Abstract
Most physical and other natural systems are complex entities that are composed of a large number of interacting individual elements. It is a surprising fact that they often obey the so-called scaling laws that relate an observable quantity to a measure of the size of the system. Here, we describe the discovery of universal superlinear metabolic scaling laws in human cancers. This dependence underpins increasing tumour aggressiveness, owing to evolutionary dynamics, that leads to an explosive growth as the disease progresses. We validated this dynamic using longitudinal volumetric data of different histologies from large cohorts of patients with cancer. To explain our observations we tested complex, biologically inspired mathematical models that describe the key processes that govern tumour growth. Our models predict that the emergence of superlinear allometric scaling laws is an inherently three-dimensional phenomenon. Moreover, the scaling laws that we identified allowed us to define a set of metabolic metrics with prognostic value, which adds clinical utility to our findings.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data for Figs. 1,2,4 are available for this paper. All other data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
Code availability
The mesoscopic simulator code is available for download from http://matematicas.uclm.es/molab/DiscrSimulator1.zip. Source data are provided with this paper.
References
Kleiber, M. Body size and metabolism. Hilgardia 6, 315–351 (1932).
West, G. Scale: The Universal Laws of Growth Innovation, Sustainability, and the Pace of Life in Organisms, Cities, Economies and Companies (Penguin, 2017).
West, G. B., Brown, J. H. & Enquist, B. J. A general model for the origin of allometric scaling laws in biology. Science 276, 122–126 (1997).
Savage, V. M. et al. The predominance of quarter-power scaling in biology. Funct. Ecol. 18, 257–282 (2004).
Reich, P. B., Tjoelker, M. G., Machado, J. L. & Oleksyn, J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 439, 457–461 (2006).
Enquist, B. J. et al. Biological scaling: does the exception prove the rule? Nature 445, E9–10 (2007).
Guiot, C., Degiorgis, P. G., Delsanto, P. P., Gabriele, P. & Deisboeck, T. S. Does tumour growth follow a universal law? J. Theor. Biol. 225, 147–151 (2003).
Herman, A. B., Savage, V. M. & West, G. B. A quantitative theory of solid tumor growth, metabolic rate and vascularization. PLoS ONE 6, e22973 (2011).
Milotti, E., Vyshemirsky, V., Sega, M., Stella, S. & Chignola, R. Metabolic scaling in solid tumours. Sci. Rep. 3, 1938 (2013).
Palm, W. & Thompson, C. B. Nutrient acquisition strategies in mammalian cells. Nature 546, 234–242 (2017).
Zhu, J. & Thompson, C. B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 20, 436–450 (2019).
De Grado, T. R., Reiman, R. E., Price, D. T., Wang, S. & Coleman, R. E. Pharmacokinetics and radiation dosimetry of 18F-fluorocholine. J. Nucl. Med. 43, 92–96 (2002).
Barwick, T., Bencherif, B., Mountz, J. M. & Avril, N. Molecular PET and PET/CT imaging of tumour cell proliferation using F-18 fluoro-L-thymidine: a comprehensive evaluation. Nucl. Med. Commun. 30, 908–917 (2009).
DeLong, J. P., Okie, J. G., Moses, M. E., Sibly, R. M. & Brown, J. H. Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proc. Natl Acad. Sci. USA 107, 12941–12945 (2010).
De Palma, M., Biziato, D. & Petrova, T. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017).
West, G. B., Brown, J. H. & Enquist, B. J. A general model for ontogenetic growth. Nature 413, 628–631 (2001).
Gerlee, P. The model muddle: in search of tumor growth laws. Cancer Res. 73, 2407–2411 (2013).
Rodriguez-Brenes, I. A., Komarova, N. L. & Wodarz, D. Tumor growth dynamics: insights into evolutionary processes. Trends Ecol. Evol. 28, 597–604 (2013).
Benzekry, S. et al. Classical mathematical models for description and prediction of experimental tumour growth. PLoS Comput. Biol. 10, e1003800 (2014).
Talkington, A. & Durret, R. Estimating tumour growth rates in vivo. Bull. Math. Biol. 77, 1934–1954 (2015).
Gengenbacher, N., Singhal, M. & Augustin, H. G. Preclinical mouse solid tumour models: status quo, challenges and perspectives. Nat. Rev. Cancer 17, 751–765 (2017).
Mandonnet, E. et al. Continuous growth of mean tumour diameter in a subset of grade II gliomas. Ann. Neurol. 53, 524–528 (2003).
Van Havenbergh, T., Carvalho, G., Tatagiba, M., Plets, C. & Samii, M. Natural history of petroclival meningiomas. Neurosurgery 52, 55–64 (2003).
Heesterman, B. L. et al. Mathematical models for tumour growth and the reduction of overtreatment. J. Neurol. Surg. B 80, 72–78 (2019).
Nguyen, D. X. et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138, 51–62 (2009).
Gargini, R. et al. The IDH-TAU-EGFR triad defines the neovascular landscape of diffuse gliomas. Sci. Transl. Med. 12, eaax1501 (2020).
Hallastschek, O. & Fisher, D. S. Acceleration of evolutionary spread by long-range dispersal. Proc. Natl Acad. Sci. USA 111, E4911–E4919 (2014).
Deforet, M., Carmona-Fontaine, C., Korolev, K. S. & Xavier, J. B. Evolution at the edge of expanding populations. Am. Nat. 194, 291–305 (2019).
Waclaw, B. et al. A spatial model predicts that dispersal and cell turnover limit intratumour heterogeneity. Nature 525, 261–264 (2015).
Komarova, N. L. Spatial interactions and cooperation can change the speed of evolution of complex phenotypes. Proc. Natl Acad. Sci. USA 111, 10789–10795 (2014).
Durrett, R., Foo, J., Leder, K., Mayberry, J. & Michor, F. Evolutionary dynamics of tumor progression with random fitness values. Theor. Popul. Biol. 78, 54–66 (2010).
McFarland, C. D., Mirny, L. A. & Korolev, K. S. Tug-of-war between driver and passenger mutations in cancer and other adaptive processes. Proc. Natl Acad. Sci. USA 111, 15138–15143 (2014).
Robertson-Tessi, M., Gillies, R. J., Gatenby, R. A. & Anderson, A. R. Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res. 75, 1567–1579 (2015).
Clark, K. et al. The cancer imaging archive (TCIA): maintaining and operating a public information repository. J. Dig. Imag. 26, 1045–1057 (2013).
Vallieres, M. et al. Radiomics strategies for risk assessment of tumour failure in head-and-neck cancer. Sci. Rep. 7, 10117 (2017).
Kostakoglu, L. et al. A phase II study of 3′-Deoxy-3′-18F-Fluorothymidine PET in the assessment of early response of breast cancer to neoadjuvant chemotherapy: results from ACRIN 6688. J. Nucl. Med. 56, 1681–1689 (2015).
Detterbeck, F. C., Boffa, D. J., Kim, A. W. & Tanoue, L. T. The eighth edition lung cancer stage classification. Chest 151, 193–203 (2017).
Pérez-Beteta, J. et al. Tumour surface regularity at MR imaging predicts survival and response to surgery in patients with glioblastoma. Radiology 288, 218–225 (2018).
Pallud, J. et al. Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Ann. Neurol. 60, 380–383 (2006).
Valiente, M. et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014).
Harrell, F. E. et al. Evaluating the yield of medical tests. JAMA 247, 2543–2546 (1982).
Acknowledgements
This research has been supported by the James S. McDonnell Foundation 21st Century Science Initiative in Mathematical and Complex Systems Approaches for Brain Cancer (collaborative awards 220020560 and 220020450), Ministerio de Economía y Competitividad/FEDER, Spain (grant no. MTM2015-71200-R), Junta de Comunidades de Castilla-La Mancha (grant no. SBPLY/17/180501/000154). Research in the Brain Metastasis Group is supported by MINECO grant MINECO-Retos SAF2017-89643-R (M.V.), Bristol-Myers Squibb Melanoma Research Alliance Young Investigator Award 2017 (498103) (M.V.), Beug Foundation’s Prize for Metastasis Research 2017 (M.V.), Fundación Ramón Areces (CIVP19S8163) (M.V.), Worldwide Cancer Research (19-0177) (M.V.), H2020-FETOPEN (828972) (M.V.), Fundació La Marató de tv3 (141), Clinic and Laboratory Integration Program CRI Award 2018 (54545) (M.V.), AECC Coordinated Translational Groups 2017 (GCTRA16015SEOA) (M.V.), LAB AECC 2019 (LABAE19002VALI) (M.V.), La Caixa INPhINIT Fellowship (LCF/BQ/IN17/11620028) (P.G.-G.), La Caixa-Severo Ochoa International PhD Program Fellowship (LCF/BQ/SO16/52270014) (L.Z.). M.V. is a member of the European Molecular Biology Organization Young Investigators programme (4053). We would like to acknowledge J. Cervera and J. C. Peñalver from the IVO Foundation (Valencia, Spain).
Author information
Authors and Affiliations
Contributions
V.M.P.-G. designed the research, collected and processed data, performed the fittings, and developed and simulated the mathematical models. J.J.B. collected data, processed data, performed fitting tasks and developed the automatic segmentation algorithm. J.P.-B. collected and processed data. O.L.-T. collected and processed data and fitted the longitudinal volumetric data. M.V., L.Z., P.G.-G., P.S.-G., E.H.-S.M. and R.H. performed the experiments in the animal models. E.A., P.S., M.M., D.A., A.O.d.M., A.A.R., F.V. and A.F.H.M. collected data. G.A.J.L. collected and processed data. M.P.-C. processed data. G.F.C., J.J.B., J.J., J.B.-B., Y.A. and A.M. developed and simulated the mathematical models. D.M.-G. performed the survival studies. A.M.G.V. designed the research, and collected and processed data. V.M.P.-G. and G.F.C. drafted the manuscript. All of the authors edited and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
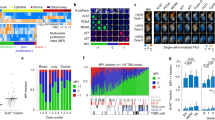
Extended Data Fig. 1 Two human cancer animal models display superlinear growth dynamics.
Two human cancer animal models display superlinear growth dynamics. Group 1 (G1) data correspond to untreated nude mice injected with the human lung adenocarcinoma brain tropic model H2030-BrM (see methods). Group data (G2) correspond to primary glioma cells (L0627) expressing the luciferase reporter gene injected into the brain of nude mice (see methods). Bioluminiscence images for G1 for some mice are shown in panel A. Total tumour mass growth curves for G1 showed superlinear dynamics with best fitting exponent β = 1.25 (for G2 it was β = 1.3). (B, upper panel). Errors relative to best fit were found to be substantially smaller with the optimal superlinear fits than for both the linear and sublinear fits (exponents 1 and 0.75 respectively) (B, lower panel).
Supplementary information
Supplementary Information
Supplementary text, figures, tables and references.
Source data
Source Data Fig. 1
Data of TLA versus MTV for the different tumour histologies considered.
Source Data Fig. 2
Data of tumour volume versus time for the different patient groups and tumour histologies shown in the figure.
Source Data Fig. 4
Survival data for the different groups and tumour histologies shown in the figure.
Rights and permissions
About this article
Cite this article
Pérez-García, V.M., Calvo, G.F., Bosque, J.J. et al. Universal scaling laws rule explosive growth in human cancers. Nat. Phys. 16, 1232–1237 (2020). https://doi.org/10.1038/s41567-020-0978-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-020-0978-6
This article is cited by
-
Growth speed of large brain metastases between diagnostic and radiosurgical planning MRI and predictors of rapid tumor growth
Japanese Journal of Radiology (2024)
-
A Mathematical Model of Stroma-Supported Allometric Tumor Growth
Bulletin of Mathematical Biology (2024)
-
Computational markers for personalized prediction of outcomes in non-small cell lung cancer patients with brain metastases
Clinical & Experimental Metastasis (2024)
-
Knowledge-based mechanistic modeling accurately predicts disease progression with gefitinib in EGFR-mutant lung adenocarcinoma
npj Systems Biology and Applications (2023)
-
Stochastic Fluctuations Drive Non-genetic Evolution of Proliferation in Clonal Cancer Cell Populations
Bulletin of Mathematical Biology (2023)