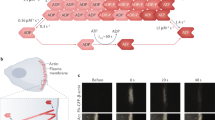
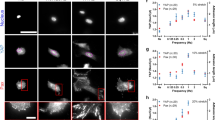
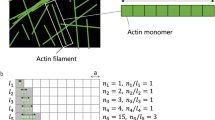
Abstract
How cells with diverse morphologies and cytoskeletal architectures modulate their mechanical behaviours to drive robust collective motion within tissues is poorly understood. During wound repair within epithelial monolayers in vitro, cells coordinate the assembly of branched and bundled actin networks to regulate the total mechanical work produced by collective cell motion. Using traction force microscopy, we show that the balance of actin network architectures optimizes the wound closure rate and the magnitude of the mechanical work. These values are constrained by the effective power exerted by the monolayer, which is conserved and independent of actin architectures. Using a cell-based physical model, we show that the rate at which mechanical work is done by the monolayer is limited by the transformation between actin network architectures and differential regulation of cell–substrate friction. These results and our proposed mechanisms provide a robust physical model for how cells collectively coordinate their non-equilibrium behaviours to dynamically regulate tissue-scale mechanical output.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data that support plots and other findings within this manuscript are available from the corresponding authors upon reasonable request.
Code availability
Custom codes that were used to analyse experimental data within this manuscript are available from the corresponding authors upon reasonable request.
References
Haigo, S. L. & Bilder, D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331, 1071–1074 (2011).
Edwards, K. A., Demsky, M., Montague, R. A., Weymouth, N. & Kiehart, D. P. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev. Biol. 191, 103–117 (1997).
Simske, J. S. & Hardin, J. Getting into shape: epidermal morphogenesis in Caenorhabditis elegans embryos. Bioessays 23, 12–23 (2001).
Nabeshima, K., Inoue, T., Shimao, Y., Kataoka, H. & Koono, M. Cohort migration of carcinoma cells: differentiated colorectal carcinoma cells move as coherent cell clusters or sheets. Histol. Histopathol. 14, 1183–1197 (1999).
Friedl, P. et al. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 55, 4557–4560 (1995).
Gaggioli, C. et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392–1400 (2007).
Friedl, P. & Gilmour, D. Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell. Biol. 10, 445–457 (2009).
Trepat, X. et al. Physical forces during collective cell migration. Nat. Phys. 5, 426–430 (2009).
Tambe, D. T. et al. Collective cell guidance by cooperative intercellular forces. Nat. Mater. 10, 469–475 (2011).
Maruthamuthu, V., Sabass, B., Schwarz, U. S. & Gardel, M. L. Cell–ECM traction force modulates endogenous tension at cell–cell contacts. Proc. Natl Acad. Sci. USA 108, 4708–4713 (2011).
Schmidt, G. H., Winton, D. J. & Ponder, B. A. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development 103, 785–790 (1988).
Brugues, A. et al. Forces driving epithelial wound healing. Nat. Phys. 10, 684–691 (2014).
Zhao, M., Song, B., Pu, J., Forrester, J. V. & McCaig, C. D. Direct visualization of a stratified epithelium reveals that wounds heal by unified sliding of cell sheets. FASEB J. 17, 397–406 (2003).
Bement, W. M., Mandato, C. A. & Kirsch, M. N. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr. Biol. 9, 579–587 (1999).
Farooqui, R. & Fenteany, G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J. Cell Sci. 118, 51–63 (2005).
Nusrat, A., Delp, C. & Madara, J. L. Intestinal epithelial restitution - characterization of a cell-culture model and mapping of cytoskeletal elements in migrating cells. J. Clin. Investig. 89, 1501–1511 (1992).
Tamada, M., Perez, T. D., Nelson, W. J. & Sheetz, M. P. Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J. Cell Biol. 176, 27–33 (2007).
Bement, W. M., Forscher, P. & Mooseker, M. S. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J. Cell Biol. 121, 565–578 (1993).
Murrell, M., Kamm, R. & Matsudaira, P. Substrate viscosity enhances correlation in epithelial sheet movement. Biophys. J. 101, 297–306 (2011).
Anon, E. et al. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc. Natl Acad. Sci. USA 109, 10891–10896 (2012).
Ravasio, A. et al. Gap geometry dictates epithelial closure efficiency. Nat. Commun. 6, 7683 (2015).
Vedula, S. R. et al. Mechanics of epithelial closure over non-adherent environments. Nat. Commun. 6, 6111 (2015).
Califano, J. P. & Reinhart-King, C. A. Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell Mol. Bioeng. 3, 68–75 (2010).
Han, S. J., Bielawski, K. S., Ting, L. H., Rodriguez, M. L. & Sniadecki, N. J. Decoupling substrate stiffness, spread area, and micropost density: a close spatial relationship between traction forces and focal adhesions. Biophys. J. 103, 640–648 (2012).
Harkness, T. et al. High-content imaging with micropatterned multiwell plates reveals influence of cell geometry and cytoskeleton on chromatin dynamics. Biotechnol. J. 10, 1555–1567 (2015).
Limouze, J., Straight, A. F., Mitchison, T. & Sellers, J. R. Specificity of blebbistatin, an inhibitor of myosin II. J. Muscle Res. Cell Motil. 25, 337–341 (2004).
Rizvi, S. A. et al. Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly. Chem. Biol. 16, 1158–1168 (2009).
Nolen, B. J. et al. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature 4640, 1031–1034 (2009).
Burnette, D. T. et al. A role for actin arcs in the leading-edge advance of migrating cells. Nat. Cell Biol. 13, 371–U388 (2011).
Honda, H. & Eguchi, G. How much does the cell boundary contract in a monolayered cell sheet. J. Theor. Biol. 84, 575–588 (1980).
Farhadifar, R., Roper, J. C., Algouy, B., Eaton, S. & Julicher, F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
Fletcher, A. G., Osterfield, M., Baker, R. E. & Shvartsman, S. Y. Vertex models of epithelial morphogenesis. Biophys. J. 106, 2291–2304 (2014).
Krzyszczyk, P. & Wolgemuth, C. W. Mechanosensing can result from adhesion molecule dynamics. Biophys. J. 101, L53–L55 (2011).
Cochet-Escartin, O., Ranft, J., Silberzan, P. & Marcq, P. Border forces and friction control epithelial closure dynamics. Biophys. J. 106, 65–73 (2014).
Brown, R. A., Prajapati, R., McGrouther, D. A., Yannas, I. V. & Eastwood, M. Tensional homeostasis in dermal fibroblasts: mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 175, 323–332 (1998).
Chien, S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 292, H1209–H1224 (2007).
du Roure, O. et al. Force mapping in epithelial cell migration. Proc. Natl Acad. Sci. USA 102, 2390–2395 (2005).
Mertz, A. F. et al. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc. Natl Acad. Sci. USA 110, 842–847 (2013).
Mertz, A. F. et al. Scaling of traction forces with the size of cohesive cell colonies. Phys. Rev. Lett. 108, 198101 (2012).
Bi, D., Yang, X., Marchetti, M. C. & Manning, M. L. Motility-driven glass and jamming transitions in biological tissues. Phys. Rev. X 6, 021011 (2016).
Razzell, W., Wood, W. & Martin, P. Recapitulation of morphogenetic cell shape changes enables wound re-epithelialisation. Development 141, 1814–1820 (2014).
Staddon, M. F. et al. Cooperation of dual modes of cell motility promotes epithelial stress relaxation to accelerate wound healing. PLoS Comput. Biol. 14, e1006502 (2018).
Mitrossilis, D. et al. Single-cell response to stiffness exhibits muscle-like behavior. Proc. Natl Acad. Sci. USA 106, 18243–18248 (2009).
Yeung, T. et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton 60, 24–34 (2005).
Sabass, B., Gardel, M. L., Waterman, C. M. & Schwarz, U. S. High resolution traction force microscopy based on experimental and computational advances. Biophys. J. 94, 207–220 (2008).
Thevenaz, P., Ruttimann, U. E. & Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 7, 27–41 (1998).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Brakke, K. A. The surface evolver. Exp. Math. 1, 141–165 (1992).
Kuipers, D. et al. Epithelial repair is a two-stage process driven first by dying cells and then by their neighbours. J. Cell Sci. 127, 1229–1241 (2014).
Acknowledgements
We acknowledge funding ARO MURI W911NF-14-1-0403 to M.P.M., D.R.K., C.S., V.A. and A.P.T. D.S.S. acknowledges support from National Science Foundation (NSF) Fellowship grant number DGE1122492. We acknowledge funding CMMI-1525316 and NIH RO1 GM126256 to M.P.M. and NIH U54 CA209992 to M.P.M. and M.S.Y. We also acknowledge fellowship support from the Yale Endowed Fund to V.A. M.F.S. is supported by a UK Engineering and Physical Sciences Research Council (EPSRC) PhD studentship at University College London (UCL). S.B. is supported by Royal Society Tata University Research Fellowship grant number URF/R1/180187, and acknowledges support from a Strategic Fellowship at UCL. S.B. and M.P.M. also acknowledge support from Human Frontiers Science Program (HFSP) grant number RGY0073/2018. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the NSF, NIH, HFSP, Royal Society, or EPSRC.
Author information
Authors and Affiliations
Contributions
M.P.M. designed and conceived the experimental work. S.B. designed and conceived the computational model. V.A., A.P.T., A.F. and M.S.Y. acquired experimental data. M.P.M., A.P.T., D.S.S., C.S. and V.A. analysed experimental data. M.F.S. implemented the model and performed simulations. D.B. provided computational tools and design. M.P.M. and S.B. contributed analytical tools. M.P.M., A.P.T., S.B., M.F.S. and D.K. wrote the paper. V.A. and A.P.T. are equal co-first authors, listed alphabetically, and A.J.F. and M.F.S. are equal co-second authors, listed alphabetically.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Physics thanks Dimitrije Stamenovic and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–4, Supplementary Equations, Supplementary Figures 1–10 and Supplementary References 1–10.
Supplementary Video 1
Retraction of cell monolayer after ablation. DIC images of cell monolayer during wounding. Radial velocity vectors calculated from PIV measurements of DIC show a net outward flow, caused by a retraction of the monolayer. Scale bar is 20 μm.
Supplementary Video 2
Depolymerization of F-actin during monolayer retraction. Caco2 cell transiently transfected with LifeAct-GFP. Upon ablation, the cell retracts and forms both lamellipodial and purse string F-actin architectures. Concomitantly, cytoskeletal F-actin structures depolymerize. Scale bar is 25 μm.
Supplementary Video 3
Assembly of lamellipodia and purse string—early times. Ablation of epithelial sheets of Caco2 cells singly transfected with LifeAct-GFP. At the wound boundary, a cell assembles F-actin lamellipodium (left) and purse string (right) architectures. Scale bar is 20 μm.
Supplementary Video 4
Assembly of lamellipodia and purse string—late times. Ablation of epithelial sheets of Caco2 cells singly transfected with LifeAct-GFP. At the wound boundary, a cell assembles F-actin lamellipodial (left) and purse string (right) architectures. Scale bar is 20 μm.
Supplementary Video 5
Enhanced contrast of purse string during closure. Ablation of epithelial sheets of Caco2 cells singly transfected with LifeAct-GFP. At the wound boundary, a cell assembles an F-actin purse string architecture. Actin intensity is scaled to show details of the actin purse string. Scale bar is 20 μm.
Supplementary Video 6
Pharmacological controls. Wounds induced on 12.2 kPa substrates. CK666 and SMIFH2 inhibitors (left) and calcium–magnesium switch (right). Scale bar is 20 μm.
Supplementary Video 7
Wound closure for pharmacological perturbations. Wounds induced on 24 kPa substrates for the control (left), SMIFH2 (middle) and CK666 (right). Scale bar is 20 μm.
Supplementary Video 8
Pharmacological controls. Wounds induced on 12.2 kPa substrates. Nocodazole treatment to interfere with microtubule polymerization (left) and Y-27632 to biochemically inhibit ROCK1 and ROCK2 protein kinases (right). Scale bar is 20 μm.
Supplementary Video 9
Vertex model wound closure. Simulated wounds where the cells at the leading edge switch from lamellipodial crawling to purse string with a rate kPS. kPS = 1.5 h–1 (left) leads to primarily lamellipodial closure, whereas kPS = 3.5 h–1 (right) results in a complete transition to purse-string closure.
Supplementary Video 10
Instance of lamellipodial cell overcoming the leading purse string. Actin fluorescence images and traction stress maps for the rare case of a lamellipodial protrusion of a sub-marginal cell passing the purse string. Stresses localize to actin structures at wound edge. Scale bar is 20 μm.
Rights and permissions
About this article
Cite this article
Ajeti, V., Tabatabai, A.P., Fleszar, A.J. et al. Wound healing coordinates actin architectures to regulate mechanical work. Nat. Phys. 15, 696–705 (2019). https://doi.org/10.1038/s41567-019-0485-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-019-0485-9
This article is cited by
-
Computational modeling and simulation of epithelial wound closure
Scientific Reports (2023)
-
Geometric constraint-triggered collagen expression mediates bacterial-host adhesion
Nature Communications (2023)
-
Free and Interfacial Boundaries in Individual-Based Models of Multicellular Biological systems
Bulletin of Mathematical Biology (2023)
-
Collective curvature sensing and fluidity in three-dimensional multicellular systems
Nature Physics (2022)
-
Dysregulation of TSP2-Rac1-WAVE2 axis in diabetic cells leads to cytoskeletal disorganization, increased cell stiffness, and dysfunction
Scientific Reports (2022)