Abstract
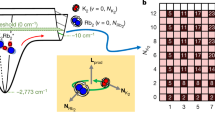
In many chemical reactions, the transformation from reactants to products is mediated by transient intermediate complexes. For gas-phase reactions involving molecules with a few atoms, these complexes typically live on the order of 10 ps or less before dissociating, and are therefore rarely influenced by external processes. Here, we demonstrate that the transient intermediate complex K2Rb2*, formed from collisions between ultracold KRb molecules, undergoes rapid photo-excitation in the presence of a continuous-wave laser source at 1,064 nm, a wavelength commonly used to confine ultracold molecules. These excitations are facilitated by the exceptionally long lifetime of the complex under ultracold conditions. Indeed, by monitoring the change in the complex population after the sudden removal of the excitation light, we directly measure the lifetime of the complex to be 360 ± 30 ns, in agreement with our calculations based on the Rice–Ramsperger–Kassel–Marcus (RRKM) statistical theory. Our results shed light on the origin of the two-body loss widely observed in ultracold molecule experiments. Additionally, the long complex lifetime, coupled with the observed photo-excitation pathway, opens up the possibility to spectroscopically probe the structure of the complex with high resolution, thus elucidating the reaction dynamics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The computer codes used for theoretical calculations in this study are available from T.K. (tijs.karman@cfa.harvard.edu) upon reasonable request.
References
Light, J. C. Statistical theory of bimolecular exchange reactions. Discuss. Faraday Soc. 44, 14–29 (1967).
Herschbach, D. Reactive scattering. Faraday Discuss. Chem. Soc. 55, 233–251 (1973).
Troe, J. The Polanyi Lecture. The colourful world of complex-forming bimolecular reactions. J. Chem. Soc. Faraday Trans. 90, 2303–2317 (1994).
Su, Y.-T., Huang, Y.-H., Witek, H. A. & Lee, Y.-P. Infrared absorption spectrum of the simplest Criegee intermediate CH2OO. Science 340, 174–176 (2013).
Bjork, B. J. et al. Direct frequency comb measurement of OD + CO → DOCO kinetics. Science 354, 444–448 (2016).
Continetti, R. E. & Guo, H. Dynamics of transient species via anion photodetachment. Chem. Soc. Rev. 46, 7650–7667 (2017).
Osborn, D. L. Reaction mechanisms on multiwell potential energy surfaces in combustion (and atmospheric) chemistry. Annu. Rev. Phys. Chem. 68, 233–260 (2017).
Zewail, A. H. Femtochemistry: atomic-scale dynamics of the chemical bond. J. Phys. Chem. A 104, 5660–5694 (2000).
Mayle, M., Quéméner, G., Ruzic, B. P. & Bohn, J. L. Scattering of ultracold molecules in the highly resonant regime. Phys. Rev. A 87, 012709 (2013).
Hu, M.-G. et al. Direct observation of bimolecular reactions of ultracold KRb molecules. Science 366, 1111–1115 (2019).
Quemener, G. & Julienne, P. S. Ultracold molecules under control! Chem. Rev. 112, 4949–5011 (2012).
Balakrishnan, N. Perspective: ultracold molecules and the dawn of cold controlled chemistry. J. Chem. Phys. 145, 150901 (2016).
Lique, F. et al. Cold Chemistry: Molecular Scattering and Reactivity Near Absolute Zero (Royal Society of Chemistry, 2017).
Takekoshi, T. et al. Ultracold dense samples of dipolar RbCs molecules in the rovibrational and hyperfine ground state. Phys. Rev. Lett. 113, 205301 (2014).
Ye, X., Guo, M., González-Martínez, M. L., Quéméner, G. & Wang, D. Collisions of ultracold 23Na87Rb molecules with controlled chemical reactivities. Sci. Adv. 4, eaaq0083 (2018).
Gregory, P. D. et al. Sticky collisions of ultracold RbCs molecules. Nat. Commun. 10, 3104 (2019).
Park, J. W., Will, S. A. & Zwierlein, M. W. Ultracold dipolar gas of fermionic 23Na40K molecules in their absolute ground state. Phys. Rev. Lett. 114, 205302 (2015).
Santos, L., Shlyapnikov, G., Zoller, P. & Lewenstein, M. Bose–Einstein condensation in trapped dipolar gases. Phys. Rev. Lett. 85, 1791–1794 (2000).
Büchler, H. P. et al. Strongly correlated 2D quantum phases with cold polar molecules: controlling the shape of the interaction potential. Phys. Rev. Lett. 98, 060404 (2007).
Levinsen, J., Cooper, N. R. & Shlyapnikov, G. V. Topological px + ipy superfluid phase of fermionic polar molecules. Phys. Rev. A 84, 013603 (2011).
Baranov, M. A., Dalmonte, M., Pupillo, G. & Zoller, P. Condensed matter theory of dipolar quantum gases. Chem. Rev. 112, 5012–5061 (2012).
Christianen, A., Zwierlein, M. W., Groenenboom, G. C. & Karman, T. Photoinduced two-body loss of ultracold molecules. Phys. Rev. Lett. 123, 123402 (2019).
Christianen, A., Karman, T. & Groenenboom, G. C. Quasiclassical method for calculating the density of states of ultracold collision complexes. Phys. Rev. A 100, 032708 (2019).
Levine, R. D. Molecular Reaction Dynamics (Cambridge Univ. Press, 2009).
Liu, Y., Grimes, D. D., Hu, M.-G. & Ni, K.-K. Probing ultracold chemistry using ion spectrometry. Phys. Chem. Chem. Phys. 22, 4861–4874 (2020).
Vadla, C. et al. Comparison of theoretical and experimental red and near infrared absorption spectra in overheated potassium vapour. Eur. Phys. J. D 37, 37–49 (2006).
Edvardsson, D., Lunell, S. & Marian, C. M. Calculation of potential energy curves for Rb2 including relativistic effects. Mol. Phys. 101, 2381–2389 (2003).
Sato, H. Photodissociation of simple molecules in the gas phase. Chem. Rev. 101, 2687–2726 (2001).
Noll, R. J., Yi, S. S. & Weisshaar, J. C. Bimolecular Ni+(2D5/2) + C3H8 reaction dynamics in real time. J. Phys. Chem. A 102, 386–394 (1998).
Scherer, N., Sipes, C., Bernstein, R. & Zewail, A. Real-time clocking of bimolecular reactions: application to H + CO2. J. Chem. Phys. 92, 5239–5259 (1990).
Ospelkaus, S. et al. Quantum-state controlled chemical reactions of ultracold potassium-rubidium molecules. Science 327, 853–857 (2010).
Huber, K.-P. Molecular Spectra and Molecular Structure: IV. Constants of Diatomic Molecules (Springer, 2013).
Amiot, C. Laser-induced fluorescence of Rb2: the (1)\({}^{1}{\Sigma }_{g}^{+}(X)\), (2)\({}^{1}{\Sigma }_{g}^{+}(X)\), (1)1Πu(B), (1)1Πg, and (2)1Πg(C) electronic states. J. Chem. Phys. 93, 8591–8604 (1990).
Werner, H.-J. et al. Molpro, version 2019.2, a package of ab initio programs (Molpro, 2019); http://www.molpro.net
De Marco, L. et al. A degenerate Fermi gas of polar molecules. Science 363, 853–856 (2019).
Yao, N. Y., Zaletel, M. P., Stamper-Kurn, D. M. & Vishwanath, A. A quantum dipolar spin liquid. Nat. Phys. 14, 405–410 (2018).
Gregory, P. D., Blackmore, J. A., Bromley, S. L. & Cornish, S. L. Loss of ultracold 87Rb 133Cs molecules via optical excitation of long-lived two-body collision complexes. Phys. Rev. Lett. 124, 163402 (2020).
Fuentealba, P., Preuss, H., Stoll, H. & Von Szentpály, L. A proper account of core-polarization with pseudopotentials: single valence-electron alkali compounds. Chem. Phys. Lett. 89, 418–422 (1982).
Christianen, A., Karman, T., Vargas-Hernández, R. A., Groenenboom, G. C. & Krems, R. V. Six-dimensional potential energy surface for NaK–NaK collisions: Gaussian process representation with correct asymptotic form. J. Chem. Phys. 150, 064106 (2019).
Byrd, J. N., Montgomery, J. A. Jr & Côté, R. Structure and thermochemistry of K2Rb, KRb2, and K2Rb2. Phys. Rev. A 82, 010502 (2010).
Yang, D. et al. A global full-dimensional potential energy surface for the K2Rb2 complex and its lifetime. J. Phys. Chem. Lett. 11, 2605–2610 (2020).
Acknowledgements
We thank L. Zhu for experimental assistance. This work is supported by DOE YIP and the David and Lucile Packard Foundation. M.A.N. is supported by an HQI postdoctoral fellowship. T.K. is supported by NWO Rubicon grant no. 019.172EN.007 and the NSF through ITAMP. H.G. acknowledges a MURI grant from ARO (W911NF-19-1-0283) and a Humboldt Research Award.
Author information
Authors and Affiliations
Contributions
The experimental work and data analysis were carried out by Y.L., M.-G.H., M.A.N., D.D.G. and K.-K.N. Theoretical calculations were performed by T.K., and H.G. aided in the analysis of the results. All authors contributed to interpreting the results and writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
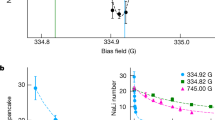
Extended Data Fig. 1 Continued formation of products at high ODT intensities.
Steady-state K2+ (red circles) and Rb2+ (blue circles) ion counts at ODT light intensities in the 11.3–46.2 kW/cm2 range, normalized by the number of experimental cycles (~ 80 for each data point). The error bars represent shot noise. The dashed lines indicate the levels to which the ion counts plateau, obtained by averaging, within each dataset, the values of the points at ODT intensities larger than 15 kW/cm2. (Inset) Timing schemes of the ODT (red and blue) and the pulsed UV ionization laser (purple) used for the measurements presented here. The red (blue) trace corresponds to a high (low) duty cycle modulation of the ODT. The instantaneous ODT intensity, I, is inversely proportional to the duty cycle, while the time-averaged ODT intensity, Iavg is constant for all measurements.
Source data
Source Data Fig. 1
K2+, Rb2+ (Fig. 2a) and K2Rb2+ (Fig. 2b) ion counts as a function of ODT intensity.
Source Data Fig. 4
Fig. 4a: oscilloscope traces of the 1,064-nm and UV ionization light intensities for the complex lifetime measurement. Fig. 4b: K2Rb2+ ion counts as a function of the delay between ODT turn-off edge and the UV ionization pulse.
Source Data Extended Data Fig. 1
K2+ and Rb2+ ion counts as a function of ODT intensity.
Rights and permissions
About this article
Cite this article
Liu, Y., Hu, MG., Nichols, M.A. et al. Photo-excitation of long-lived transient intermediates in ultracold reactions. Nat. Phys. 16, 1132–1136 (2020). https://doi.org/10.1038/s41567-020-0968-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-020-0968-8
This article is cited by
-
A Feshbach resonance in collisions between triplet ground-state molecules
Nature (2023)
-
Magnetic trapping of ultracold molecules at high density
Nature Physics (2023)
-
Evidence for the association of triatomic molecules in ultracold 23Na40K + 40K mixtures
Nature (2022)
-
Evaporation of microwave-shielded polar molecules to quantum degeneracy
Nature (2022)
-
Precision test of statistical dynamics with state-to-state ultracold chemistry
Nature (2021)