Abstract
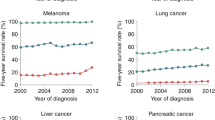
Metastatic dissemination occurs very early in the malignant progression of a cancer but the clinical manifestation of metastases often takes years. In recent decades, 5-year survival of patients with many solid cancers has increased due to earlier detection, local disease control and adjuvant therapies. As a consequence, we are confronted with an increase in late relapses as more antiproliferative cancer therapies prolong disease courses, raising questions about how cancer cells survive, evolve or stop growing and finally expand during periods of clinical latency. I argue here that the understanding of early metastasis formation, particularly of the currently invisible phase of metastatic colonization, will be essential for the next stage in adjuvant therapy development that reliably prevents metachronous metastasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Eyles, J. et al. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 120, 2030–2039 (2010).
McCreery, M. Q. et al. Evolution of metastasis revealed by mutational landscapes of chemically induced skin cancers. Nat. Med. 21, 1514–1520 (2015).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature https://doi.org/10.1038/nature20785 (2016).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Werner-Klein, M. et al. Genetic alterations driving metastatic colony formation are acquired outside of the primary tumour in melanoma. Nat. Commun. 9, 595 (2018).
Rhim, A. D. et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 146, 647–651 (2014).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 69, 363–385 (2019).
Manola, J., Atkins, M., Ibrahim, J. & Kirkwood, J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J. Clin. Oncol. 18, 3782–3793 (2000).
Balch, C. M. et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J. Clin. Oncol. 19, 3622–3634 (2001).
Miller, K. D. et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 66, 271–289 (2016).
Kolmel, K. F., Kulle, B., Lippold, A. & Seebacher, C. Survival probabilities and hazard functions of malignant melanoma in Germany 1972-1996, an analysis of 10433 patients. Evolution of gender differences and malignancy. Eur. J. Cancer 38, 1388–1394 (2002).
Crowley, N. J. & Seigler, H. F. Late recurrence of malignant melanoma. Analysis of 168 patients. Ann. Surg. 212, 173–177 (1990).
Faries, M. B., Steen, S., Ye, X., Sim, M. & Morton, D. L. Late recurrence in melanoma: clinical implications of lost dormancy. J. Am. Coll. Surg. 217, 27–34 (2013).
Spratt, J. S., Meyer, J. S. & Spratt, J. A. Rates of growth of human neoplasms: part II. J. Surg. Oncol. 61, 68–83 (1996).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
Kennecke, H. et al. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. 28, 3271–3277 (2010).
Jatoi, I., Anderson, W. F., Jeong, J. H. & Redmond, C. K. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J. Clin. Oncol. 29, 2301–2304 (2011).
Copson, E. et al. Prospective observational study of breast cancer treatment outcomes for UK women aged 18–40 years at diagnosis: the POSH study. J. Natl Cancer Inst. 105, 978–988 (2013).
Pan, H. et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N. Engl. J. Med. 377, 1836–1846 (2017).
Reddy, S. M. et al. Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. Br. J. Cancer 118, 17–23 (2018).
Brustugun, O. T. et al. Substantial nation-wide improvement in lung cancer relative survival in Norway from 2000 to 2016. Lung Cancer 122, 138–145 (2018).
Noroxe, D. S. & Sorensen, J. B. Ultra-late relapse with a single cerebellar metastasis 10 years after complete surgery for stage IIA non-small cell lung cancer (bronchioalveolar carcinoma). J. Thorac. Oncol. 7, 764–765 (2012).
Martini, N. et al. Factors influencing ten-year survival in resected stages I to IIIa non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 117, 32–36 (1999).
Maeda, R. et al. Long-term outcome and late recurrence in patients with completely resected stage IA non-small cell lung cancer. J. Thorac. Oncol. 5, 1246–1250 (2010).
Collins, V. P., Loeffler, R. K. & Tivey, H. Observations on growth rates of human tumors. Am. J. Roentgenol. Radium Ther. Nucl. Med. 76, 988–1000 (1956).
Friberg, S. & Mattson, S. On the growth rates of human malignant tumors: implications for medical decision making. J. Surg. Oncol. 65, 284–297 (1997).
Klein, C. A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302–312 (2009).
Berghoff, A. S. et al. Differential role of angiogenesis and tumour cell proliferation in brain metastases according to primary tumour type: analysis of 639 cases. Neuropathol. Appl. Neurobiol. 41, e41–e55 (2015).
Berghoff, A. S. et al. Prognostic significance of Ki67 proliferation index, HIF1 alpha index and microvascular density in patients with non-small cell lung cancer brain metastases. Strahlenther. Onkol. 190, 676–685 (2014).
Finlay, I. G., Meek, D., Brunton, F. & McArdle, C. S. Growth rate of hepatic metastases in colorectal carcinoma. Br. J. Surg. 75, 641–644 (1988).
Spratt, J. S. Jr. & Spratt, T. L. Rates of growth of pulmonary metastases and host survival. Ann. Surg. 159, 161–171 (1964).
Hadfield, G. The dormant cancer cell. Br. Med. J. 4888, 607–610 (1954).
Willis, R. A. The Spread of Tumours in the Human Body (J. & A. Churchill, 1934).
Klein, C. A. Framework models of tumor dormancy from patient-derived observations. Curr. Opin. Genet. Dev. 21, 42–49 (2011).
Klein, C. A. & Holzel, D. Systemic cancer progression and tumor dormancy: mathematical models meet single cell genomics. Cell Cycle 5, 1788–1798 (2006).
Ulmer, A. et al. Quantitative measurement of melanoma spread in sentinel lymph nodes and survival. PLoS Med. 11, e1001604 (2014).
Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1, 99–101 (1889).
Aleckovic, M., McAllister, S. S. & Polyak, K. Metastasis as a systemic disease: molecular insights and clinical implications. Biochim. Biophys. Acta Rev. Cancer 1872, 89–102 (2019).
Klein, C. A. Selection and adaptation during metastatic cancer progression. Nature 501, 365–372 (2013).
Schumacher, S. et al. Disseminated tumour cells with highly aberrant genomes are linked to poor prognosis in operable oesophageal adenocarcinoma. Br. J. Cancer 117, 725–733 (2017).
Stoecklein, N. H. et al. Direct genetic analysis of single disseminated cancer cells for prediction of outcome and therapy selection in esophageal cancer. Cancer Cell 13, 441–453 (2008).
Werner-Klein, M. et al. Interleukin-6 trans-signaling is a candidate mechanism to drive progression of human DCCs during clinical latency. Nat. Commun. https://doi.org/10.1038/s41467-020-18701-4 (2020).
Holcomb, I. N. et al. Genomic alterations indicate tumor origin and varied metastatic potential of disseminated cells from prostate cancer patients. Cancer Res. 68, 5599–5608 (2008).
Schardt, J. A. et al. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell 8, 227–239 (2005).
Schmidt-Kittler, O. et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc. Natl Acad. Sci. USA 100, 7737–7742 (2003).
Weckermann, D. et al. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J. Clin. Oncol. 27, 1549–1556 (2009).
Hu, Z. et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 51, 1113–1122 (2019).
Ishaque, N. et al. Whole genome sequencing puts forward hypotheses on metastasis evolution and therapy in colorectal cancer. Nat. Commun. 9, 4782 (2018).
Kroigard, A. B. et al. Genomic analyses of breast cancer progression reveal distinct routes of metastasis emergence. Sci. Rep. 7, 43813 (2017).
Kroigard, A. B. et al. Identification of metastasis driver genes by massive parallel sequencing of successive steps of breast cancer progression. PLoS ONE 13, e0189887 (2018).
Leung, M. L. et al. Single-cell DNA sequencing reveals a late-dissemination model in metastatic colorectal cancer. Genome Res. 27, 1287–1299 (2017).
Sanborn, J. Z. et al. Phylogenetic analyses of melanoma reveal complex patterns of metastatic dissemination. Proc. Natl Acad. Sci. USA 112, 10995–11000 (2015).
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx renal. Cell 173, 581–594 e512 (2018).
Yates, L. R. et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell 32, 169–184 e167 (2017).
Kuipers, J., Jahn, K. & Beerenwinkel, N. Advances in understanding tumour evolution through single-cell sequencing. Biochim. Biophys. Acta Rev. Cancer 1867, 127–138 (2017).
Kuipers, J., Jahn, K., Raphael, B. J. & Beerenwinkel, N. Single-cell sequencing data reveal widespread recurrence and loss of mutational hits in the life histories of tumors. Genome Res. 27, 1885–1894 (2017).
Reeves, M. Q., Kandyba, E., Harris, S., Del Rosario, R. & Balmain, A. Multicolour lineage tracing reveals clonal dynamics of squamous carcinoma evolution from initiation to metastasis. Nat. Cell Biol. 20, 699–709 (2018).
Heyde, A., Reiter, J. G., Naxerova, K. & Nowak, M. A. Consecutive seeding and transfer of genetic diversity in metastasis. Proc. Natl Acad. Sci. USA 116, 14129–14137 (2019).
Cheung, K. J. et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863 (2016).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Szczerba, B. M. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 (2019).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Burnet, M. Cancer; a biological approach. I. The processes of control. Br. Med. J. 1, 779–786 (1957).
Mittal, D., Gubin, M. M., Schreiber, R. D. & Smyth, M. J. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr. Opin. Immunol. 27, 16–25 (2014).
Alspach, E. et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 574, 696–701 (2019).
Senovilla, L. et al. An immunosurveillance mechanism controls cancer cell ploidy. Science 337, 1678–1684 (2012).
Malladi, S. et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 165, 45–60 (2016).
Malaise, M. et al. KLRG1+ NK cells protect T-bet-deficient mice from pulmonary metastatic colorectal carcinoma. J. Immunol. 192, 1954–1961 (2014).
Chuang, H. N. et al. Carcinoma cells misuse the host tissue damage response to invade the brain. Glia 61, 1331–1346 (2013).
Celia-Terrassa, T. & Kang, Y. Metastatic niche functions and therapeutic opportunities. Nat. Cell Biol. 20, 868–877 (2018).
Hoye, A. M. & Erler, J. T. Structural ECM components in the premetastatic and metastatic niche. Am. J. Physiol. Cell Physiol. 310, C955–C967 (2016).
Smith, H. A. & Kang, Y. Determinants of organotropic metastasis. Annu. Rev. Cancer Biol. 1, 403–423 (2017).
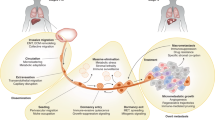
Giancotti, F. G. Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764 (2013).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
Ghajar, C. M. Metastasis prevention by targeting the dormant niche. Nat. Rev. Cancer 15, 238–247 (2015).
Aslakson, C. J. & Miller, F. R. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405 (1992).
Aslakson, C. J., Rak, J. W., Miller, B. E. & Miller, F. R. Differential influence of organ site on three subpopulations of a single mouse mammary tumor at two distinct steps in metastasis. Int. J. Cancer 47, 466–472 (1991).
Bragado, P. et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat. Cell Biol. 15, 1351–1361 (2013).
Gao, H. et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell 150, 764–779 (2012).
Lu, X. et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011).
Morris, V. L., Tuck, A. B., Wilson, S. M., Percy, D. & Chambers, A. F. Tumor progression and metastasis in murine D2 hyperplastic alveolar nodule mammary tumor cell lines. Clin. Exp. Metastasis 11, 103–112 (1993).
Ossowski, L. & Reich, E. Experimental model for quantitative study of metastasis. Cancer Res. 40, 2300–2309 (1980).
Ossowski, L. & Reich, E. Loss of malignancy during serial passage of human carcinoma in culture and discordance between malignancy and transformation parameters. Cancer Res. 40, 2310–2315 (1980).
Ossowski, L. & Reich, E. Changes in malignant phenotype of a human carcinoma conditioned by growth environment. Cell 33, 323–333 (1983).
Rak, J. W., McEachern, D. & Miller, F. R. Sequential alteration of peanut agglutinin binding-glycoprotein expression during progression of murine mammary neoplasia. Br. J. Cancer 65, 641–648 (1992).
Suzuki, M., Mose, E. S., Montel, V. & Tarin, D. Dormant cancer cells retrieved from metastasis-free organs regain tumorigenic and metastatic potency. Am. J. Pathol. 169, 673–681 (2006).
Wheeler, S. E. et al. Spontaneous dormancy of metastatic breast cancer cells in an all human liver microphysiologic system. Br. J. Cancer 111, 2342–2350 (2014).
Endo, H. et al. The induction of MIG6 under hypoxic conditions is critical for dormancy in primary cultured lung cancer cells with activating EGFR mutations. Oncogene 36, 2824–2834 (2017).
Fluegen, G. et al. Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nat. Cell Biol. 19, 120–132 (2017).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Carlson, P. et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 (2019).
Shiozawa, Y. et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Invest. 121, 1298–1312 (2011).
Shiozawa, Y. et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia 12, 116–127 (2010).
Kobayashi, A. et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J. Exp. Med. 208, 2641–2655 (2011).
Yu-Lee, L. Y. et al. Osteoblast-secreted factors mediate dormancy of metastatic prostate cancer in the bone via activation of the TGFbetaRIII-p38MAPK-pS249/T252RB pathway. Cancer Res. 78, 2911–2924 (2018).
Lee, E. et al. Growth arrest-specific 6 (GAS6) promotes prostate cancer survival by G1 arrest/S phase delay and inhibition of apoptosis during chemotherapy in bone marrow. J. Cell Biochem. 117, 2815–2824 (2016).
Cackowski, F. C. et al. Mer tyrosine kinase regulates disseminated prostate cancer cellular dormancy. J. Cell Biochem. 118, 891–902 (2017).
Ren, D. et al. Wnt5a induces and maintains prostate cancer cells dormancy in bone. J. Exp. Med. 216, 428–449 (2019).
Johnson, R. W. et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat. Cell Biol. 18, 1078–1089 (2016).
Lee, I. K., Vansaun, M. N., Shim, J. H., Matrisian, L. M. & Gorden, D. L. Increased metastases are associated with inflammation and matrix metalloproteinase-9 activity at incision sites in a murine model of peritoneal dissemination of colorectal cancer. J. Surg. Res. 180, 252–259 (2013).
Roy, L. D. et al. Collagen induced arthritis increases secondary metastasis in MMTV-PyV MT mouse model of mammary cancer. BMC Cancer 11, 365 (2011).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science https://doi.org/10.1126/science.aao4227 (2018).
Bouchard, G. et al. Pre-irradiation of mouse mammary gland stimulates cancer cell migration and development of lung metastases. Br. J. Cancer 109, 1829–1838 (2013).
El Rayes, T. et al. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc. Natl Acad. Sci. USA 112, 16000–16005 (2015).
De Cock, J. M. et al. Inflammation triggers Zeb1-dependent escape from tumor latency. Cancer Res. 76, 6778–6784 (2016).
Oskarsson, T. et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874 (2011).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2012).
Gao, H. et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166, 47–62 (2016).
Ombrato, L. et al. Metastatic-niche labelling reveals parenchymal cells with stem features. Nature 572, 603–608 (2019).
Montagner, M. et al. Crosstalk with lung epithelial cells regulates Sfrp2-mediated latency in breast cancer dissemination. Nat. Cell Biol. 22, 289–296 (2020).
Campbell, J. P. et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 10, e1001363 (2012).
Decker, A. M. et al. Sympathetic signaling reactivates quiescent disseminated prostate cancer cells in the bone marrow. Mol. Cancer Res. 15, 1644–1655 (2017).
Zhang, X. et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 10, 788 (2019).
Liao, C. P. et al. Loss of MAOA in epithelia inhibits adenocarcinoma development, cell proliferation and cancer stem cells in prostate. Oncogene 37, 5175–5190 (2018).
Wu, J. B. et al. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J. Clin. Invest. 124, 2891–2908 (2014).
Woodward, W. A. Inflammatory breast cancer: unique biological and therapeutic considerations. Lancet Oncol. 16, e568–e576 (2015).
Lim, B., Woodward, W. A., Wang, X., Reuben, J. M. & Ueno, N. T. Inflammatory breast cancer biology: the tumour microenvironment is key. Nat. Rev. Cancer 18, 485–499 (2018).
Masuda, H. et al. Long-term treatment efficacy in primary inflammatory breast cancer by hormonal receptor- and HER2-defined subtypes. Ann. Oncol. 25, 384–391 (2014).
Riethmuller, G. & Klein, C. A. Early cancer cell dissemination and late metastatic relapse: clinical reflections and biological approaches to the dormancy problem in patients. Semin. Cancer Biol. 11, 307–311 (2001).
Buell, J. F. et al. Donor transmitted malignancies. Ann. Transpl. 9, 53–56 (2004).
Lipshutz, G. S. et al. Death from donor-transmitted malignancy despite emergency liver retransplantation. Liver Transpl. 9, 1102–1107 (2003).
Dib, L. L., Soares, A. L., Sandoval, R. L. & Nannmark, U. Breast metastasis around dental implants: a case report. Clin. Implant. Dent. Relat. Res. 9, 112–115 (2007).
Pfammatter, C., Lindenmuller, I. H., Lugli, A., Filippi, A. & Kuhl, S. Metastases and primary tumors around dental implants: a literature review and case report of peri-implant pulmonary metastasis. Quintessence Int. 43, 563–570 (2012).
Hirshberg, A., Leibovich, P., Horowitz, I. & Buchner, A. Metastatic tumors to postextraction sites. J. Oral. Maxillofac. Surg. 51, 1334–1337 (1993).
El Sharouni, S. Y., Kal, H. B. & Battermann, J. J. Accelerated regrowth of non-small-cell lung tumours after induction chemotherapy. Br. J. Cancer 89, 2184–2189 (2003).
Yoo, H. et al. Growth rates of metastatic brain tumors in nonsmall cell lung cancer. Cancer 113, 1043–1047 (2008).
Behrenbruch, C. et al. Surgical stress response and promotion of metastasis in colorectal cancer: a complex and heterogeneous process. Clin. Exp. Metastasis 35, 333–345 (2018).
Demicheli, R., Retsky, M. W., Hrushesky, W. J., Baum, M. & Gukas, I. D. The effects of surgery on tumor growth: a century of investigations. Ann. Oncol. 19, 1821–1828 (2008).
Gottschalk, A., Sharma, S., Ford, J., Durieux, M. E. & Tiouririne, M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth. Analg. 110, 1636–1643 (2010).
Krall, J. A. et al. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci. Transl Med. https://doi.org/10.1126/scitranslmed.aan3464 (2018).
Bianchini, F., Kaaks, R. & Vainio, H. Overweight, obesity, and cancer risk. Lancet Oncol. 3, 565–574 (2002).
Biganzoli, E. et al. Recurrence dynamics of breast cancer according to baseline body mass index. Eur. J. Cancer 87, 10–20 (2017).
Zhang, M. et al. Time-varying effects of prognostic factors associated with long-term survival in breast cancer. Endocr. Relat. Cancer 25, 509–521 (2018).
Olson, O. C., Quail, D. F. & Joyce, J. A. Obesity and the tumor microenvironment. Science 358, 1130–1131 (2017).
Quail, D. F. et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat. Cell Biol. 19, 974–987 (2017).
Diessner, J. et al. Evaluation of clinical parameters influencing the development of bone metastasis in breast cancer. BMC Cancer 16, 307 (2016).
Giuliani, N. et al. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp. Gerontol. 36, 547–557 (2001).
Sanoff, H. K. et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J. Natl Cancer Inst. 106, dju057 (2014).
Davalos, A. R., Coppe, J. P., Campisi, J. & Desprez, P. Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 29, 273–283 (2010).
Martin, M. et al. Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: results from the GEICAM/2003-10 study. J. Clin. Oncol. 33, 3788–3795 (2015).
Sonnenblick, A. & Piccart, M. Adjuvant systemic therapy in breast cancer: quo vadis? Ann. Oncol. 26, 1629–1634 (2015).
Abravanel, D. L. et al. Notch promotes recurrence of dormant tumor cells following HER2/neu-targeted therapy. J. Clin. Invest. 125, 2484–2496 (2015).
Havas, K. M. et al. Metabolic shifts in residual breast cancer drive tumor recurrence. J. Clin. Invest. 127, 2091–2105 (2017).
Rambow, F. et al. Toward minimal residual disease-directed therapy in melanoma. Cell 174, 843–855 e819 (2018).
Davies, C. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013).
Al-Mubarak, M. et al. Extended adjuvant tamoxifen for early breast cancer: a meta-analysis. PLoS ONE 9, e88238 (2014).
Early Breast Cancer Trialists’ Collaborative Group. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 386, 1353–1361 (2015).
Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365, 1687–1717 (2005).
Peto, R. et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 379, 432–444 (2012).
Jatoi, I. et al. Time-varying effects of breast cancer adjuvant systemic therapy. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djv304 (2016).
Chavez-MacGregor, M., Clarke, C. A., Lichtensztajn, D. Y. & Giordano, S. H. Delayed initiation of adjuvant chemotherapy among patients with breast cancer. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2015.3856 (2015).
Gagliato Dde, M. et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J. Clin. Oncol. 32, 735–744 (2014).
Werner-Klein, M. & Klein, C. A. Therapy resistance beyond cellular dormancy. Nat. Cell Biol. 21, 117–119 (2019).
Goldhirsch, A. et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet 382, 1021–1028 (2013).
Kramar, A. et al. Trastuzumab duration effects within patient prognostic subgroups in the PHARE trial. Ann. Oncol. 25, 1563–1570 (2014).
Mavroudis, D. et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 26, 1333–1340 (2015).
Di Leo, A. et al. HER2 and TOP2A as predictive markers for anthracycline-containing chemotherapy regimens as adjuvant treatment of breast cancer: a meta-analysis of individual patient data. Lancet Oncol. 12, 1134–1142 (2011).
Guarneri, V. et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann. Oncol. 24, 2990–2994 (2013).
Niikura, N. et al. Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. J. Clin. Oncol. 30, 593–599 (2012).
Twelves, C. et al. “New” metastases are associated with a poorer prognosis than growth of pre-existing metastases in patients with metastatic breast cancer treated with chemotherapy. Breast Cancer Res. 17, 150 (2015).
Mathew, A. & Romond, E. H. Systemic therapy for HER2-positive early-stage breast cancer. Curr. Probl. Cancer 40, 106–116 (2016).
Naume, B. et al. Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. J. Clin. Oncol. 32, 3848–3857 (2014).
Gimotty, P. A. et al. Biologic and prognostic significance of dermal Ki67 expression, mitoses, and tumorigenicity in thin invasive cutaneous melanoma. J. Clin. Oncol. 23, 8048–8056 (2005).
Parise, C. A., Bauer, K. R., Brown, M. M. & Caggiano, V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 15, 593–602 (2009).
Sundquist, M., Brudin, L. & Tejler, G. Improved survival in metastatic breast cancer 1985–2016. Breast 31, 46–50 (2017).
Nishimura, R. et al. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp. Ther. Med. 1, 747–754 (2010).
Nakashima, K. et al. Does breast cancer growth rate really depend on tumor subtype? Measurement of tumor doubling time using serial ultrasonography between diagnosis and surgery. Breast Cancer 26, 206–214 (2019).
Jennings, S. G., Winer-Muram, H. T., Tann, M., Ying, J. & Dowdeswell, I. Distribution of stage I lung cancer growth rates determined with serial volumetric CT measurements. Radiology 241, 554–563 (2006).
Engel, J. et al. The process of metastasisation for breast cancer. Eur. J. Cancer 39, 1794–1806 (2003).
Holzel, D., Eckel, R., Emeny, R. T. & Engel, J. Distant metastases do not metastasize. Cancer Metastasis Rev. 29, 737–750 (2010).
Fidler, I. J. Selection of successive tumour lines for metastasis. Nat. New Biol. 242, 148–149 (1973).
Vaage, J. Metastasizing potentials of mouse mammary tumors and their metastases. Int. J. Cancer 41, 855–858 (1988).
Riethdorf, S., Wikman, H. & Pantel, K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int. J. Cancer 123, 1991–2006 (2008).
Hutchinson, J. N., Jin, J., Cardiff, R. D., Woodgett, J. R. & Muller, W. J. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 64, 3171–3178 (2004).
Liu, H. et al. MYC suppresses cancer metastasis by direct transcriptional silencing of alphav and beta3 integrin subunits. Nat. Cell Biol. 14, 567–574 (2012).
Rack, B. et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/dju066 (2014).
Meng, S. et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 10, 8152–8162 (2004).
Braun, S. et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J. Clin. Oncol. 18, 80–86 (2000).
Pantel, K. et al. Immunocytological detection of bone marrow micrometastasis in operable non-small cell lung cancer. Cancer Res. 53, 1027–1031 (1993).
Klauber-DeMore, N., Van Zee, K. J., Linkov, I., Borgen, P. I. & Gerald, W. L. Biological behavior of human breast cancer micrometastases. Clin. Cancer Res. 7, 2434–2439 (2001).
Acknowledgements
The author thanks S. Pausch for help with the figures, T. Perry for critical reading of the manuscript and M. Guzvic for hints about relevant references.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author is a member of the scientific advisory board of HiberCell.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Klein, C.A. Cancer progression and the invisible phase of metastatic colonization. Nat Rev Cancer 20, 681–694 (2020). https://doi.org/10.1038/s41568-020-00300-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-020-00300-6
This article is cited by
-
Dormancy of cutaneous melanoma
Cancer Cell International (2024)
-
Emerging roles of ADP-dependent glucokinase in prostate cancer
Military Medical Research (2024)
-
Systematic pan-cancer analyses of the potential function of the Golgi scaffold protein PAQR3
Scientific Reports (2024)
-
Targeting cancer cell dormancy
Nature Reviews Cancer (2024)
-
Lipids as mediators of cancer progression and metastasis
Nature Cancer (2024)