Abstract
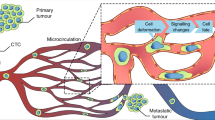
Metastasis is a dynamic succession of events involving the dissemination of tumour cells to distant sites within the body, ultimately reducing the survival of patients with cancer. To colonize distant organs and, therefore, systemically disseminate within the organism, cancer cells and associated factors exploit several bodily fluid systems, which provide a natural transportation route. Indeed, the flow mechanics of the blood and lymphatic circulatory systems can be co-opted to improve the efficiency of cancer cell transit from the primary tumour, extravasation and metastatic seeding. Flow rates, vessel size and shear stress can all influence the survival of cancer cells in the circulation and control organotropic seeding patterns. Thus, in addition to using these fluids as a means to travel throughout the body, cancer cells exploit the underlying physical forces within these fluids to successfully seed distant metastases. In this Review, we describe how circulating tumour cells and tumour-associated factors leverage bodily fluids, their underlying forces and imposed stresses during metastasis. As the contribution of bodily fluids and their mechanics raises interesting questions about the biology of the metastatic cascade, an improved understanding of this process might provide a new avenue for targeting cancer cells in transit.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Massagué, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Martin, J. D., Seano, G. & Jain, R. K. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu. Rev. Physiol. 81, 505–534 (2019).
Mohammadi, H. & Sahai, E. Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol. 20, 766 (2018).
Northey, J. J., Przybyla, L. & Weaver, V. M. Tissue force programs cell fate and tumor aggression. Cancer Discov. 7, 1224–1237 (2017).
Wirtz, D., Konstantopoulos, K. & Searson, P. C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011). This review provides one of the first important discussions of how mechanical forces control interactions between cancer cells and their microenvironment, including blood flow, and how such forces are essential to the metastatic process.
Koumoutsakos, P., Pivkin, I. & Milde, F. The fluid mechanics of cancer and its therapy. Annu. Rev. Fluid Mech. 45, 325–355 (2013).
Swartz, M. A. & Lund, A. W. Lymphatic and interstitial flow in the tumour microenvironment: linking mechanobiology with immunity. Nat. Rev. Cancer 12, 210–219 (2012).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Brown, M. et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science 359, 1408–1411 (2018). This paper shows that tumour cells experimentally delivered to lymph nodes can efficiently disseminate by invading local blood vessels; lymph node blood vessels are, therefore, also used by cancer cells for efficient dissemination to distant organs.
Naxerova, K. et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science 357, 55–60 (2017).
Pereira, E. R. et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science 359, 1403–1407 (2018). This paper uses photoconversion of tumour cells to track the fate of metastatic cells located in the lymph nodes, demonstrating that such cells efficiently leave lymph nodes via local blood vessels to seed distant organs.
Weiss, L., Bronk, J., Pickren, J. W. & Lane, W. W. Metastatic patterns and target organ arterial blood flow. Invasion Metastasis 1, 126–135 (1981). This study compares the metastatic rate of eight target organs with their arterial blood flow, in the context of colorectal cancer and oesophageal squamous cell carcinoma; the authors observed that the frequency of organ metastasis correlated with blood flow.
Headley, M. B. et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature 531, 513–517 (2016). Using intravital imaging of metastatic dissemination to the lung, this study reveals that shear flow fragments CTCs, thereby generating immune-interacting intermediates that define the efficiency of metastatic cell seeding.
Follain, G. et al. Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev. Cell 45, 33–52.e12 (2018). This paper provides the first demonstration that metastatic dissemination occurs in vascular regions with permissive flow profiles, and further shows that metastatic extravasation is facilitated by endothelial remodelling, which is dependent on blood flow forces.
Broggi, M. A. S. et al. Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients. J. Exp. Med. 216, 1091–1107 (2019).
García-Silva, S. et al. Use of extracellular vesicles from lymphatic drainage as surrogate markers of melanoma progression and BRAF V600E mutation. J. Exp. Med. 216, 1061–1070 (2019).
Bessonov, N., Sequeira, A., Simakov, S., Vassilevskii, Y. & Volpert, V. Methods of blood flow modelling. Math. Model. Nat. Phenom. 11, 1–25 (2016).
Freund, J. B. Numerical simulation of flowing blood cells. Annu. Rev. Fluid Mech. 46, 67–95 (2014).
Dixon, J. B. et al. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation 13, 597–610 (2006).
Peng, S.-L., Shih, C.-T., Huang, C.-W., Chiu, S.-C. & Shen, W.-C. Optimized analysis of blood flow and wall shear stress in the common carotid artery of rat model by phase-contrast MRI. Sci. Rep. 7, 5253 (2017).
Reneman, R. S. & Hoeks, A. P. G. Wall shear stress as measured in vivo: consequences for the design of the arterial system. Med. Biol. Eng. Comput. 46, 499–507 (2008).
Stylianopoulos, T., Munn, L. L. & Jain, R. K. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: from mathematical modeling to bench to bedside. Trends Cancer 4, 292–319 (2018).
Levick, J. R. & Michel, C. C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 87, 198–210 (2010).
Darcy, H. Les fontaines publiques de la ville de Dijon. Exposition et application des principes à suivre et des formules à employer dans les questions de distribution d’eau: ouvrage terminé par un appendice relatif aux fournitures d’eau de plusieurs villes au filtrage des eaux et à la fabrication des tuyaux de fonte, de plomb, de tole et de bitume. (Dalmont, 1856).
De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017).
Stacker, S. A. et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 14, 159–172 (2014).
Torcellan, T. et al. In vivo photolabeling of tumor-infiltrating cells reveals highly regulated egress of T-cell subsets from tumors. Proc. Natl Acad. Sci. USA 114, 5677–5682 (2017).
Baish, J. W., Netti, P. A. & Jain, R. K. Transmural coupling of fluid flow in microcirculatory network and interstitium in tumors. Microvasc. Res. 53, 128–141 (1997).
Jain, R. K. Delivery of molecular medicine to solid tumors: lessons from in vivo imaging of gene expression and function. J. Control. Release 74, 7–25 (2001).
Liu, L. J. & Schlesinger, M. Interstitial hydraulic conductivity and interstitial fluid pressure for avascular or poorly vascularized tumors. J. Theor. Biol. 380, 1–8 (2015).
Roose, T., Netti, P. A., Munn, L. L., Boucher, Y. & Jain, R. K. Solid stress generated by spheroid growth estimated using a linear poroelasticity model. Microvasc. Res. 66, 204–212 (2003).
Sefidgar, M., Soltani, M., Raahemifar, K. & Bazmara, H. Effect of fluid friction on interstitial fluid flow coupled with blood flow through solid tumor microvascular network. Comput. Math. Methods Med. 2015, 673426 (2015).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Stylianopoulos, T. et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl Acad. Sci. USA 109, 15101–15108 (2012).
Milosevic, M. et al. Interstitial permeability and elasticity in human cervix cancer. Microvasc. Res. 75, 381–390 (2008).
Baxter, L. T. & Jain, R. K. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc. Res. 37, 77–104 (1989).
Chary, S. R. & Jain, R. K. Direct measurement of interstitial convection and diffusion of albumin in normal and neoplastic tissues by fluorescence photobleaching. Proc. Natl Acad. Sci. USA. 86, 5385–5389 (1989).
Jain, R. K. Transport of molecules, particles, and cells in solid tumors. Annu. Rev. Biomed. Eng. 1, 241–263 (1999).
Swartz, M. A. & Fleury, M. E. Interstitial flow and its effects in soft tissues. Annu. Rev. Biomed. Eng. 9, 229–256 (2007).
Stylianopoulos, T. The solid mechanics of cancer and strategies for improved therapy. J. Biomech. Eng. 139, 021004 (2017).
Cornelison, R. C., Brennan, C. E., Kingsmore, K. M. & Munson, J. M. Convective forces increase CXCR4-dependent glioblastoma cell invasion in GL261 murine model. Sci. Rep. 8, 17057 (2018).
Huang, Y. L., Tung, C., Zheng, A., Kim, B. J. & Wu, M. Interstitial flows promote amoeboid over mesenchymal motility of breast cancer cells revealed by a three dimensional microfluidic model. Integr. Biol. 7, 1402–1411 (2015).
Harney, A. S. et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by Tie2hi macrophage-derived VEGFA. Cancer Discov. 5, 932–943 (2015).
Li, R. et al. Interstitial flow promotes macrophage polarization toward an M2 phenotype. Mol. Biol. Cell 29, 1927–1940 (2018).
Arwert, E. N. et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 23, 1239–1248 (2018).
Pisano, M., Triacca, V., Barbee, K. A. & Swartz, M. A. An in vitro model of the tumor–lymphatic microenvironment with simultaneous transendothelial and luminal flows reveals mechanisms of flow enhanced invasion. Integr. Biol. 7, 525–533 (2015).
Sänger, N. et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer 129, 2522–2526 (2011).
Hu, Z. et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 51, 1113–1122 (2019).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Hüsemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Harper, K. L. et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592 (2016).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014). This paper shows that CTCs can be found as clusters in the bloodstream that display increased metastatic potential, and further shows that plakoglobin mediates intravascular cluster formation through intercellular adhesion.
Nathanson, S. D. Insights into the mechanisms of lymph node metastasis. Cancer 98, 413–423 (2003).
Wong, S. Y. & Hynes, R. O. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle 5, 812–817 (2006).
Jones, D., Pereira, E. R. & Padera, T. P. Growth and immune evasion of lymph node metastasis. Front. Oncol. 8, 36 (2018).
Faries, M. B. et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N. Engl. J. Med. 376, 2211–2222 (2017).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Cox, T. R. et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 (2015).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Peinado, H. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891 (2012).
Zomer, A. et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 (2015). This study uses intravital imaging to track the local and distant dissemination of EVs, demonstrating that their uptake can shape metastatic fitness.
Weber, M. et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339, 328–332 (2013).
Moravec, R., Divi, R. & Verma, M. Detecting circulating tumor material and digital pathology imaging during pancreatic cancer progression. World J. Gastrointest. Oncol. 9, 235–250 (2017).
Pantel, K. & Alix-Panabières, C. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat. Rev. Clin. Oncol. 16, 409–424 (2019).
Morishita, M. et al. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin–lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J. Pharm. Sci. 104, 705–713 (2015).
Saunderson, S. C., Dunn, A. C., Crocker, P. R. & McLellan, A. D. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 123, 208–216 (2014).
Diehl, F. et al. Circulating mutant DNA to assess tumor dynamics. Nat. Med. 14, 985–990 (2008).
Imai, T. et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 4, 26238 (2015).
Hyenne, V. et al. Studying the fate of tumor extracellular vesicles at high spatiotemporal resolution using the zebrafish embryo. Dev. Cell 48, 554–572.e7 (2019).
Chennakrishnaiah, S. et al. Leukocytes as a reservoir of circulating oncogenic DNA and regulatory targets of tumor-derived extracellular vesicles. J. Thromb. Haemost. 16, 1800–1813 (2018).
Toy, R., Hayden, E., Shoup, C., Baskaran, H. & Karathanasis, E. The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 22, 115101 (2011).
Müller, K., Fedosov, D. A. & Gompper, G. Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci. Rep. 4, 4871 (2014).
Zhang, H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332–343 (2018).
Di Vizio, D. et al. Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res. 69, 5601–5609 (2009).
De Jong, W. H. et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials 29, 1912–1919 (2008).
Regmi, S., Fu, A. & Luo, K. Q. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci. Rep. 7, 39975 (2017).
Srinivasan, S., Vannberg, F. O. & Dixon, J. B. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Sci. Rep. 6, 24436 (2016).
Hood, J. L., San, R. S. & Wickline, S. A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801 (2011).
Pucci, F. et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle–B cell interactions. Science 352, 242–246 (2016).
Moalli, F. et al. Intravital and whole-organ imaging reveals capture of melanoma-derived antigen by lymph node subcapsular macrophages leading to widespread deposition on follicular dendritic cells. Front. Immunol 6, 114 (2015).
Miyamura, Y. et al. Drainage of tumor-derived DNA into sentinel lymph nodes in breast cancer patients. Pathol. Oncol. Res. 25, 1635–1643 (2019).
Olmeda, D. et al. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature 546, 676–680 (2017).
Liu, Y. & Cao, X. Characteristics and significance of the pre-metastatic niche. Cancer Cell 30, 668–681 (2016).
Zhou, W. et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 25, 501–515 (2014).
Treps, L. et al. Extracellular vesicle-transported Semaphorin3A promotes vascular permeability in glioblastoma. Oncogene 35, 2615–2623 (2016).
Tominaga, N. et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood–brain barrier. Nat. Commun. 6, 6716 (2015).
Verweij, F. J. et al. Live tracking of inter-organ communication by endogenous exosomes in vivo. Dev. Cell 48, 573–589.e4 (2019).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Metastasis: dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Anton, H. et al. Pulse propagation by a capacitive mechanism drives embryonic blood flow. Development 140, 4426–4434 (2013).
Ku, D. N. Blood flow in arteries. Annu. Rev. Fluid Mech. 29, 399–434 (1997).
Tanaka, T. et al. Inertial migration of cancer cells in blood flow in microchannels. Biomed. Microdevices 14, 25–33 (2012).
Jolly, M. K. et al. Inflammatory breast cancer: a model for investigating cluster-based dissemination. NPJ Breast Cancer 3, 21 (2017).
Rejniak, K. A. Circulating tumor cells: when a solid tumor meets a fluid microenvironment. Adv. Exp. Med. Biol. 936, 93–106 (2016).
Anderson, K. J., de Guillebon, A., Hughes, A. D., Wang, W. & King, M. R. Effect of circulating tumor cell aggregate configuration on hemodynamic transport and wall contact. Math. Biosci. 294, 181–194 (2017).
Hong, Y., Fang, F. & Zhang, Q. Circulating tumor cell clusters: what we know and what we expect [Review]. Int. J. Oncol. 49, 2206–2216 (2016).
Gassmann, P., Hemping-Bovenkerk, A., Mees, S. T. & Haier, J. Metastatic tumor cell arrest in the liver–lumen occlusion and specific adhesion are not exclusive. Int. J. Colorectal Dis. 24, 851–858 (2009).
Kienast, Y. et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116–122 (2010). This paper uses longitudinal real-time intravital imaging to provide a clear description of intravascular steps during brain metastasis formation; the authors show that arrest of CTCs at vascular branch points of microvessels is a key step in metastatic progression, offering a very useful in vivo approach for tracking intravascular behaviour of CTCs.
Mitchell, M. J. & King, M. R. Computational and experimental models of cancer cell response to fluid shear stress. Front. Oncol. 3, 44 (2013).
Prothero, J. & Burton, A. C. The physics of blood flow in capillaries. Biophys. J. 7, 565–579 (1961).
Tawhai, M. H. & Burrowes, K. S. Modelling pulmonary blood flow. Respir. Physiol. Neurobiol. 163, 150–157 (2008).
Mauroy, B. Following red blood cells in a pulmonary capillary. ESAIM. Proceedings 23, 48–65 (2008).
Rejniak, K. A. Investigating dynamical deformations of tumor cells in circulation: predictions from a theoretical model. Front. Oncol. 2, 111 (2012).
King, M. R. et al. A physical sciences network characterization of circulating tumor cell aggregate transport. Am. J. Physiol. Cell Physiol. 308, C792–C802 (2015).
Takeishi, N., Imai, Y., Yamaguchi, T. & Ishikawa, T. Flow of a circulating tumor cell and red blood cells in microvessels. Phys. Rev. E. Stat. Nonlin. Soft Matter Phys. 92, 063011 (2015).
Phillips, K. G. et al. The thrombotic potential of circulating tumor microemboli: computational modeling of circulating tumor cell-induced coagulation. Am. J. Physiol. Cell Physiol. 308, C229–C236 (2015).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency. Am. J. Pathol. 153, 865–873 (1998).
Cameron, M. D. et al. Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res. 60, 2541–2546 (2000).
van der Weyden, L. et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature 541, 233–236 (2017).
Basnet, H. et al. Flura-seq identifies organ-specific metabolic adaptations during early metastatic colonization. eLife 8, e43627 (2019).
Chang, S.-F. et al. Tumor cell cycle arrest induced by shear stress: roles of integrins and Smad. Proc. Natl Acad. Sci. USA 105, 3927–3932 (2008).
Regmi, S., Fung, T. S., Lim, S. & Luo, K. Q. Fluidic shear stress increases the anti-cancer effects of ROS-generating drugs in circulating tumor cells. Breast Cancer Res. Treat. 172, 297–312 (2018).
Mitchell, M. J. et al. Lamin A/C deficiency reduces circulating tumor cell resistance to fluid shear stress. Am. J. Physiol. Cell Physiol. 309, C736–C746 (2015). This study shows that tumour cells are resistant to shear forces and display reduced apoptosis and necrosis relative to normal cells; the paper further shows that such resistance is mediated by lamin A/C, which acts as a structural component and facilitates survival.
Lien, S.-C. et al. Mechanical regulation of cancer cell apoptosis and autophagy: roles of bone morphogenetic protein receptor, Smad1/5, and p38 MAPK. Biochim. Biophys. Acta 1833, 3124–3133 (2013).
Mitchell, M. J. & King, M. R. Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. New J. Phys. 15, 015008 (2013).
Barnes, J. M., Nauseef, J. T. & Henry, M. D. Resistance to fluid shear stress is a conserved biophysical property of malignant cells. PLOS ONE 7, e50973 (2012).
Terasaki, M., Miyake, K. & McNeil, P. L. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle–vesicle fusion events. J. Cell Biol. 139, 63–74 (1997).
Lammerding, J. et al. Lamins A and C but not Lamin B1 regulate nuclear mechanics. J. Biol. Chem. 281, 25768–25780 (2006).
Woroniuk, A. et al. STEF/TIAM2-mediated Rac1 activity at the nuclear envelope regulates the perinuclear actin cap. Nat. Commun. 9, 2124 (2018).
Gong, C. et al. Potentiated DNA damage response in circulating breast tumor cells confers resistance to chemotherapy. J Biol. Chem. 290, 14811–14825 (2015).
Zheng, Y. et al. Expression of β-globin by cancer cells promotes cell survival during blood-borne dissemination. Nat. Commun. 8, 14344 (2017).
Fu, A. et al. High expression of MnSOD promotes survival of circulating breast cancer cells and increases their resistance to doxorubicin. Oncotarget 7, 50239–50257 (2016).
Piskounova, E. et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 527, 186–191 (2015).
Strilic, B. & Offermanns, S. Intravascular survival and extravasation of tumor cells. Cancer Cell 32, 282–293 (2017).
Hamidi, H. & Ivaska, J. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer 18, 533–548 (2018).
Desgrosellier, J. S. et al. An integrin αvβ3-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat. Med. 15, 1163–1169 (2009).
Alanko, J. et al. Integrin endosomal signalling suppresses anoikis. Nat. Cell Biol. 17, 1412–1421 (2015).
Aslan, B. et al. The ZNF304–integrin axis protects against anoikis in cancer. Nat. Commun. 6, 7351 (2015).
Douma, S. et al. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 430, 1034–1039 (2004).
Sun, B. et al. Midkine promotes hepatocellular carcinoma metastasis by elevating anoikis resistance of circulating tumor cells. Oncotarget 8, 32523–32535 (2017).
Lee, H. J. et al. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 8, 14122 (2017).
Lee, H. J., Ewere, A., Diaz, M. F. & Wenzel, P. L. TAZ responds to fluid shear stress to regulate the cell cycle. Cell Cycle 17, 147–153 (2018).
Derynck, R. & Weinberg, R. A. EMT and cancer: more than meets the eye. Dev. Cell 49, 313–316 (2019).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial–mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Rizvi, I. et al. Flow induces epithelial–mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc. Natl Acad. Sci. USA. 110, E1974–E1983 (2013). This study uses microfluidic approaches to show that flow forces increase EMT of tumour cells and favour their motility and aggressiveness.
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Baccelli, I. et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544 (2013).
Beerling, E. et al. Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep. 14, 2281–2288 (2016).
Erami, Z. et al. Intravital FRAP imaging using an E-cadherin–GFP mouse reveals disease- and drug-dependent dynamic regulation of cell–cell junctions in live tissue. Cell Rep. 14, 152–167 (2016).
Ting, D. T. et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 8, 1905–1918 (2014).
Choi, H. Y. et al. Hydrodynamic shear stress promotes epithelial–mesenchymal transition by downregulating ERK and GSK3β activities. Breast Cancer Res. 21, 6 (2019).
Xin, Y. et al. Mechanics and actomyosin-dependent survival/chemoresistance of suspended tumor cells in shear flow. Biophys. J. 116, 1803–1814 (2019).
Moazzam, F., DeLano, F. A., Zweifach, B. W. & Schmid-Schönbein, G. W. The leukocyte response to fluid stress. Proc. Natl Acad. Sci. USA 94, 5338–5343 (1997).
Guido, S. & Tomaiuolo, G. Microconfined flow behavior of red blood cells in vitro. Comptes Rendus Phys. 10, 751–763 (2009).
Xiao, L. L., Liu, Y., Chen, S. & Fu, B. M. Effects of flowing RBCs on adhesion of a circulating tumor cell in microvessels. Biomech. Model. Mechanobiol. 16, 597–610 (2017).
Padmanaban, V. et al. E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573, 439–444 (2019).
Zhuang, X. & Long, E. O. CD28 homolog is a strong activator of natural killer cells for lysis of B7H7+ tumor cells. Cancer Immunol. Res. 7, 939–951 (2019).
Cheung, K. J. et al. Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc. Natl Acad. Sci. USA 113, E854–E863 (2016).
Liu, X. et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient-derived breast cancer models. Cancer Discov. 9, 96–113 (2019).
Chaffer, C. L. et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell 154, 61–74 (2013).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Au, S. H. et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl Acad. Sci. USA 113, 4947–4952 (2016). This paper shows that clusters of CTCs can successfully flow through vessel constrictions; they reduce their hydrodynamic resistance by reorganizing into single-file chain-like clusters.
Ao, Z. et al. Identification of cancer associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res. 75, 4681–4687 (2015).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010).
McCarroll, J. A. et al. Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front. Physiol. 5, 141 (2014).
Kärre, K. Express yourself or die: peptides, MHC molecules, and NK cells. Science 267, 978–979 (1995).
Spicer, J. D. et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 72, 3919–3927 (2012).
Szczerba, B. M. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 (2019). This paper shows that clusters of CTCs are accompanied by neutrophils; the presence of neutrophils was associated with cell cycle progression in CTCs within the bloodstream, thereby promoting their metastatic potential.
Strell, C., Lang, K., Niggemann, B., Zaenker, K. S. & Entschladen, F. Surface molecules regulating rolling and adhesion to endothelium of neutrophil granulocytes and MDA-MB-468 breast carcinoma cells and their interaction. Cell. Mol. Life Sci. 64, 3306–3316 (2007).
Chen, M. B. et al. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc. Natl Acad. Sci. USA. 115, 7022–7027 (2018).
Bambace, N. M. & Holmes, C. E. The platelet contribution to cancer progression. J. Thromb. Haemost. 9, 237–249 (2011).
Gasic, G. J., Gasic, T. B. & Stewart, C. C. Antimetastatic effects associated with platelet reduction. Proc. Natl Acad. Sci. USA 61, 46–52 (1968).
Ilkan, Z. et al. Evidence for shear-mediated Ca2+ entry through mechanosensitive cation channels in human platelets and a megakaryocytic cell line. J. Biol. Chem. 292, 9204–9217 (2017).
Labelle, M., Begum, S. & Hynes, R. O. Direct signaling between platelets and cancer cells induces an epithelial–mesenchymal-like transition and promotes metastasis. Cancer Cell 20, 576–590 (2011).
Labelle, M., Begum, S. & Hynes, R. O. Platelets guide the formation of early metastatic niches. Proc. Natl Acad. Sci. USA 111, E3053–E3061 (2014).
Frenette, P. S., Johnson, R. C., Hynes, R. O. & Wagner, D. D. Platelets roll on stimulated endothelium in vivo: an interaction mediated by endothelial P-selectin. Proc. Natl Acad. Sci. USA 92, 7450–7454 (1995).
Reymond, N., d’Água, B. B. & Ridley, A. J. Crossing the endothelial barrier during metastasis. Nat. Rev. Cancer 13, 858–870 (2013).
Haemmerle, M. et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat. Commun. 8, 310 (2017).
Echtler, K. et al. Platelet GPIIb supports initial pulmonary retention but inhibits subsequent proliferation of melanoma cells during hematogenic metastasis. PLOS ONE 12, e0172788 (2017).
Erpenbeck, L., Nieswandt, B., Schön, M., Pozgajova, M. & Schön, M. P. Inhibition of platelet GPIb α and promotion of melanoma metastasis. J. Invest. Dermatol. 130, 576–586 (2010).
Valastyan, S. & Weinberg, R. A. Tumor metastasis: molecular insights and evolving paradigms. Cell 147, 275–292 (2011).
Al-Mehdi, A. B. et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med. 6, 100–102 (2000).
Chen, M. B., Whisler, J. A., Jeon, J. S. & Kamm, R. D. Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integr. Biol. 5, 1262–1271 (2013).
Stoletov, K., Montel, V., Lester, R. D., Gonias, S. L. & Klemke, R. High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proc. Natl Acad. Sci. USA 104, 17406–17411 (2007).
Bell, G. I. A theoretical model for adhesion between cells mediated by multivalent ligands. Cell Biochem. Biophys. 1, 133–147 (1979).
Bell, G. I., Dembo, M. & Bongrand, P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys. J. 45, 1051–1064 (1984).
Marshall, B. T. et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature 423, 190–193 (2003).
Alon, R. & Feigelson, S. W. Chemokine-triggered leukocyte arrest: force-regulated bi-directional integrin activation in quantal adhesive contacts. Curr. Opin. Cell Biol. 24, 670–676 (2012).
Aigner, S. et al. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J. 12, 1241–1251 (1998).
Schaefer, A. & Hordijk, P. L. Cell-stiffness-induced mechanosignaling—a key driver of leukocyte transendothelial migration. J. Cell Sci. 128, 2221–2230 (2015).
Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 15, 692–704 (2015).
Kong, F. et al. Cyclic mechanical reinforcement of integrin–ligand interactions. Mol. Cell 49, 1060–1068 (2013).
Osmani, N. et al. Metastatic tumor cells exploit their adhesion repertoire to counteract shear forces during intravascular arrest. Cell Rep. 28, 2491–2500.e5 (2019).
Liu, T.-L. et al. Observing the cell in its native state: imaging subcellular dynamics in multicellular organisms. Science 360, eaaq1392 (2018).
Stoletov, K. et al. Visualizing extravasation dynamics of metastatic tumor cells. J. Cell Sci. 123, 2332–2341 (2010).
Hiratsuka, S. et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc. Natl Acad. Sci. USA 108, 3725–3730 (2011).
Fan, R. et al. Circulatory shear flow alters the viability and proliferation of circulating colon cancer cells. Sci. Rep. 6, 27073 (2016).
Entenberg, D. et al. A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nat. Methods 15, 73–80 (2018). This study provides methodologies for stably tracking the formation of lung metastases in a mouse model, enabling the accurate tracking of the intravascular arrival of CTCs, extravasation, growth and progression to micrometastases.
Lautscham, L. A. et al. Migration in confined 3D environments is determined by a combination of adhesiveness, nuclear volume, contractility, and cell stiffness. Biophys. J. 109, 900–913 (2015).
Raab, M. et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362 (2016).
Denais, C. M. et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016).
Infante, E. et al. LINC complex–Lis1 interplay controls MT1-MMP matrix digest-on-demand response for confined tumor cell migration. Nat. Commun. 9, 2443 (2018).
Leong, H. S. et al. Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Rep 8, 1558–1570 (2014).
Williams, K. C. et al. Invadopodia are chemosensing protrusions that guide cancer cell extravasation to promote brain tropism in metastasis. Oncogene 38, 3598–3615 (2019).
Warren, S. C. et al. Removing physiological motion from intravital and clinical functional imaging data. eLife 7, e35800 (2018). This study highlights the application of intravital Förster resonance energy transfer imaging to characterize signalling dynamics that might be involved in cancer cell extravasation and arrival at secondary sites; the publication also characterizes a novel software tool that can be used to correct for sample motion, a major challenge commonly encountered in intravital imaging due to animal respiration or heartbeat.
Lapis, K., Paku, S. & Liotta, L. A. Endothelialization of embolized tumor cells during metastasis formation. Clin. Exp. Metastasis 6, 73–89 (1988).
Lam, C. K., Yoo, T., Hiner, B., Liu, Z. & Grutzendler, J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 465, 478–482 (2010).
Ghajar, C. M. et al. The perivascular niche regulates breast tumor dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Carlson, P. et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 (2019).
Heyder, C. et al. Realtime visualization of tumor cell/endothelial cell interactions during transmigration across the endothelial barrier. J. Cancer Res. Clin. Oncol. 128, 533–538 (2002).
Strilic, B. et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536, 215–218 (2016).
Miyamoto, D. T., Ting, D. T., Toner, M., Maheswaran, S. & Haber, D. A. Single-cell analysis of circulating tumor cells as a window into tumor heterogeneity. Cold Spring Harb. Symp. Quant. Biol. 81, 269–274 (2016).
Hu, L., Lee, M., Campbell, W., Perez-Soler, R. & Karpatkin, S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood 104, 2746–2751 (2004).
Goertz, L. et al. Heparins that block VEGF-A-mediated von Willebrand factor fiber generation are potent inhibitors of hematogenous but not lymphatic metastasis. Oncotarget 7, 68527–68545 (2016).
Bauer, A. T. et al. von Willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood 125, 3153–3163 (2015).
Sloan, E. K. et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 70, 7042–7052 (2010).
Le, C. P. et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 7, 10634 (2016).
Kim, T.-H. et al. Cancer cells become less deformable and more invasive with activation of β-adrenergic signaling. J. Cell Sci. 129, 4563–4575 (2016).
Shaashua, L. et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 23, 4651–4661 (2017).
Tanaka, N. et al. Prognonstic impact of renin–angiotensin system blockade in localised upper-tract urothelial carcinoma. Br. J. Cancer 106, 290–296 (2012).
Lever, A. F. et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet 352, 179–184 (1998).
Pinter, M. & Jain, R. K. Targeting the renin–angiotensin system to improve cancer treatment: implications for immunotherapy. Sci. Transl. Med. 9, eaan5616 (2017).
Diop-Frimpong, B., Chauhan, V. P., Krane, S., Boucher, Y. & Jain, R. K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl Acad. Sci. USA 108, 2909–2914 (2011).
Chauhan, V. P. et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516 (2013).
Vennin, C. et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci. Transl. Med. 9, eaai8504 (2017).
Bhowmick, T., Berk, E., Cui, X., Muzykantov, V. R. & Muro, S. Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J. Control. Release 157, 485–492 (2012).
Raghavan, V., Rbaibi, Y., Pastor-Soler, N. M., Carattino, M. D. & Weisz, O. A. Shear stress-dependent regulation of apical endocytosis in renal proximal tubule cells mediated by primary cilia. Proc. Natl Acad. Sci. USA 111, 8506–8511 (2014).
Jain, R. K., Martin, J. D. & Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014).
Pathak, A. P., Artemov, D., Neeman, M. & Bhujwalla, Z. M. Lymph node metastasis in breast cancer xenografts is associated with increased regions of extravascular drain, lymphatic vessel area, and invasive phenotype. Cancer Res. 66, 5151–5158 (2006).
Polacheck, W. J., Charest, J. L. & Kamm, R. D. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl Acad. Sci. USA 108, 11115–11120 (2011).
Polacheck, W. J., German, A. E., Mammoto, A., Ingber, D. E. & Kamm, R. D. Mechanotransduction of fluid stresses governs 3D cell migration. Proc. Natl Acad. Sci. USA 111, 2447–2452 (2014).
Angeli, S. & Stylianopoulos, T. Biphasic modeling of brain tumor biomechanics and response to radiation treatment. J. Biomech. 49, 1524–1531 (2016).
Shi, Z.-D. & Tarbell, J. M. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann. Biomed. Eng. 39, 1608–1619 (2011).
Shields, J. D. et al. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 11, 526–538 (2007).
Qazi, H., Shi, Z.-D. & Tarbell, J. M. Fluid shear stress regulates the invasive potential of glioma cells via modulation of migratory activity and matrix metalloproteinase expression. PLOS ONE 6, e20348 (2011).
Butcher, D. T., Alliston, T. & Weaver, V. M. A tense situation: forcing tumour progression. Nat. Rev. Cancer 9, 108–122 (2009).
Blatter, C. et al. In vivo label-free measurement of lymph flow velocity and volumetric flow rates using Doppler optical coherence tomography. Sci. Rep. 6, 29035 (2016).
Hagendoorn, J. et al. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ. Res. 95, 204–209 (2004).
Rane, S. et al. Clinical feasibility of noninvasive visualization of lymphatic flow with principles of spin labeling MR imaging: implications for lymphedema assessment. Radiology 269, 893–902 (2013).
Northcott, J. M., Dean, I. S., Mouw, J. K. & Weaver, V. M. Feeling stress: the mechanics of cancer progression and aggression. Front. Cell Dev. Biol. 6, 17 (2018).
Dixon, J. B., Gashev, A. A., Zawieja, D. C., Moore, J. E. & Coté, G. L. Image correlation algorithm for measuring lymphocyte velocity and diameter changes in contracting microlymphatics. Ann. Biomed. Eng. 35, 387–396 (2007).
Aird, W. C. Spatial and temporal dynamics of the endothelium. J. Thromb. Haemost. 3, 1392–1406 (2005).
Gray, K. M. & Stroka, K. M. Vascular endothelial cell mechanosensing: new insights gained from biomimetic microfluidic models. Semin. Cell Dev. Biol. 71, 106–117 (2017).
Kamiya, A., Bukhari, R. & Togawa, T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull. Math. Biol. 46, 127–137 (1984).
Aaslid, R., Markwalder, T.-M. & Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 57, 769–774 (1982).
Bishop, C. C., Powell, S., Rutt, D. & Browse, N. L. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 17, 913–915 (1986).
Fronek, A., Coel, M. & Berstein, E. F. Quantitative ultrasonographic studies of lower extremity flow velocities in health and disease. Circulation 53, 957–960 (1976).
Hennerici, M., Rautenberg, W., Sitzer, G. & Schwartz, A. Transcranial Doppler ultrasound for the assessment of intracranial arterial flow velocity—Part 1. Examination technique and normal values. Surg. Neurol. 27, 439–448 (1987).
Reinitz, A., DeStefano, J., Ye, M., Wong, A. D. & Searson, P. C. Human brain microvascular endothelial cells resist elongation due to shear stress. Microvasc. Res. 99, 8–18 (2015).
Segadal, L. & Matre, K. Blood velocity distribution in the human ascending aorta. Circulation 76, 90–100 (1987).
Brookes, M. Arteriolar blockade: a method of measuring blood flow rates in the skeleton. J. Anat. 106, 557–563 (1970).
Ashikawa, K., Kanatsuka, H., Suzuki, T. & Takishima, T. Phasic blood flow velocity pattern in epimyocardial microvessels in the beating canine left ventricle. Circ. Res. 59, 704–711 (1986).
Coffman, J. D. & Lempert, J. A. Venous flow velocity, venous volume and arterial blood flow. Circulation 52, 141–145 (1975).
Briers, J. D. Laser speckle contrast analysis (LASCA): a nonscanning, full-field technique for monitoring capillary blood flow. J. Biomed. Opt. 1, 174 (1996).
Intaglietta, M., Silverman, N. R. & Tompkins, W. R. Capillary flow velocity measurements in vivo and in situ by television methods. Microvasc. Res. 10, 165–179 (1975).
Ivanov, K. P., Kalinina, M. K. & Levkovich, Yu. I. Blood flow velocity in capillaries of brain and muscles and its physiological significance. Microvasc. Res. 22, 143–155 (1981).
Miller, J. S. et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774 (2012).
Grigoryan, B. et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464 (2019).
Dondossola, E. et al. Intravital microscopy of osteolytic progression and therapy response of cancer lesions in the bone. Sci. Transl. Med. 10, eaao5726 (2018).
Price, T. T. et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 8, 340ra73 (2016).
He, W., Wang, H., Hartmann, L. C., Cheng, J.-X. & Low, P. S. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc. Natl Acad. Sci. USA 104, 11760–11765 (2007).
Hodgkinson, C. L. et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat. Med. 20, 897–903 (2014).
Girotti, M. R. et al. Application of sequencing, liquid biopsies, and patient-derived xenografts for personalized medicine in melanoma. Cancer Discov. 6, 286–299 (2016).
Pereira-Veiga, T. et al. CTCs-derived xenograft development in a triple negative breast cancer case. Int. J. Cancer 144, 2254–2265 (2019).
Greystoke, A. et al. Development of a circulating miRNA assay to monitor tumor burden: from mouse to man. Mol. Oncol. 10, 282–291 (2016).
Vishnoi, M. et al. Targeting USP7 identifies a metastasis-competent state within bone marrow-resident melanoma CTCs. Cancer Res. 78, 5349–5362 (2018).
Williamson, S. C. et al. Vasculogenic mimicry in small cell lung cancer. Nat. Commun. 7, 13322 (2016).
Gkountela, S. et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176, 98–112.e14 (2019).
Lee, A. M., Tormoen, G. W., Kanso, E., McCarty, O. J. T. & Newton, P. K. Modeling and simulation of procoagulant circulating tumor cells in flow. Front. Oncol. 2, 108 (2012).
Harlepp, S., Thalmann, F., Follain, G., Goetz, J. G. & Théry, M. Hemodynamic forces can be accurately measured in vivo with optical tweezers. Mol. Biol. Cell 28, 3252–3260 (2017).
Meng, F. & Sachs, F. Visualizing dynamic cytoplasmic forces with a compliance-matched FRET sensor. J. Cell Sci. 124, 261–269 (2011).
Verma, D. et al. Interplay between cytoskeletal stresses and cell adaptation under chronic flow. PLOS ONE 7, e44167 (2012).
Ye, N. et al. Direct observation of α-actinin tension and recruitment at focal adhesions during contact growth. Exp. Cell Res. 327, 57–67 (2014).
Yamashita, S., Tsuboi, T., Ishinabe, N., Kitaguchi, T. & Michiue, T. Wide and high resolution tension measurement using FRET in embryo. Sci. Rep. 6, 28535 (2016).
Conway, D. E. et al. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23, 1024–1030 (2013).
Cai, D. et al. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 157, 1146–1159 (2014).
Stabley, D. R., Jurchenko, C., Marshall, S. S. & Salaita, K. S. Visualizing mechanical tension across membrane receptors with a fluorescent sensor. Nat. Methods 9, 64–67 (2012).
Arsenovic, P. T. & Conway, D. E. in The LINC Complex (eds Gundersen, G. G. & Worman, H. J.) 1840, 59–71 (Springer, 2018).
Arsenovic, P. T. et al. Nesprin-2G, a component of the nuclear LINC complex, is subject to myosin-dependent rension. Biophys. J. 110, 34–43 (2016).
Arsenovic, P. T., Bathula, K. & Conway, D. E. A protocol for using Förster resonance energy transfer (FRET)-force biosensors to measure mechanical forces across the nuclear LINC complex. J. Vis. Exp. https://doi.org/10.3791/54902 (2017).
Seong, J. et al. Visualization of src activity at different compartments of the plasma membrane by FRET imaging. Chem. Biol. 16, 48–57 (2009).
Seong, J. et al. Detection of focal adhesion kinase activation at membrane microdomains by fluorescence resonance energy transfer. Nat. Commun. 2, 406 (2011).
Itoh, R. E. et al. Activation of Rac and Cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 22, 6582–6591 (2002).
Yoshizaki, H. et al. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. J. Cell Biol. 162, 223–232 (2003).
Johnsson, A.-K. E. et al. The rac-FRET mouse reveals tight spatiotemporal control of rac activity in primary cells and tissues. Cell Rep. 6, 1153–1164 (2014).
Nobis, M. et al. A rhoA-FRET biosensor mouse for intravital imaging in normal tissue homeostasis and disease contexts. Cell Rep. 21, 274–288 (2017).
Antonacci, G. & Braakman, S. Biomechanics of subcellular structures by non-invasive Brillouin microscopy. Sci. Rep. 6, 37217 (2016).
Palombo, F. et al. Biomechanics of fibrous proteins of the extracellular matrix studied by Brillouin scattering. J. R. Soc. Interface 11, 20140739 (2014).
Palombo, F., Madami, M., Stone, N. & Fioretto, D. Mechanical mapping with chemical specificity by confocal Brillouin and Raman microscopy. Analyst 139, 729–733 (2014).
Acknowledgements
D.H. acknowledges support from the Cancer Institute of New South Wales (CINSW) Fellowship and project grants from the National Breast Cancer Foundation (NBCF), St. Vincent’s Clinic Foundation and Sydney Catalyst (the Translational Cancer Research Centre of central Sydney and regional New South Wales). S.W. acknowledges support from National Health and Medical Research Council (NHMRC) Project Grants and Suttons. P.T. acknowledges support from an NHMRC fellowship, NHMRC project grants and a Len Ainsworth Pancreatic Cancer Fellowship. This project was made possible by an Avner Pancreatic Cancer Foundation Grant. The authors thank all members of the Goetz Lab for helpful discussions. Work related to this Review in the Goetz Lab (G.F., N.O., V.H., S.H. and J.G.G.) is funded by the French National Cancer Institute (INCa) and Plan Cancer, and by institutional funds from INSERM and the University of Strasbourg. N.O. acknowledges support from Plan Cancer 2014–2019 (OptoMetaTrap) and the Association pour la Recherche Contre le Cancer (ARC). G.F. is supported by La Ligue Contre le Cancer and the University of Strasbourg.
Author information
Authors and Affiliations
Contributions
G.F., D.H., N.O., S.H., V.H., P.T. and J.G.G. researched data for the article. All authors made substantial contributions to the discussion of content, wrote the manuscript and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review informationNature Reviews Cancer thanks D. Wirtz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Fluids
-
Substances devoid of rigidity that continuously deform and do not resist shear stress applied to them.
- Interstitial fluid
-
Fluid originating from blood capillaries’ leakiness that is present around cells, bringing nutrients to these cells and flowing towards lymphatic draining sites, giving rise to interstitial fluid flow.
- Circulating tumour cells
-
(CTCs). Tumour cells that have left their primary tumour and entered the blood or lymphatic circulation (intravasation); to survive and to travel throughout the body, they co-opt this new fluid environment that they are exposed to.
- Soluble factors
-
Proteins (including protein ligands or extracellular matrix-modifying proteins) that are secreted by tumour cells to induce the remodelling of distant sites to facilitate metastatic seeding.
- Extracellular vesicles
-
(EVs). Lipid bilayer-delimited particles that serve as cargo for the transfer of nucleic acids, proteins or lipids to distant sites; their membrane composition provides a fingerprint for the targeted priming of specific organs.
- Intravascular arrest
-
The process that precedes cancer cell extravasation, whereby circulating tumour cells become physically occluded in microvessels and/or actively adhere to the inner endothelial lining of blood vessels at secondary sites.
- Pre-metastatic niches
-
(PMNs). A new paradigm for the initiation of metastasis; these are pre-metastatic microenvironments that are seeded and/or educated by tumour-secreted factors, inducing phenotypes that can help secondary tumour development.
- Reynolds number
-
(Re). A dimensionless number proportional to fluid velocity V, density ρ, the displacement length L and the viscosity η. It allows one to classify flow types and to determine whether the fluid flow is laminar or turbulent (Re > 1000).
- Laminar flow
-
In fluid dynamics, fluid particles transported in a laminar flow follow smooth directions in layers, with little or no mixing.
- Viscosity
-
A measure of the resistance of a fluid to deformation at a given rate that quantifies the frictional force generated by layers of fluids that are in motion. Gases, water and other liquids are considered Newtonian fluids because they display a linear correlation between shear stress and the rate of deformation. When fluids display a non-linear relation between stress and the deformation rate, they are considered non-Newtonian — this is the case for blood.
- Turbulent flow
-
In contrast to laminar flow, turbulent flow is characterized by chaotic changes in pressure and flow velocity that create mixing of the flow paths of displaced particles.
- Darcy’s law
-
A physical law that, in the context of cancer progression, is linked to interstitial fluid and to hydraulic conductivity of the environment arising from a convective fluid displacement.
- Hydraulic conductance
-
The capacity of a porous material to allow fluid to cross under the effect of pressure differences.
- Interstitial fluid pressure
-
(IFP). The hydrostatic pressure in the cellular interstice, which, in solid tumours, is often due to vessel leakage and lymphatic drainage.
- Lymphangiogenesis
-
The remodelling of existing lymphatic networks to give rise to new lymphatic vessels.
- Solid stress
-
A stress exerted by the solid constituents of a tissue that accumulate within solid structural components (for example, extracellular matrix and tumour and stromal cells) during tumour growth.
- Convective flow
-
A flow directed towards a gradient; in the case of a solid tumour, this is a pressure gradient.
- Shear stress
-
A pressure that creates deformation when moving tangentially to a surface; for example, shear stress is generated when fluids flow over an endothelial surface.
- Margination
-
A physical process that brings cells or vesicles close to the endothelial wall.
- Exomeres
-
A type of extracellular vesicle that is characterized by small size (~35 nm) and the absence of a membrane; exomeres are enriched in metabolic enzymes and hypoxia-related, microtubule-related and coagulation proteins.
- Oncosomes
-
A subtype of large membrane-derived extracellular vesicles (microvesicles) secreted by cancer cells that transfer oncogenic molecules to other cells.
- Hagen–Poiseuille equation
-
This equation describes the velocity in a laminar regime for a viscous fluid moving in a cylindrical tube as a quadratic distribution.
- Inertia
-
The extent to which cells and other material can maintain their motion against the flow.
- Viscoelasticity
-
This term defines a material that exhibits elastic and viscous behaviour when placed under stress.
- Fluid-structure behaviour
-
This behaviour occurs when the fluid deforms a physical structure, which will, in turn, modify the fluid flow.
- Ramification
-
A process that occurs in the vasculature when a stem vessel is branching out into two smaller-sized vessels.
- Metastable
-
A state of least energy until more external energy is added to the system; this state seems to be stable, although it is, in theory, unstable and capable of changing to a more stable state.
- Thrombocytopenia
-
A condition characterized by low platelet count.
- Bell’s model
-
A statistical model taking into account the chemical rates of formation and dissociation between ligands and receptors as well as the statistical distribution of these ligand–receptor pairs on two cell surfaces when external forces are applied.
- Diapedesis
-
The transmigration process by which circulating cells exit the bloodstream using either the transcellular (through endothelial cells) or the paracellular (between endothelial cells through cell–cell junctions) route.
- Catch bonds
-
Receptor–ligand interactions, the strength and lifetime of which increases under applied force.
- Cytoplasts
-
Nucleus-free portions of cells that are released from arrested circulating tumour cells when facing high shear forces.
- Necroptosis
-
A process that induces cell death through controlled necrosis.
- Endothelial remodelling
-
A process that represents the ability of endothelial cells to sense and respond to stimuli (such as fluid flow, shear stress and trafficking of immune cells) by remodelling their contact adhesion to move towards specific reorganization, leading to flow homeostasis.
- Liquid biopsy
-
The collection of non-solid tissues, such as blood, and screening it for disease-related parameters, such as circulating tumour cells. In contrast to biopsy of solid internal tissues, liquid biopsy is minimally invasive and suited for repeated assessment in the same patient.
Rights and permissions
About this article
Cite this article
Follain, G., Herrmann, D., Harlepp, S. et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat Rev Cancer 20, 107–124 (2020). https://doi.org/10.1038/s41568-019-0221-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-019-0221-x
This article is cited by
-
Linking cell mechanical memory and cancer metastasis
Nature Reviews Cancer (2024)
-
Evidence and therapeutic implications of biomechanically regulated immunosurveillance in cancer and other diseases
Nature Nanotechnology (2024)
-
Experimental measurement and numerical modeling of deformation behavior of breast cancer cells passing through constricted microfluidic channels
Microsystems & Nanoengineering (2024)
-
KLF5 regulates actin remodeling to enhance the metastasis of nasopharyngeal carcinoma
Oncogene (2024)
-
Survival mechanisms of circulating tumor cells and their implications for cancer treatment
Cancer and Metastasis Reviews (2024)