
Christen, S. et al. Breast cancer-derived lung metastasis show increased pyruvate carboxylase-dependent anaplerosis. Cell Rep. 17, 837–848 (2016).
Elia, I. et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 568, 117–121 (2019). This article demonstrates that breast cancer cells are dependent on pyruvate uptake to initiate collagen-based extracellular matrix remodelling for the establishment of a metastatic niche and subsequent metastatic growth in the lung.
Gilkes, D. M. et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 73, 3285–3296 (2013).
Shinde, A., Wilmanski, T., Chen, H., Teegarden, D. & Wendt, M. K. Pyruvate carboxylase supports the pulmonary tropism of metastatic breast cancer. Breast Cancer Res. 20, 76–76 (2018).
Rinaldi, G. et al. In vivo evidence for serine biosynthesis-defined sensitivity of lung metastasis, but not of primary breast tumors, to mTORC1 inhibition. Mol. Cell https://doi.org/10.1016/j.molcel.2020.11.027 (2020).
Elia, I. et al. Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nat. Commun. 8, 15267 (2017).
Diers, A. R., Broniowska, K. A., Chang, C. F. & Hogg, N. Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition. Biochem. J. 444, 561–571 (2012).
Corbet, C. et al. Interruption of lactate uptake by inhibiting mitochondrial pyruvate transport unravels direct antitumor and radiosensitizing effects. Nat. Commun. 9, 1208 (2018).
Faubert, B. et al. Lactate metabolism in human lung tumors. Cell 171, 358–371.e9 (2017).
Elia, I., Schmieder, R., Christen, S. & Fendt, S.-M. Organ-specific cancer metabolism and its potential for therapy. Handb. Exp. Pharmacol. 233, 321–353 (2016).
Yang, L. et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol. Syst. Biol. 10, 728 (2014).
Rodrigues, M. F. et al. Enhanced OXPHOS, glutaminolysis and β-oxidation constitute the metastatic phenotype of melanoma cells. Biochem. J. 473, 703 (2016).
Xiang, L. et al. Glutaminase 1 expression in colorectal cancer cells is induced by hypoxia and required for tumor growth, invasion, and metastatic colonization. Cell Death Dis. 10, 40 (2019).
Du, F. et al. SOX12 promotes colorectal cancer cell proliferation and metastasis by regulating asparagine synthesis. Cell Death Dis. 10, 239 (2019).
Zhang, C. et al. Glutaminase 2 is a novel negative regulator of small GTPase Rac1 and mediates p53 function in suppressing metastasis. eLife 5, e10727–e10727 (2016).
Kuo, T.-C. et al. Glutaminase 2 stabilizes Dicer to repress Snail and metastasis in hepatocellular carcinoma cells. Cancer Lett. 383, 282–294 (2016).
Sugano, K. M. K., Ohtani, H., Nagahara, H., Shibutani, M. & Hirakawa, K. Expression of xCT as a predictor of disease recurrence in patients with colorectal cancer. Anticancer Res. 35, 677–682 (2015).
Dornier, E. et al. Glutaminolysis drives membrane trafficking to promote invasiveness of breast cancer cells. Nat. Commun. 8, 2255 (2017).
Yae, T. et al. Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat. Commun. 3, 883 (2012).
Chen, R. S. et al. Disruption of xCT inhibits cancer cell metastasis via the caveolin-1/β-catenin pathway. Oncogene 28, 599 (2008).
Jin, H. et al. Serum metabolomic signatures of lymph node metastasis of esophageal squamous cell carcinoma. J. Proteome Res. 13, 4091–4103 (2014).
Toyoshima, K. et al. Analysis of circulating tumor cells derived from advanced gastric cancer. Int. J. Cancer 137, 991–998 (2015).
Liu, G. et al. Glutamate dehydrogenase is a novel prognostic marker and predicts metastases in colorectal cancer patients. J. Transl Med. 13, 144 (2015).
Jin, L. et al. The PLAG1–GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2–AMPK signaling in LKB1-deficient lung cancer. Mol. Cell 69, 87–99.e7 (2018).
Wang, Q. et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 236, 278–289 (2015).
Shelton, L. M., Huysentruyt, L. C. & Seyfried, T. N. Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model. Int. J. Cancer 127, 2478–2485 (2010).
Lanzardo, S. et al. Immunotargeting of antigen xCT attenuates stem-like cell behavior and metastatic progression in breast cancer. Cancer Res. 76, 62 (2016).
Gaschler, M. M. & Stockwell, B. R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 482, 419–425 (2017).
Peck, B. & Schulze, A. Lipid desaturation — the next step in targeting lipogenesis in cancer? FEBS J. 283, 2767–2778 (2016).
Rohrig, F. & Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 16, 732–749 (2016).
Chen, M. et al. An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nat. Genet. 50, 206–218 (2018).
Pandey, V., Vijayakumar, M. V., Ajay, A. K., Malvi, P. & Bhat, M. K. Diet-induced obesity increases melanoma progression: involvement of Cav-1 and FASN. Int. J. Cancer 130, 497–508 (2012).
Jiralerspong, S. & Goodwin, P. J. Obesity and breast cancer prognosis: evidence, challenges, and opportunities. J. Clin. Oncol. 34, 4203–4216 (2016).
O’Flanagan, C. H. et al. Metabolic reprogramming underlies metastatic potential in an obesity-responsive murine model of metastatic triple negative breast cancer. NPJ Breast Cancer 3, 26 (2017).
Sant’Anna-Silva, A. C. B. et al. Metabolic profile of oral squamous carcinoma cell lines relies on a higher demand of lipid metabolism in metastatic cells. Front. Oncol. 8, 13 (2018).
Pascual, G. et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45 (2017). This article identifies CD36+ metastasis-initiating cells in human oral carcinoma and other human cancer types and shows that blocking CD36 inhibits metastasis formation.
Antalis, C. J., Uchida, A., Buhman, K. K. & Siddiqui, R. A. Migration of MDA-MB-231 breast cancer cells depends on the availability of exogenous lipids and cholesterol esterification. Clin. Exp. Metastasis 28, 733–741 (2011).
Nath, A. & Chan, C. Genetic alterations in fatty acid transport and metabolism genes are associated with metastatic progression and poor prognosis of human cancers. Sci. Rep. 6, 18669 (2016).
Nath, A., Li, I., Roberts, L. R. & Chan, C. Elevated free fatty acid uptake via CD36 promotes epithelial–mesenchymal transition in hepatocellular carcinoma. Sci. Rep. 5, 14752 (2015).
Ladanyi, A. et al. Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 37, 2285–2301 (2018).
Yang, P. et al. Dietary oleic acid-induced CD36 promotes cervical cancer cell growth and metastasis via up-regulation Src/ERK pathway. Cancer Lett. 438, 76–85 (2018).
Zaoui, M. et al. Breast-associated adipocytes secretome induce fatty acid uptake and invasiveness in breast cancer cells via CD36 independently of body mass index, menopausal status and mammary density. Cancers 11, 2012 (2019).
Xu, W. H. et al. Elevated CD36 expression correlates with increased visceral adipose tissue and predicts poor prognosis in ccRCC patients. J. Cancer 10, 4522–4531 (2019).
Hale, J. S. et al. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cell 32, 1746–1758 (2014).
Wang, R. et al. Fatty-acid receptor CD36 functions as a hydrogen sulfide-targeted receptor with its Cys333–Cys272 disulfide bond serving as a specific molecular switch to accelerate gastric cancer metastasis. EBioMedicine 45, 108–123 (2019).
Pan, J. et al. CD36 mediates palmitate acid-induced metastasis of gastric cancer via AKT/GSK-3β/β-catenin pathway. J. Exp. Clin. Cancer Res. 38, 52–52 (2019).
Sp, N. et al. Nobiletin inhibits CD36-dependent tumor angiogenesis, migration, invasion, and sphere formation through the CD36/STAT3/NF-κB signaling axis. Nutrients 10, 772 (2018).
Casciano, J. C. et al. MYC regulates fatty acid metabolism through a multigenic program in claudin-low triple negative breast cancer. Br. J. Cancer 122, 868–884 (2020).
Watt, M. J. et al. Suppressing fatty acid uptake has therapeutic effects in preclinical models of prostate cancer. Sci. Transl Med. 11, eaau5758 (2019).
Deng, M. et al. CD36 promotes the epithelial–mesenchymal transition and metastasis in cervical cancer by interacting with TGF-β. J. Transl Med. 17, 352 (2019).
Gharpure, K. M. et al. FABP4 as a key determinant of metastatic potential of ovarian cancer. Nat. Commun. 9, 2923 (2018).
Kawaguchi, K. et al. High expression of fatty acid-binding protein 5 promotes cell growth and metastatic potential of colorectal cancer cells. FEBS Open Bio 6, 190–199 (2016).
Wu, G. et al. FABP5 is correlated with poor prognosis and promotes tumour cell growth and metastasis in clear cell renal cell carcinoma. Eur. J. Pharmacol. 862, 172637 (2019).
Ohata, T. et al. Fatty acid-binding protein 5 function in hepatocellular carcinoma through induction of epithelial–mesenchymal transition. Cancer Med. 6, 1049–1061 (2017).
Wang, W. et al. FABP5 correlates with poor prognosis and promotes tumor cell growth and metastasis in cervical cancer. Tumor Biol. 37, 14873–14883 (2016).
Pan, J., Dai, Q., Zhang, T. & Li, C. Palmitate acid promotes gastric cancer metastasis via FABP5/SP1/UCA1 pathway. Cancer Cell Int. 19, 69 (2019).
Carbonetti, G. et al. FABP5 coordinates lipid signaling that promotes prostate cancer metastasis. Sci. Rep. 9, 18944 (2019).
Ku, C.-Y., Liu, Y.-H., Lin, H.-Y., Lu, S.-C. & Lin, J.-Y. Liver fatty acid-binding protein (L-FABP) promotes cellular angiogenesis and migration in hepatocellular carcinoma. Oncotarget 7, 18229–18246 (2016).
Zhang, M. et al. Adipocyte-derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 8, 1006–1025 (2018).
Seguin, F. et al. The fatty acid synthase inhibitor orlistat reduces experimental metastases and angiogenesis in B16-F10 melanomas. Br. J. Cancer 107, 977 (2012).
Zaytseva, Y. Y. et al. Inhibition of fatty acid synthase attenuates CD44-associated signaling and reduces metastasis in colorectal cancer. Cancer Res. 72, 1504 (2012).
Jafari, N. et al. De novo fatty acid synthesis-driven sphingolipid metabolism promotes metastatic potential of colorectal cancer. Mol. Cancer Res. 17, 140–152 (2019).
Vriens, K. et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 566, 403–406 (2019).
Triki, M. et al. mTOR signaling and SREBP activity increase FADS2 expression and can activate sapienate biosynthesis. Cell Rep. 31, 107806 (2020).
Ran, H. et al. Stearoyl-CoA desaturase-1 promotes colorectal cancer metastasis in response to glucose by suppressing PTEN. J. Exp. Clin. Cancer Res. 37, 54 (2018).
Zhao, J. et al. Exogenous lipids promote the growth of breast cancer cells via CD36. Oncol. Rep. 38, 2105–2115 (2017).
Li, J. et al. Lipid desaturation is a metabolic marker and therapeutic target of ovarian cancer stem cells. Cell Stem Cell 20, 303–314.e5 (2017).
Vivas-García, Y. et al. Lineage-restricted regulation of SCD and fatty acid saturation by MITF controls melanoma phenotypic plasticity. Mol. Cell 77, 120–137.e9 (2020). This work reveals that SCD1 is regulated by MITF being implicated in melanoma proliferation. SCD1 inhibition leads to endoplasmic reticulum stress response and enhanced invasion and metastasis formation.
Bellenghi, M. et al. SCD5-induced oleic acid production reduces melanoma malignancy by intracellular retention of SPARC and cathepsin B. J. Pathol. 236, 315–325 (2015).
Lee, H. J. et al. Cholesterol esterification inhibition suppresses prostate cancer metastasis by impairing the Wnt/β-catenin pathway. Mol. Cancer Res. 16, 974 (2018).
Li, J. et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene 35, 6378–6388 (2016).
Bi, M. et al. Effect of inhibiting ACAT-1 expression on the growth and metastasis of Lewis lung carcinoma. Oncol. Lett. 18, 1548–1556 (2019).
Wang, Y. Y. et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2, e87489 (2017).
Miranda, F. et al. Salt-inducible kinase 2 couples ovarian cancer cell metabolism with survival at the adipocyte-rich metastatic niche. Cancer Cell 30, 273–289 (2016).
Xiong, Y. et al. CPT1A regulates breast cancer-associated lymphangiogenesis via VEGF signaling. Biomed. Pharmacother. 106, 1–7 (2018).
Pucci, S. et al. Carnitine palmitoyl transferase-1A (CPT1A): a new tumor specific target in human breast cancer. Oncotarget 7, 19982–19996 (2016).
Wang, C. et al. Elevated level of mitochondrial reactive oxygen species via fatty acid β-oxidation in cancer stem cells promotes cancer metastasis by inducing epithelial–mesenchymal transition. Stem Cell Res. Ther. 10, 175 (2019).
Gomes, A. P. et al. Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature 585, 283–287 (2020). This study shows that age-dependent metabolic change, that is, methylmalonic acid accumulation in the blood, promotes cancer progression by inducing Sox4.
Notarnicola, M. et al. Serum lipid profile in colorectal cancer patients with and without synchronous distant metastases. Oncology 68, 371–374 (2005).
Liu, Y.-L. et al. Association of serum lipid profile with distant metastasis in breast cancer patients. Zhonghua Zhong Liu Za Zhi 34, 129–131 (2012).
Acharya, S., Rai, P., Hallikeri, K., Anehosur, V. & Kale, J. Serum lipid profile in oral squamous cell carcinoma: alterations and association with some clinicopathological parameters and tobacco use. Int. J. Oral. Maxillofac. Surg. 45, 713–720 (2016).
Wang, Y. N. et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene 37, 6025–6040 (2018).
Jiang, L. et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016).
Lignitto, L. et al. Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell 178, 316–329.e18 (2019).
Yu, G. et al. Organelle-derived acetyl-CoA promotes prostate cancer cell survival, migration, and metastasis via activation of calmodulin kinase II. Cancer Res. 78, 2490 (2018).
McCoy, F. et al. Metabolic activation of CaMKII by coenzyme A. Mol. Cell 52, 325–339 (2013).
Ubellacker, J. M. et al. Metastasis through lymph protects melanoma cells from ferroptosis. Nature 158, 113–118 (2020). This work reveals that metastasizing melanoma cells in lymphatic vessels experience less oxidative stress than cells in the blood because they are protected from ferroptosis, and thus are more efficient to form metastases.
Shang, C. et al. LNMICC promotes nodal metastasis of cervical cancer by reprogramming fatty acid metabolism. Cancer Res. 78, 877 (2018).
Mukherjee, A. et al. Adipocyte-induced FABP4 expression in ovarian cancer cells promotes metastasis and mediates carboplatin resistance. Cancer Res. 80, 1748–1761 (2020).
Jiang, L. et al. Up-regulated FASN expression promotes transcoelomic metastasis of ovarian cancer cell through epithelial–mesenchymal transition. Int. J. Mol. Sci. 15, 11539–11554 (2014).
Li, J. et al. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J. Hepatol. 63, 1378–1389 (2015).
Liu, G. et al. Lung fibroblasts promote metastatic colonization through upregulation of stearoyl-CoA desaturase 1 in tumor cells. Oncogene 37, 1519–1533 (2018).
Blomme, A. et al. Myoferlin regulates cellular lipid metabolism and promotes metastases in triple-negative breast cancer. Oncogene 36, 2116 (2016).
Lee, C.-K. et al. Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science 363, 644 (2019).
Lu, J. The Warburg metabolism fuels tumor metastasis. Cancer Metastasis Rev. 38, 157–164 (2019).
Lehuédé, C., Dupuy, F., Rabinovitch, R., Jones, R. G. & Siegel, P. M. Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Res. 76, 5201 (2016).
Lu, M. et al. ACOT12-dependent alteration of acetyl-CoA drives hepatocellular carcinoma metastasis by epigenetic induction of epithelial–mesenchymal transition. Cell Metab. 29, 886–900.e5 (2019).
Sun, L. et al. Decreased expression of acetyl-CoA synthase 2 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Cancer Sci. 108, 1338–1346 (2017).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Pollari, S. et al. Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 125, 421–430 (2011).
Zhu, J. et al. High expression of PHGDH predicts poor prognosis in non-small cell lung cancer. Transl Oncol. 9, 592–599 (2016).
Song, Z., Feng, C., Lu, Y., Lin, Y. & Dong, C. PHGDH is an independent prognosis marker and contributes cell proliferation, migration and invasion in human pancreatic cancer. Gene 642, 43–50 (2018).
Kim, H. M., Jung, W. H. & Koo, J. S. Site-specific metabolic phenotypes in metastatic breast cancer. J. Transl Med. 12, 354 (2014).
Samanta, D. et al. PHGDH expression is required for mitochondrial redox homeostasis, breast cancer stem cell maintenance, and lung metastasis. Cancer Res. 76, 4430 (2016).
Ngo, B. et al. Limited environmental serine and glycine confer brain metastasis sensitivity to PHGDH inhibition. Cancer Discov. 12, 1352–1373 (2020).
Cao, Y. et al. Glutamic pyruvate transaminase GPT2 promotes tumorigenesis of breast cancer cells by activating sonic hedgehog signaling. Theranostics 7, 3021–3033 (2017).
Pavlova, N. N. et al. As extracellular glutamine levels decline, asparagine becomes an essential amino acid. Cell Metab. 27, 428–438.e5 (2018).
Knott, S. R. V. et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378 (2018). This work finds limiting asparagine bioavailability by silencing ASNS or treatment with L-asparaginase, or dietary asparagine restriction, reduces metastasis formation without affecting primary tumour growth.
Tanner, J. J., Fendt, S.-M. & Becker, D. F. The proline cycle as a potential cancer therapy target. Biochemistry 57, 3433–3444 (2018).
Loayza-Puch, F. et al. Tumour-specific proline vulnerability uncovered by differential ribosome codon reading. Nature 530, 490 (2016).
Wang, D. et al. PYCR1 promotes the progression of non-small-cell lung cancer under the negative regulation of miR-488. Biomed. Pharmacother. 111, 588–595 (2019).
Fang, E. et al. Therapeutic targeting of MZF1-AS1/PARP1/E2F1 axis inhibits proline synthesis and neuroblastoma progression. Adv. Sci. 0, 1900581 (2019).
Sahu, N. et al. Proline starvation induces unresolved ER stress and hinders mTORC1-dependent tumorigenesis. Cell Metab. 24, 753–761 (2016).
Fendt, S.-M. Metabolic vulnerabilities of metastasizing cancer cells. BMC Biol. 17, 54 (2019).
Lunt, S. Y. & Fendt, S.-M. Metabolism — a cornerstone of cancer initiation, progression, immune evasion and treatment response. Curr. Opin. Syst. Biol. 8, 67–72 (2018).
Wang, Y.-n. et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene 37, 6025–6040 (2018).
Kamarajugadda, S. et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol. Cell. Biol. 32, 1893–1907 (2012).
Kruse, N. J. & Bornstein, P. The metabolic requirements for transcellular movement and secretion of collagen. J. Biol. Chem. 250, 4841–4847 (1975).
Park, J. H. et al. Fatty acid oxidation-driven Src links mitochondrial energy reprogramming and oncogenic properties in triple-negative breast cancer. Cell Rep. 14, 2154–2165 (2016).
Wright, H. J. et al. CDCP1 drives triple-negative breast cancer metastasis through reduction of lipid-droplet abundance and stimulation of fatty acid oxidation. Proc. Natl Acad. Sci. USA 114, E6556 (2017).
Andrzejewski, S. et al. PGC-1α promotes breast cancer metastasis and confers bioenergetic flexibility against metabolic drugs. Cell Metab.26, 778–787 (2017).
Dupuy, F. et al. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 22, 577–589 (2015).
Loo, J. M. et al. Extracellular metabolic energetics can promote cancer progression. Cell 160, 393–406 (2015).
Payne, C. E. et al. A novel selective and orally bioavailable Nav 1.8 channel blocker, PF-01247324, attenuates nociception and sensory neuron excitability. Br. J. Pharmacol. 172, 2654–2670 (2015).
Fernandes, M., Rosel, D. & Brábek, J. Solid cancer: the new tumour spread endpoint opens novel opportunities. Br. J. Cancer 121, 513–514 (2019).
Chaika, N. V. et al. Differential expression of metabolic genes in tumor and stromal components of primary and metastatic loci in pancreatic adenocarcinoma. PLoS ONE 7, e32996 (2012).
Davis, R. T. et al. Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat. Cell Biol. 22, 310–320 (2020).
Karaayvaz, M. et al. Unravelling subclonal heterogeneity and aggressive disease states in TNBC through single-cell RNA-seq. Nat. Commun. 9, 3588 (2018).
Basnet, H. et al. Flura-seq identifies organ-specific metabolic adaptations during early metastatic colonization. eLife 8, e43627 (2019).
Chen, E. I. et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 67, 1472 (2007).
Fischer, G. M. et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 9, 628–645 (2019).
Davidson, S. M. et al. Environment impacts the metabolic dependencies of Ras-driven non-small cell lung cancer. Cell Metab. 23, 517–528 (2016).
Hensley, C. T. et al. Metabolic heterogeneity in human lung tumors. Cell 164, 681–694 (2016).
Maher, E. A. et al. Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 25, 1234–1244 (2012).
Fernández-García, J., Altea-Manzano, P., Pranzini, E. & Fendt, S.-M. Stable isotopes for tracing mammalian-cell metabolism in vivo. Trends Biochem. Sci. 45, 185–201 (2020).
Buescher, J. M. et al. A roadmap for interpreting 13C metabolite labeling patterns from cells. Curr. Opin. Biotechnol. 34, 189–201 (2015).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015).
Hao, Y. et al. Investigation of lipid metabolism dysregulation and the effects on immune microenvironments in pan-cancer using multiple omics data. BMC Bioinf. 20, 195 (2019).
Berod, L. et al. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med. 20, 1327 (2014).
Wang, H. et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 21, 298–308 (2020).
Su, P. et al. Enhanced lipid accumulation and metabolism are required for the differentiation and activation of tumor-associated macrophages. Cancer Res. 80, 1438–1450 (2020).
Niavarani, S. R. et al. Lipid accumulation impairs natural killer cell cytotoxicity and tumor control in the postoperative period. BMC Cancer 19, 823 (2019).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018).
Pan, Y. et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543, 252 (2017).
García-Mulero, S. et al. Lung metastases share common immune features regardless of primary tumor origin. J. Immunother. Cancer 8, e000491 (2020).
Acknowledgements
The authors regret the inability to cite all studies that have shaped the understanding of cancer metastasis metabolism. S.-M.F. acknowledges funding from the European Research Council under the ERC Consolidator Grant Agreement n. 771486 — MetaRegulation, FWO — Odysseus II, FWO research projects (G098120N, G088318N), KU Leuven — Methusalem Co-Funding and Fonds Baillet Latour. G.B. acknowledges funding from the Flemish cancer society Stichting tegen Kanker (STK 1303), the Flemish government FWO (G0A0818N) and the National Institutes of Health (NIH)/National Cancer Institute (NCI) (R01CA201537).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
S.-M.F. has received funding from Bayer, Merck and BlackBelt Therapeutics and has consulted for Fund+. G.B. declares no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks S.A. Benitah, S.J. Morrison and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Metastatic virulence genes
-
Genes or factors that confer proliferation and/or survival advantages to metastasizing cancer cells at the secondary site without affecting primary tumours.
- Metabolic plasticity
-
A metabolite can be used for multiple purposes.
- Metabolic flexibility
-
Several metabolites can be used for the same purpose.
- Divergence in metabolism
-
Divergent properties appear in distinct molecular subsets of cancer and contribute to metabolic heterogeneity.
- Anaplerosis
-
Refilling of the tricarboxylic acid (TCA) cycle with carbon.
- Glutaminolysis
-
Full oxidation of glutamine.
- Peroxidation
-
A chemical reaction between mainly unsaturated fatty acids and the reactive forms of oxygen.
- Nutrient inflexibility
-
A dependence on one nutrient despite the fact that multiple nutrients can lead to the production of a certain metabolite.
- Reductive carboxylation
-
A metabolic pathway in which α-ketoglutarate is converted to citrate through a reaction with carbon dioxide.
- Glucose fermentation
-
A biological process in which glucose is converted to lactate.
Rights and permissions
About this article
Cite this article
Bergers, G., Fendt, SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer 21, 162–180 (2021). https://doi.org/10.1038/s41568-020-00320-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-020-00320-2
This article is cited by
-
Joint analysis of mutational and transcriptional landscapes in human cancer reveals key perturbations during cancer evolution
Genome Biology (2024)
-
C6orf15 promotes liver metastasis via WNT/β-catenin signalling in colorectal cancer
Cancer Cell International (2024)
-
Beyond the barrier: the immune-inspired pathways of tumor extravasation
Cell Communication and Signaling (2024)
-
Cancer metabolism and carcinogenesis
Experimental Hematology & Oncology (2024)
-
Hypoxia promotes metastasis by relieving miR-598-3p-restricted glycolysis in gastric cancer
Journal of Translational Medicine (2024)