Abstract
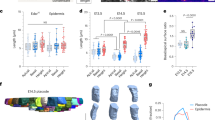
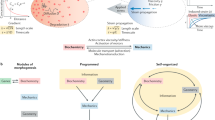
Organ development involves complex shape transformations driven by active mechanical stresses that sculpt the growing tissue1,2. Epithelial gland morphogenesis is a prominent example where cylindrical branches transform into spherical alveoli during growth3,4,5. Here we show that this shape transformation is induced by a local change from anisotropic to isotropic tension within the epithelial cell layer of developing human mammary gland organoids. By combining laser ablation with optical force inference and theoretical analysis, we demonstrate that circumferential tension increases at the expense of axial tension through a reorientation of cells that correlates with the onset of persistent collective rotation around the branch axis. This enables the tissue to locally control the onset of a generalized Rayleigh–Plateau instability, leading to spherical tissue buds6. The interplay between cell motion, cell orientation and tissue tension is a generic principle that may turn out to drive shape transformations in other cell tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Microscopy data that support the findings of this study are available in Zenodo at https://doi.org/10.5281/zenodo.5076123. Source data are provided with this paper. All other relevant data supporting the key findings of this study are available within the article and Supplementary Information or from the corresponding authors upon reasonable request.
Code availability
The code used for analysing the data of this study is available in Zenodo at https://doi.org/10.5281/zenodo.5076123.
References
Petridou, N. I., Grigolon, S., Salbreux, G., Hannezo, E. & Heisenberg, C.-P. Fluidization-mediated tissue spreading by mitotic cell rounding and non-canonical Wnt signalling. Nat. Cell Biol. 21, 169–178 (2019).
Karzbrun, E., Kshirsagar, A., Cohen, S. R., Hanna, J. H. & Reiner, O. Human brain organoids on a chip reveal the physics of folding. Nat. Phys. 14, 515–522 (2018).
Wang, S., Sekiguchi, R., Daley, W. P. & Yamada, K. M. Patterned cell and matrix dynamics in branching morphogenesis. J. Cell Biol. 216, 559–570 (2017).
Linnemann, J. R. et al. Quantification of regenerative potential in primary human mammary epithelial cells. Development 142, 3239–3251 (2015).
Rios, A. C. et al. Intraclonal plasticity in mammary tumors revealed through large-scale single-cell resolution 3D imaging. Cancer Cell 35, 618–632.e6 (2019).
Rayleigh, L. On the instability of jets. Proc. Lond. Math. Soc. s1–10, 4–13 (1878).
Inman, J. L., Robertson, C., Mott, J. D. & Bissell, M. J. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development 142, 1028–1042 (2015).
Bar-Ziv, R., Tlusty, T., Moses, E., Safran, S. A. & Bershadsky, A. Pearling in cells: a clue to understanding cell shape. Proc. Natl Acad. Sci. USA 96, 10140–10145 (1999).
Bar-Ziv, R. & Moses, E. Instability and ‘pearling’ states produced in tubular membranes by competition of curvature and tension. Phys. Rev. Lett. 73, 1392–1395 (1994).
Dong, B., Hannezo, E. & Hayash, S. Balance between apical membrane growth and luminal matrix resistance determines epithelial tubule shape. Cell Rep. 7, 941–950 (2014).
Pérez-González, C. et al. Active wetting of epithelial tissues. Nat. Phys. 15, 79–88 (2019).
Tetley, R. J. et al. Tissue fluidity promotes epithelial wound healing. Nat. Phys. 15, 1195–1203 (2019).
He, B., Doubrovinski, K., Polyakov, O. & Wieschaus, E. Apical constriction drives tissue-scale hydrodynamic flow to mediate cell elongation. Nature 508, 392–396 (2014).
Maître, J.-L. et al. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science 338, 253–256 (2012).
Chatterjee, S. J., Halaoui, R., Deagle, R. C., Rejon, C. & McCaffrey, L. Numb regulates cell tension required for mammary duct elongation. Biol. Open 8, bio042341 (2019).
Parmar, H. & Cunha, G. R. Epithelial–stromal interactions in the mouse and human mammary gland in vivo. Endocr. Relat. Cancer 11, 437–458 (2004).
Buchmann, B. et al. Mechanical plasticity of collagen directs invasive branching morphogenesis in human mammary gland organoids. Nat. Commun. 12, 2759 (2021).
Alcaraz, J. et al. Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening. Integr. Biol. 3, 1153–1166 (2011).
Wisdom, K. M. et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 9, 4144 (2018).
Hall, M. S. et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc. Natl Acad. Sci. USA 113, 14043–14048 (2016).
Lecuit, T., Lenne, P.-F. & Munro, E. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 27, 157–184 (2011).
Brodland, G. W. et al. Video force microscopy reveals the mechanics of ventral furrow in vagination in Drosophila. Proc. Natl Acad. Sci. USA 107, 22111–22116 (2010).
Brodland, G. W. et al. CellFIT: a cellular force-inference toolkit using curvilinear cell boundaries. PLoS ONE 9, 1–15 (2014).
Kong, W. et al. Experimental validation of force inference in epithelia from cell to tissue scale. Sci. Rep. 9, 14647 (2019).
Bischofs, I. B. & Schwarz, U. S. Cell organization in soft media due to active mechanosensing. Proc. Natl Acad. Sci. USA 100, 9274–9279 (2003).
Tanner, K., Mori, H., Mroue, R., Bruni-Cardoso, A. & Bissell, M. J. Coherent angular motion in the establishment of multicellular architecture of glandular tissues. Proc. Natl Acad. Sci. USA 109, 1973–1978 (2012).
Thüroff, F., Goychuk, A., Reiter, M. & Frey, E. Bridging the gap between single-cell migration and collective dynamics. eLife 8, e46842 (2019).
Tambe, D. T. et al. Collective cell guidance by cooperative intercellular forces. Nat. Mater. 10, 469–475 (2011).
Jain, S. et al. The role of single-cell mechanical behaviour and polarity in driving collective cell migration. Nat. Phys. 16, 802–809 (2020).
Segerer, F. J., Thüroff, F., Alberola, A. P., Frey, E. & Rädler, J. O. Emergence and persistence of collective cell migration on small circular micropatterns. Phys. Rev. Lett. 114, 228102 (2015).
Tinevez, J.-Y. et al. Role of cortical tension in bleb growth. Proc. Natl Acad. Sci. USA 106, 18581–18586 (2009).
Villeneuve, C. et al. aPKCi triggers basal extrusion of luminal mammary epithelial cells by tuning contractility and vinculin localization at cell junctions. Proc. Natl Acad. Sci. USA 116, 24108–24114 (2019).
Colombelli, J., Grill, S. W. & Stelzer, E. H. K. Ultraviolet diffraction limited nanosurgery of live biological tissues. Rev. Sci. Instrum. 75, 472–478 (2004).
Edelstein, A. et al. Advanced methods of microscope control using μManager software. J. Biol. Methods 1, e10 (2014).
Bradski, G. The OpenCV library. Dr. Dobb’s J. Software Tools 25, 120–125 (2000).
Acknowledgements
We gratefully acknowledge financial support by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 810104-PoInt) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Project ID 201269156–SFB 1032. A.G. was supported by a DFG fellowship through the Graduate School of Quantitative Biosciences Munich (QBM). We thank C. Gabka from the Nymphenburg Clinic for Plastic and Aesthetic Surgery for providing the primary human mammary gland tissue. We thank L. Ushakov for his aid in implementing the force inference algorithm.
Author information
Authors and Affiliations
Contributions
P.A.F., C.H.S. and A.R.B. designed the research. P.A.F., B.B, L.K.E. and M.K.R. performed the experiments and analysed the data. A.G., P.A.F. and E.F. performed theoretical interpretation of the experiments. P.A.F., A.G., E.F. and A.R.B. wrote the paper. A.R.B., E.F. and C.H.S. supervised the project. All the authors revised and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Physics thanks Sanjay Kumar, Mingming Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12, Table 1 and Discussion.
Supplementary Video 1
Cylindrical branches flow into the organoid body after hydrolysis of the collagen matrix.
Supplementary Video 2
Laser ablation of organoids for branches grown in the attached (left) and floating (right) configurations.
Supplementary Video 3
Laser ablation in the presence of cytochalasin D.
Supplementary Video 4
Representative examples of cell dynamics over one day. All organoids stem from the same donor (M25) and were grown in floating gels. Note that the branch shape strongly correlates with the type of motion: axial translation in cylindrical branches and rotation in nascent and mature alveoli.
Supplementary Video 5
Long-term observation of cell dynamics shows that alveologenesis and collective cell rotation are correlated (donor, M28).
Supplementary Video 6
Addition of HECD-1 antibody against E-cadherin abolishes alveolar rotation within 15–25 h (donor, M25).
Supplementary Video 7
Cell dynamics at ×25 magnification. This experiment corresponds to Supplementary Fig. 4a (donor, M25).
Supplementary Video 8
Cell dynamics at ×25 magnification. This experiment corresponds to Supplementary Fig. 4b (donor, M25).
Source data
Source Data Fig. 1
Statistical source data for Fig. 1.
Source Data Fig. 2
Statistical source data for Fig. 2.
Source Data Fig. 3
Statistical source data for Fig. 3.
Source Data SI
Source data for Supplementary Information.
Rights and permissions
About this article
Cite this article
Fernández, P.A., Buchmann, B., Goychuk, A. et al. Surface-tension-induced budding drives alveologenesis in human mammary gland organoids. Nat. Phys. 17, 1130–1136 (2021). https://doi.org/10.1038/s41567-021-01336-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-021-01336-7
This article is cited by
-
Curvature induces active velocity waves in rotating spherical tissues
Nature Communications (2023)
-
Mechanically enhanced biogenesis of gut spheroids with instability-driven morphomechanics
Nature Communications (2023)
-
Activity-induced polar patterns of filaments gliding on a sphere
Nature Communications (2022)