Abstract
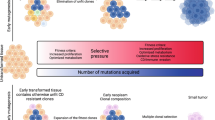
The tumour microenvironment plays a critical role in determining tumour fate. Within that environment, and indeed throughout epithelial tissues, cells experience competition with their neighbours, with those less fit being eliminated by fitter adjacent cells. Herein we discuss evidence suggesting that mutations in cancer cells may be selected for their ability to exploit cell competition to kill neighbouring host cells, thereby facilitating tumour expansion. In some instances, cell competition may help host tissues to defend against cancer, by removing neoplastic and aneuploid cells. Cancer risk factors, such as high-sugar or high-fat diet and inflammation, impact cell competition-based host defences, suggesting that their effect on tumour risk may in part be accounted for by their influence on cell competition. We propose that interventions aimed at modifying the strength and direction of cell competition could induce cancer cell killing and form the basis for novel anticancer therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
14 April 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41568-020-0261-2
References
Kastan, M. B. & Bartek, J. Cell-cycle checkpoints and cancer. Nature 432, 316–323 (2004).
Massague, J. G1 cell-cycle control and cancer. Nature 432, 298–306 (2004).
Evan, G. I. & Vousden, K. H. Proliferation, cell cycle and apoptosis in cancer. Nature 411, 342–348 (2001).
Wagstaff, L. et al. Mechanical cell competition kills cells via induction of lethal p53 levels. Nat. Commun. 7, 11373 (2016).
Suijkerbuijk, S. J., Kolahgar, G., Kucinski, I. & Piddini, E. Cell competition drives the growth of intestinal adenomas in Drosophila. Curr. Biol. 26, 428–438 (2016).
Moreno, E. & Basler, K. dMyc transforms cells into super-competitors. Cell 117, 117–129 (2004).
de la Cova, C., Abril, M., Bellosta, P., Gallant, P. & Johnston, L. A. Drosophila myc regulates organ size by inducing cell competition. Cell 117, 107–116 (2004).
Brown, S. et al. Correction of aberrant growth preserves tissue homeostasis. Nature 548, 334–337 (2017).
Hogan, C. et al. Characterization of the interface between normal and transformed epithelial cells. Nat. Cell Biol. 11, 460–467 (2009).
Kajita, M. et al. Filamin acts as a key regulator in epithelial defence against transformed cells. Nat. Commun. 5, 4428 (2014).
Ohoka, A. et al. EPLIN is a crucial regulator for extrusion of RasV12-transformed cells. J. Cell Sci. 128, 781–789 (2015).
Eisenhoffer, G. T. et al. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 484, 546–549 (2012).
Ohsawa, S., Vaughen, J. & Igaki, T. Cell extrusion: a stress-responsive force for good or evil in epithelial homeostasis. Dev. Cell 44, 284–296 (2018).
Marinari, E. et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, 542–545 (2012).
Ellis, S. J. et al. Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature 569, 497–502 (2019).
Liu, N. et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 568, 344–350 (2019).
Kolahgar, G. et al. Cell competition modifies adult stem cell and tissue population dynamics in a JAK-STAT-dependent manner. Dev. Cell 34, 297–309 (2015).
Watanabe, H. et al. Mutant p53-expressing cells undergo necroptosis via cell competition with the neighboring normal epithelial cells. Cell Rep. 23, 3721–3729 (2018).
Li, W. & Baker, N. E. Engulfment is required for cell competition. Cell 129, 1215–1225 (2007).
Ohsawa, S. et al. Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev. Cell 20, 315–328 (2011).
Sun, Q. et al. Competition between human cells by entosis. Cell Res. 24, 1299–1310 (2014).
Hamann, J. C. et al. Entosis is induced by glucose starvation. Cell Rep. 20, 201–210 (2017).
Morata, G. & Ripoll, P. Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol. 42, 211–221 (1975).
Simpson, P. Parameters of cell competition in the compartments of the wing disc of Drosophila. Dev. Biol. 69, 182–193 (1979).
Simpson, P. & Morata, G. Differential mitotic rates and patterns of growth in compartments in the Drosophila wing. Dev. Biol. 85, 299–308 (1981).
Oliver, E. R., Saunders, T. L., Tarle, S. A. & Glaser, T. Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development 131, 3907–3920 (2004).
Baker, N. E. Mechanisms of cell competition emerging from Drosophila studies. Curr. Opin. Cell Biol. 48, 40–46 (2017).
Di Gregorio, A., Bowling, S. & Rodriguez, T. A. Cell competition and its role in the regulation of cell fitness from development to cancer. Dev. Cell 38, 621–634 (2016).
Amoyel, M. & Bach, E. A. Cell competition: how to eliminate your neighbours. Development 141, 988–1000 (2014).
Gutierrez-Martinez, A., Guinevere Sew, W. Q., Molano-Fernandez, M., Carretero-Junquera, M. & Herranz, H. Mechanisms of oncogenic cell competition - paths of victory. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.05.015 (2019).
Maruyama, T. & Fujita, Y. Cell competition in mammals - novel homeostatic machinery for embryonic development and cancer prevention. Curr. Opin. Cell Biol. 48, 106–112 (2017).
Claveria, C. & Torres, M. Cell competition: mechanisms and physiological roles. Annu. Rev. Cell Dev. Biol. 32, 411–439 (2016).
Vincent, J. P., Fletcher, A. G. & Baena-Lopez, L. A. Mechanisms and mechanics of cell competition in epithelia. Nat. Rev. Mol. Cell Biol. 14, 581–591 (2013).
Neto-Silva, R. M., de Beco, S. & Johnston, L. A. Evidence for a growth-stabilizing regulatory feedback mechanism between Myc and Yorkie, the Drosophila homolog of Yap. Dev. Cell 19, 507–520 (2010).
Johnston, L. A., Prober, D. A., Edgar, B. A., Eisenman, R. N. & Gallant, P. Drosophila myc regulates cellular growth during development. Cell 98, 779–790 (1999).
Norman, M. et al. Loss of Scribble causes cell competition in mammalian cells. J. Cell Sci. 125, 59–66 (2012).
Igaki, T., Pagliarini, R. A. & Xu, T. Loss of cell polarity drives tumor growth and invasion through JNK activation in Drosophila. Curr. Biol. 16, 1139–1146 (2006).
Brumby, A. M. & Richardson, H. E. Scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769–5779 (2003).
Moitrier, S. et al. Collective stresses drive competition between monolayers of normal and Ras-transformed cells. Soft Matter 15, 537–545 (2019).
Kajita, M. et al. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J. Cell Sci. 123, 171–180 (2010).
Takagi, M. et al. Accumulation of the myosin-II-spectrin complex plays a positive role in apical extrusion of Src-transformed epithelial cells. Genes Cell 23, 974–981 (2018).
Tamori, Y. et al. Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 8, e1000422 (2010).
Ballesteros-Arias, L., Saavedra, V. & Morata, G. Cell competition may function either as tumour-suppressing or as tumour-stimulating factor in Drosophila. Oncogene 33, 4377–4384 (2013).
Rodrigues, A. B. et al. Activated STAT regulates growth and induces competitive interactions independently of Myc, Yorkie, Wingless and ribosome biogenesis. Development 139, 4051–4061 (2012).
Vincent, J. P., Kolahgar, G., Gagliardi, M. & Piddini, E. Steep differences in wingless signaling trigger Myc-independent competitive cell interactions. Dev. Cell 21, 366–374 (2011).
Bondar, T. & Medzhitov, R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6, 309–322 (2010).
Marusyk, A., Porter, C. C., Zaberezhnyy, V. & DeGregori, J. Irradiation selects for p53-deficient hematopoietic progenitors. PLOS Biol. 8, e1000324 (2010).
Chen, C. L., Schroeder, M. C., Kango-Singh, M., Tao, C. & Halder, G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc. Natl Acad. Sci. USA 109, 484–489 (2012).
Menendez, J., Perez-Garijo, A., Calleja, M. & Morata, G. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc. Natl Acad. Sci. USA 107, 14651–14656 (2010).
Tyler, D. M., Li, W., Zhuo, N., Pellock, B. & Baker, N. E. Genes affecting cell competition in Drosophila. Genetics 175, 643–657 (2007).
Moreno, E., Basler, K. & Morata, G. Cells compete for Decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755–759 (2002).
Adachi-Yamada, T. & O'Connor, M. B. Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev. Biol. 251, 74–90 (2002).
Pinal, N., Calleja, M. & Morata, G. Pro-apoptotic and pro-proliferation functions of the JNK pathway of Drosophila: roles in cell competition, tumorigenesis and regeneration. Open Biol. 9, 180256 (2019).
Johnston, L. A. Socializing with MYC: cell competition in development and as a model for premalignant cancer. Cold Spring Harb. Perspect. Med. 4, a014274 (2014).
Baillon, L. & Basler, K. Reflections on cell competition. Semin. Cell Dev. Biol. 32, 137–144 (2014).
Valon, L. & Levayer, R. Dying under pressure: cellular characterisation and in vivo functions of cell death induced by compaction. Biol. Cell 111, 51–66 (2019).
Rhiner, C. et al. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev. Cell 18, 985–998 (2010).
Alpar, L., Bergantinos, C. & Johnston, L. A. Spatially restricted regulation of Spätzle/Toll signaling during cell competition. Dev. Cell 46, 706–719 e705 (2018).
Meyer, S. N. et al. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 346, 1258236 (2014).
Yamamoto, M., Ohsawa, S., Kunimasa, K. & Igaki, T. The ligand Sas and its receptor PTP10D drive tumour-suppressive cell competition. Nature 542, 246–250 (2017).
Germani, F., Hain, D., Sternlicht, D., Moreno, E. & Basler, K. The Toll pathway inhibits tissue growth and regulates cell fitness in an infection-dependent manner. Elife 7, https://doi.org/10.7554/eLife.39939 (2018).
Kolahi, K. S. & Mofrad, M. R. Mechanotransduction: a major regulator of homeostasis and development. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 625–639 (2010).
Ingallina, E. et al. Mechanical cues control mutant p53 stability through a mevalonate-RhoA axis. Nat. Cell Biol. 20, 28–35 (2018).
Shraiman, B. I. Mechanical feedback as a possible regulator of tissue growth. Proc. Natl Acad. Sci. USA 102, 3318–3323 (2005).
Gudipaty, S. A., Conner, C. M., Rosenblatt, J. & Montell, D. J. Unconventional ways to live and die: cell death and survival in development, homeostasis, and disease. Annu. Rev. Cell Dev. Biol. 34, 311–332 (2018).
Wyatt, T. P. et al. Emergence of homeostatic epithelial packing and stress dissipation through divisions oriented along the long cell axis. Proc. Natl Acad. Sci. USA 112, 5726–5731 (2015).
Curran, S. et al. Myosin II controls junction fluctuations to guide epithelial tissue ordering. Dev. Cell 43, 480–492 e486 (2017).
Khalilgharibi, N. et al. Stress relaxation in epithelial monolayers is controlled by the actomyosin cortex. Nat. Phys. 15, 839–847 (2019).
Levayer, R., Dupont, C. & Moreno, E. tissue crowding induces caspase-dependent competition for space. Curr. Biol. 26, 670–677 (2016).
Moreno, E., Valon, L., Levillayer, F. & Levayer, R. Competition for space induces cell elimination through compaction-driven ERK downregulation. Curr. Biol. 29, 23–34 e28 (2019).
Zhang, G. et al. p53 pathway is involved in cell competition during mouse embryogenesis. Proc. Natl Acad. Sci. USA 114, 498–503 (2017).
Dejosez, M., Ura, H., Brandt, V. L. & Zwaka, T. P. Safeguards for cell cooperation in mouse embryogenesis shown by genome-wide cheater screen. Science 341, 1511–1514 (2013).
Bowling, S. et al. P53 and mTOR signalling determine fitness selection through cell competition during early mouse embryonic development. Nat. Commun. 9, 1763 (2018).
Kon, S. et al. Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat. Cell Biol. 19, 530–541 (2017).
Kalkat, M. et al. MYC deregulation in primary human cancers. Genes 8, 151 (2017).
Baker, N. E. & Li, W. Cell competition and its possible relation to cancer. Cancer Res. 68, 5505–5507 (2008).
Moreno, E. Is cell competition relevant to cancer? Nat. Rev. Cancer 8, 141–147 (2008).
Grifoni, D. & Bellosta, P. Drosophila Myc: a master regulator of cellular performance. Biochim. Biophys. Acta 1849, 570–581 (2015).
Paglia, S., Sollazzo, M., Di Giacomo, S., Strocchi, S. & Grifoni, D. Exploring MYC relevance to cancer biology from the perspective of cell competition. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.05.009 (2019).
Claveria, C., Giovinazzo, G., Sierra, R. & Torres, M. Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500, 39–44 (2013).
Sancho, M. et al. Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev. Cell 26, 19–30 (2013).
Villa Del Campo, C., Claveria, C., Sierra, R. & Torres, M. Cell competition promotes phenotypically silent cardiomyocyte replacement in the mammalian heart. Cell Rep. 8, 1741–1751 (2014).
Ziosi, M. et al. dMyc functions downstream of Yorkie to promote the supercompetitive behavior of hippo pathway mutant cells. PLOS Genet. 6, e1001140 (2010).
Eichenlaub, T., Cohen, S. M. & Herranz, H. Cell competition drives the formation of metastatic tumors in a Drosophila model of epithelial tumor formation. Curr. Biol. 26, 419–427 (2016).
Patel, P. H., Dutta, D. & Edgar, B. A. Niche appropriation by Drosophila intestinal stem cell tumours. Nat. Cell Biol. 17, 1182–1192 (2015).
Alcolea, M. P. & Jones, P. H. Cell competition: winning out by losing notch. Cell Cycle 14, 9–17 (2015).
Schroeder, M. C., Chen, C. L., Gajewski, K. & Halder, G. A non-cell-autonomous tumor suppressor role for Stat in eliminating oncogenic scribble cells. Oncogene 32, 4471–4479 (2013).
Moya, I. M. & Halder, G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Harvey, K. F., Zhang, X. & Thomas, D. M. The Hippo pathway and human cancer. Nat. Rev. Cancer 13, 246–257 (2013).
Liu, Z. et al. Differential YAP expression in glioma cells induces cell competition and promotes tumorigenesis. J. Cell Sci. 132, https://doi.org/10.1242/jcs.225714 (2019).
Zhan, T., Rindtorff, N. & Boutros, M. Wnt signaling in cancer. Oncogene 36, 1461–1473 (2017).
Groner, B. & von Manstein, V. Jak Stat signaling and cancer: opportunities, benefits and side effects of targeted inhibition. Mol. Cell Endocrinol. 451, 1–14 (2017).
Ryoo, H. D., Gorenc, T. & Steller, H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev. Cell 7, 491–501 (2004).
Perez, E., Lindblad, J. L. & Bergmann, A. Tumor-promoting function of apoptotic caspases by an amplification loop involving ROS, macrophages and JNK in Drosophila. Elife 6, https://doi.org/10.7554/eLife.26747 (2017).
Tannapfel, A. et al. Apoptosis, proliferation, bax, bcl-2 and p53 status prior to and after preoperative radiochemotherapy for locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 41, 585–591 (1998).
de Bruin, E. C. & Medema, J. P. Apoptosis and non-apoptotic deaths in cancer development and treatment response. Cancer Treat. Rev. 34, 737–749 (2008).
Hiraoka, N. et al. Tumour necrosis is a postoperative prognostic marker for pancreatic cancer patients with a high interobserver reproducibility in histological evaluation. Br. J. Cancer 103, 1057–1065 (2010).
Zhang, L. & Shay, J. W. Multiple roles of APC and its therapeutic implications in colorectal cancer. J. Natl Cancer Inst. 8, 109 (2017).
Cordero, J., Vidal, M. & Sansom, O. APC as a master regulator of intestinal homeostasis and transformation: from flies to vertebrates. Cell Cycle 8, 2926–2931 (2009).
Cordero, J. B., Stefanatos, R. K., Myant, K., Vidal, M. & Sansom, O. J. Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development 139, 4524–4535 (2012).
Bangi, E., Murgia, C., Teague, A. G., Sansom, O. J. & Cagan, R. L. Functional exploration of colorectal cancer genomes using Drosophila. Nat. Commun. 7, 13615 (2016).
Romano, G., Chagani, S. & Kwong, L. N. The path to metastatic mouse models of colorectal cancer. Oncogene 37, 2481–2489 (2018).
Snippert, H. J., Schepers, A. G., van Es, J. H., Simons, B. D. & Clevers, H. Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep. 15, 62–69 (2014).
Vermeulen, L. et al. Defining stem cell dynamics in models of intestinal tumor initiation. Science 342, 995–998 (2013).
Jonason, A. S. et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc. Natl Acad. Sci. USA 93, 14025–14029 (1996).
Ling, G. et al. Persistent p53 mutations in single cells from normal human skin. Am. J. Pathol. 159, 1247–1253 (2001).
Martincorena, I. et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 348, 880–886 (2015).
Lynch, M. D. et al. Spatial constraints govern competition of mutant clones in human epidermis. Nat. Commun. 8, 1119 (2017).
Klein, A. M., Brash, D. E., Jones, P. H. & Simons, B. D. Stochastic fate of p53-mutant epidermal progenitor cells is tilted toward proliferation by UV B during preneoplasia. Proc. Natl Acad. Sci. USA 107, 270–275 (2010).
Murai, K. et al. Epidermal tissue adapts to restrain progenitors carrying clonal p53 mutations. Cell Stem Cell 23, 687–699.e688 (2018).
Muller, P. A. & Vousden, K. H. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 25, 304–317 (2014).
Ziegler, A. et al. Sunburn and p53 in the onset of skin cancer. Nature 372, 773–776 (1994).
Nakazawa, H. et al. UV and skin cancer: specific p53 gene mutation in normal skin as a biologically relevant exposure measurement. Proc. Natl Acad. Sci. USA 91, 360–364 (1994).
Zhang, W. et al. UVB-induced apoptosis drives clonal expansion during skin tumor development. Carcinogenesis 26, 249–257 (2005).
Chao, D. L., Eck, J. T., Brash, D. E., Maley, C. C. & Luebeck, E. G. Preneoplastic lesion growth driven by the death of adjacent normal stem cells. Proc. Natl Acad. Sci. USA 105, 15034–15039 (2008).
Hall, P. A., M., P., Menage, H. D., Dover, R. & Lane, D. P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene 8, 203–207 (1993).
Bissell, M. J. & Hines, W. C. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 17, 320–329 (2011).
Martins, V. C. et al. Cell competition is a tumour suppressor mechanism in the thymus. Nature 509, 465–470 (2014).
Igaki, T., Pastor-Pareja, J. C., Aonuma, H., Miura, M. & Xu, T. Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 16, 458–465 (2009).
Vidal, M., Larson, D. E. & Cagan, R. L. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev. Cell 10, 33–44 (2006).
Bilder, D., Li, M. & Perrimon, N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113–116 (2000).
Zeitler, J., Hsu, C. P., Dionne, H. & Bilder, D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J. Cell Biol. 167, 1137–1146 (2004).
Mechler, B. M., McGinnis, W. & Gehring, W. J. Molecular cloning of lethal(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 4, 1551–1557 (1985).
Gateff, E. & Schneiderman, H. A. Developmental studies of a new mutant of Drosophila melanogaster-lethal malignant brain tumor. Am. Zool. 7, 760 (1967).
Okada, M., Nada, S., Yamanashi, Y., Yamamoto, T. & Nakagawa, H. CSK: a protein-tyrosine kinase involved in regulation of src family kinases. J. Biol. Chem. 266, 24249–24252 (1991).
Kajita, M. & Fujita, Y. EDAC: Epithelial defence against cancer-cell competition between normal and transformed epithelial cells in mammals. J. Biochem. 158, 15–23 (2015).
Chiba, T. et al. MDCK cells expressing constitutively active Yes-associated protein (YAP) undergo apical extrusion depending on neighboring cell status. Sci. Rep. 6, 28383 (2016).
Sasaki, A. et al. obesity suppresses cell-competition-mediated apical elimination of RasV12-transformed cells from epithelial tissues. Cell Rep. 23, 974–982 (2018).
Leung, C. T. & Brugge, J. S. Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 482, 410–413 (2012).
Rajagopalan, H. & Lengauer, C. Aneuploidy and cancer. Nature 432, 338-341 (2004).
Chunduri, N. K. & Storchova, Z. The diverse consequences of aneuploidy. Nat. Cell Biol. 21, 54–62 (2019).
Negrini, S., Gorgoulis, V. G. & Halazonetis, T. D. Genomic instability–an evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 11, 220–228 (2010).
Baker, N. E. Cell competition. Curr. Biol. 21, R11–R15 (2011).
Warner, J. R., Vilardell, J. & Sohn, J. H. Economics of ribosome biosynthesis. Cold Spring Harb. Symp. Quant. Biol. 66, 567–574 (2001).
Marygold, S. J. et al. The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol. 8, R216 (2007).
Mills, E.W. & Green R. Ribosomopathies: there’s strength in numbers. Science 358, eaan2755 (2017).
Ajore, R. et al. Deletion of ribosomal protein genes is a common vulnerability in human cancer, especially in concert with TP53 mutations. EMBO Mol. Med. 9, 498–507 (2017).
McNamee, L. M. & Brodsky, M. H. p53-independent apoptosis limits DNA damage-induced aneuploidy. Genetics 182, 423–435 (2009).
Titen, S. W. & Golic, K. G. Telomere loss provokes multiple pathways to apoptosis and produces genomic instability in Drosophila melanogaster. Genetics 180, 1821–1832 (2008).
Bolton, H. et al. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat. Commun. 7, 11165 (2016).
Pfau, S. J., Silberman, R. E., Knouse, K. A. & Amon, A. Aneuploidy impairs hematopoietic stem cell fitness and is selected against in regenerating tissues in vivo. Genes Dev. 30, 1395–1408 (2016).
Kucinski, I., Dinan, M., Kolahgar, G. & Piddini, E. Chronic activation of JNK JAK/STAT and oxidative stress signalling causes the loser cell status. Nat. Commun. 8, 136 (2017).
Tamori, Y., Suzuki, E. & Deng, W. M. Epithelial tumors originate in tumor hotspots, a tissue-intrinsic microenvironment. PLOS Biol. 14, e1002537 (2016).
Hirabayashi, S., Baranski, T. J. & Cagan, R. L. Transformed Drosophila cells evade diet-mediated insulin resistance through wingless signaling. Cell 154, 664–675 (2013).
Moi, P., C., K., Asunis, I., Cao, A. & Kan, Y. W. Isolation of NF-E2-related factor2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the f-globin locus control region. Proc. Natl Acad. Sci. USA 91, 9926–9930 (1994).
Itoh, Ken et al. An Nrf2/small Maf heterodimer mediates the induction of phase ii detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236, 313–322 (1997).
Venugopal, R. & Jaiswal, A. K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl Acad. Sci. USA 93, 14960–14965 (1996).
Cook, J. A. et al. Oxidative stress, redox, and the tumor microenvironment. Semin. Radiat. Oncol. 14, 259–266 (2004).
Sosa, V. et al. Oxidative stress and cancer: an overview. Ageing Res. Rev. 12, 376–390 (2013).
Moloney, J. N. & Cotter, T. G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 80, 50–64 (2018).
Menegon, S., Columbano, A. & Giordano, S. The dual roles of NRF2 in cancer. Trends Mol. Med. 22, 578–593 (2016).
D'Autreaux, B. & Toledano, M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 (2007).
Radisky, D. C. et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 (2005).
Santabarbara-Ruiz, P. et al. ROS-induced JNK and p38 signaling is required for unpaired cytokine activation during Drosophila regeneration. PLOS Genet. 11, e1005595 (2015).
Shen, H. M. & Liu, Z. G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 40, 928–939 (2006).
Khan, S. J., Abidi, S. N. F., Skinner, A., Tian, Y. & Smith-Bolton, R. K. The Drosophila Duox maturation factor is a key component of a positive feedback loop that sustains regeneration signaling. PLOS Genet. 13, e1006937 (2017).
Pinal, N., Martin, M., Medina, I. & Morata, G. Short-term activation of the Jun N-terminal kinase pathway in apoptosis-deficient cells of Drosophila induces tumorigenesis. Nat. Commun. 9, 1541 (2018).
Bianchini, F., Kaaks, R. & Vainio, H. Overweight, obesity, and cancer risk. Lancet Oncol. 3, 565–574 (2002).
Font-Burgada, J., Sun, B. & Karin, M. Obesity and cancer: the oil that feeds the flame. Cell Metab. 23, 48–62 (2016).
Altorki, N. K. et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer 19, 9–31 (2019).
Grzelak, C. A. & Ghajar, C. M. Metastasis 'systems' biology: how are macro-environmental signals transmitted into microenvironmental cues for disseminated tumor cells? Curr. Opin. Cell Biol. 48, 79–86 (2017).
Ishizawar, R. & Parsons, S. J. c-Src and cooperating partners in human cancer. Cancer Cell 6, 209–214 (2004).
Rothwell, P. M. et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 379, 1591–1601 (2012).
Luo, J., Solimini, N. L. & Elledge, S. J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009).
Levayer, R. Solid stress, competition for space and cancer: the opposing roles of mechanical cell competition in tumour initiation and growth. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.05.004 (2019).
Roncucci, L. & Mariani, F. Prevention of colorectal cancer: how many tools do we have in our basket? Eur. J. Intern. Med. 26, 752–756 (2015).
Moya, I. M. et al. Peritumoral activation of the Hippo pathway effectors YAP and TAZ suppresses liver cancer in mice. Science 366, 1029–1034 (2019).
Elisi, G. M. et al. Repurposing of drugs targeting YAP-TEAD functions. Cancers 10, 329 (2018).
Oku, Y. et al. Small molecules inhibiting the nuclear localization of YAP/TAZ for chemotherapeutics and chemosensitizers against breast cancers. FEBS Open Bio 5, 542–549 (2015).
Rajabi, M. & Mousa, S. A. The role of angiogenesis in cancer treatment. Biomedicines 5, 34 (2017).
Wong, P. P., Bodrug, N. & Hodivala-Dilke, K. M. Exploring novel methods for modulating tumor blood vessels in cancer treatment. Curr. Biol. 26, R1161–R1166 (2016).
Wong, P. P. et al. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell 27, 123–137 (2015).
Pagliarini, R. A. & Xu, T. A genetic screen in Drosophila for metastatic behavior. Science 302, 1227–1231 (2003).
Yamauchi, H. et al. The cell competition-based high-throughput screening identifies small compounds that promote the elimination of RasV12-transformed cells from epithelia. Sci. Rep. 5, 15336 (2015).
Madan, E. et al. Flower isoforms promote competitive growth in cancer. Nature 572, 260-264 (2019).
Porazinski, S. et al. EphA2 drives the segregation of ras-transformed epithelial cells from normal neighbors. Curr. Biol. 26, 3220–3229 (2016).
Acknowledgements
The authors thank the reviewers for their constructive and thorough reviews that greatly helped to shape the manuscript. The authors thank P. Langton for critical reading and Y. Fujita for constructive criticism of the manuscript. The authors apologize for any studies that they were unable to cite owing to space limitations. Work in the Piddini laboratory is funded by a Wellcome Trust Senior Research Fellowship (grant 205010/Z/16/Z), by a Programme Foundation Award from Cancer Research UK (grant C38607/A26831) and by a start-up fund from the University of Bristol.
Author information
Authors and Affiliations
Contributions
E.P. conceived the ideas for this article and structured the manuscript. E.P. and M.V. wrote the manuscript. E.P. and M.V. conceived the figures. M.V. designed the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks T. Igaki, G. Morata and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vishwakarma, M., Piddini, E. Outcompeting cancer. Nat Rev Cancer 20, 187–198 (2020). https://doi.org/10.1038/s41568-019-0231-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-019-0231-8
This article is cited by
-
Cell competition and cancer from Drosophila to mammals
Oncogenesis (2024)
-
Expected and unexpected effects after systemic inhibition of Hippo transcriptional output in cancer
Nature Communications (2024)
-
Clinical-mediated discovery of pyroptosis in CD8+ T cell and NK cell reveals melanoma heterogeneity by single-cell and bulk sequence
Cell Death & Disease (2023)
-
Cell competition in development, homeostasis and cancer
Nature Reviews Molecular Cell Biology (2023)
-
Reprogramming tumour-associated macrophages to outcompete cancer cells
Nature (2023)