Abstract
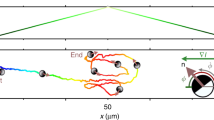
Biological microswimmers exhibit versatile strategies for sensing and navigating their environment, such as run-and-tumble and curvature modulation. Here, we report a striking phototactic behaviour of the microswimmer Euglena gracilis, where these eukaryotic cells swim in polygonal trajectories due to a sudden increase in light intensity. While smoothly curved trajectories are common for microswimmers, such quantized ones have not been reported previously. We find that this polygonal behaviour emerges from periodic switching between the flagellar beating patterns of helical swimming and spinning behaviours. We develop and experimentally validate a biophysical model that describes the phase relationship between the eyespot, cell orientation, light detection and cellular reorientation, accounting for all three behavioural states. Coordinated switching between these behaviours selects for ballistic, superdiffusive, diffusive or subdiffusive motion (including tuning the effective diffusion constant over several orders of magnitude), thereby enabling navigation in spatially structured light fields, such as edge avoidance and gradient descent. This feedback control links multiple system scales (flagellar beats, cellular behaviours and phototaxis strategies), with implications for other natural and synthetic microswimmers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data sets and computer codes generated during and analysed during the current study are available from the corresponding author on reasonable request.
Change history
09 October 2018
In the version of this Article originally published, the angular oscillation of amplitude in Fig. 4a was incorrectly labelled ζ; it should have been ξ. Also, the blue line in the top-right corner of Fig. 6d should not have been dash-dotted but solid. These have now been corrected in all versions of the Article.
References
Miller, M. B. & Bassler, B. L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199 (2001).
Berg, H. C. & Brown, D. A chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature 239, 500–504 (1972).
Friedrich, B. M. & Jülicher, F. Chemotaxis of sperm cells. Proc. Natl Acad. Sci. USA 104, 13256–13261 (2007).
Drescher, K., Goldstein, R. E. & Tuval, I. Fidelity of adaptive phototaxis. Proc. Natl Acad. Sci. USA 107, 11171–11176 (2010).
Diehn, B. Phototaxis and sensory transduction in Euglena. Science 181, 1009–1015 (1973).
Häder, D. Simulation of phototaxis in the flagellate Euglena gracilis. J. Biol. Phys. 19, 95–108 (1993).
Machemer, H. Swimming sensory cells: electrical membrane parameters, receptor properties and motor control in ciliated protozoa. Verh. Dtsch. Zool. Ges. 1977, 86–110 (1977).
Ogawa, N., Oku, H., Hashimoto, K. & Ishikawa, M. A physical model for galvanotaxis of Paramecium cell. J. Theor. Biol. 242, 314–328 (2006).
Riedel-Kruse, I. H., Chung, A. M., Dura, B., Hamilton, A. L. & Lee, B. C. Design, engineering and utility of biotic games. Lab Chip 11, 14–22 (2011).
Kantsler, V., Dunkel, J., Blayney, M. & Goldstein, R. E. Rheotaxis facilitates upstream navigation of mammalian sperm cells. eLife 3, e02403 (2014).
Arrieta, J., Barreira, A., Chioccioli, M., Polin, M. & Tuval, I. Phototaxis beyond turning: persistent accumulation and response acclimation of the microalga Chlamydomonas reinhardtii. Sci. Rep. 7, 3447 (2017).
Schaller, K., David, R. & Uhl, R. How Chlamydomonas keeps track of the light once it has reached the right phototactic orientation. Biophys. J. 73, 1562–1572 (1997).
Bennett, R. R. & Golestanian, R. A steering mechanism for phototaxis in Chlamydomonas. J. R. Soc. Interface 12, 20141164 (2015).
Sartori, P., Geyer, V. F., Scholich, A., Jülicher, F. & Howard, J. Dynamic curvature regulation accounts for the symmetric and asymmetric beats of Chlamydomonas flagella. eLife 5, e13258 (2016).
Leptos, K. C., Chioccioli, M., Furlan, S., Pesci, A. I. & Goldstein, R. E. An adaptive flagellar photoresponse determines the dynamics of accurate phototactic steering in Chlamydomonas. Preprint at https://www.biorxiv.org/content/early/2018/02/17/254714 (2018).
Hill, N. A. & Vincent, R. V. A simple model and strategies for orientation in phototactic microorganisms. J. Theor. Biol. 163, 223–235 (1993).
Giometto, A., Altermatt, F., Maritan, A., Stocker, R. & Rinaldo, A. Generalized receptor law governs phototaxis in the phytoplankton Euglena gracilis. Proc. Natl Acad. Sci. USA 112, 7045–7050 (2015).
Rossi, M., Cicconofri, G., Beran, A., Noselli, G. & DeSimone, A. Kinematics of flagellar swimming in Euglena gracilis: Helical trajectories and flagellar shapes. Proc. Natl Acad. Sci. USA 114, 13085–13090 (2017).
Wan, K. Y. & Goldstein, R. E. Time irreversibility and criticality in the motility of a flagellate microorganism. Phys. Rev. Lett. 121, 058103 (2018).
Polin, M., Tuval, I., Drescher, K., Gollub, J. P. & Goldstein, R. E. Chlamydomonas swims with two “gears” in a eukaryotic version of run-and-tumble locomotion. Science 325, 487–490 (2009).
Lauga, E., DiLuzio, W. R., Whitesides, G. M. & Stone, H. A. Swimming in circles: motion of bacteria near solid boundaries. Biophys. J. 90, 400–412 (2006).
Ozasa, K. et al. Temporal change of photophobic step-up responses of Euglena gracilis investigated through motion analysis. PLoS One 12, e0172813 (2017).
Nichols, K. M., Jacklet, A. & Rikmenspoel, R. Effects of Mg2+ and Ca2+ on photoinduced Euglena flagellar responses. J. Cell. Biol. 84, 355–363 (1980).
Arroyo, M., Heltai, L., Millán, D. & DeSimone, A. Reverse engineering the euglenoid movement. Proc. Natl Acad. Sci. USA 109, 17874–17879 (2012).
Lee, S. A. et al. Trap it!: a playful human–biology interaction for a museum installation. In Proc. 33rd Annual ACM Conference on Human Factors in Computing Systems 2593–2602 (ACM, 2015).
Cira, N. J. et al. A biotic game design project for integrated life science and engineering education. PLoS Biol. 13, e1002110 (2015).
Hossain, Z. et al. Interactive and scalable biology cloud experimentation for scientific inquiry and education. Nat. Biotechnol. 34, 1293–1298 (2016).
Hossain, Z. et al. Design guidelines and empirical case study for scaling authentic inquiry-based science learning via open online courses and interactive biology cloud labs. Int. J. Artif. Intell. Educ. https://doi.org/10.1007/s40593-017-0150-3 (2017).
Kim, H. et al. LudusScope: accessible interactive smartphone microscopy for life-science education. PLoS One 11, e0162602 (2016).
Morimoto, K. & Takemura, A. Developing teaching materials using LED for a phototaxis in Euglena. J. Res. Sci. Educ. 45, 73–77 (2005).
Ozasa, K., Lee, J., Song, S., Hara, M. & Maeda, M. Gas/liquid sensing via chemotaxis of Euglena cells confined in an isolated micro-aquarium. Lab Chip 13, 4033–4039 (2013).
Ozasa, K., Lee, J., Song, S., Hara, M. & Maeda, M. Two-dimensional optical feedback control of Euglena confined in closed-type microfluidic channels. Lab Chip 11, 1933–1940 (2011).
Lam, A. T. et al. Device and programming abstractions for spatiotemporal control of active micro-particle swarms. Lab Chip 17, 1442–1451 (2017).
Krajvovic, J., Vesteg, M. & Schwartzbach, S. D. Euglenoid flagellates: A multifaceted biotechnology platform. J. Biotechnol. 202, 135–145 (2015).
Leander, B. S., Lax, G., Karnkowska, A. & Simpson, A. G. B. Handbook of the Protists 1047–1088 (Springer, Cham, 2017).
Ascoli, C., Barbi, M., Frediani, C. & Mure, A. Measurements of Euglena motion parameters by laser light scattering. Biophys. J. 24, 585–599 (1978).
Ogawa, T. et al. The flux of Euglena gracilis cells depends on the gradient of light intensity. PLoS One 11, e0168114 (2016).
Metzler, R. & Klafter, J. The random walk’s guide to anomalous diffusion: a fractional dynamics approach. Phys. Rep. 339, 1–77 (2000).
Metzler, R., Jeon, J.-H., Cherstvy, A. G. & Barkai, E. Anomalous diffusion models and their properties: non-stationarity, non-ergodicity, and ageing at the centenary of single particle tracking. Phys. Chem. Chem. Phys. 16, 24128–24164 (2014).
Häder, D.-P. & Griebenow, K. Orientation of the green flagellate, Euglena gracilis, in a vertical column of water. FEMS Microbiol. Lett. 53, 159–167 (1988).
Shoji, E., Nishimori, H., Awazu, A., Izumi, S. & Iima, M. Localized bioconvection patterns and their initial state dependency in Euglena gracilis suspensions in an annular container. J. Phys. Soc. Jpn 83, 043001 (2014).
Wioland, H., Woodhouse, F. G., Dunkel, J. & Goldstein, R. E. Ferromagnetic and antiferromagnetic order in bacterial vortex lattices. Nat. Phys. 12, 341–345 (2016).
Riedel, I. H., Kruse, K. & Howard, J. A self-organized vortex array of hydrodynamically entrained sperm cells. Science 309, 300–303 (2005).
Dai, B. et al. Programmable artificial phototactic microswimmer. Nat. Nanotech. 11, 1087–1092 (2016).
Lozano, C., Ten Hagen, B., Löwen, H. & Bechinger, C. Phototaxis of synthetic microswimmers in optical landscapes. Nat. Commun. 7, 12828 (2016).
Palacci, J., Sacanna, S., Steinberg, A. P., Pine, D. J. & Chaikin, P. M. Living crystals of light-activated colloidal surfers. Science 339, 936–940 (2013).
Acknowledgements
We thank members of the Riedel-Kruse laboratory, B. Friedrich, J. Dunkel, N. Ouellette and A. Macdonald. This work was supported by NSF grant no. 1324753, the Stanford Discovery Innovation Fund and the Croucher Foundation (through a postdoctoral fellowship to A.C.H.T.).
Author information
Authors and Affiliations
Contributions
A.C.H.T. and I.H.R.-K. were responsible for the project idea, the theory and manuscript preparation; A.C.H.T. was responsible for the modelling, the experiments and data analysis (except Fig. 6: A.C.H.T. and A.T.L.).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–14, Supplementary Table 1, Supplementary References 1–4
Supplementary Video 1
Euglena swimming in a polygonal trajectory at intermediate light intensity. The images were sampled at 200 fps and the video is replayed at 10 × slower than the real time
Supplementary Video 2
Euglena swimming in a helical trajectory at low light intensity, recorded at 400 fps. The images were sampled at 400 fps and the video is replayed at 20 × slower than the real time
Supplementary Video 3
Euglena spinning around locally at high light intensity, recorded at 400 fps. The images were sampled at 400 fps and the video is replayed at 20 × slower than the real time
Supplementary Video 4
Various Euglena flagellar beat patterns for helical swimming, spinning, and polygonal swimming. The images are sampled at 200 fps
Supplementary Video 5
Comparison of simulation and experiment for helical swimming
Supplementary Video 6
Comparison of simulation and experiment for spinning
Supplementary Video 7
Comparison of simulation and experiment for polygonal swimming
Supplementary Video 8
Experimental tracking of Euglena exhibiting light avoidance from a light barrier
Supplementary Video 9
Experimental tracking of Euglena exhibiting spinning behaviour initially, followed by swimming in polygonal paths of increasing order, thereby expanding the search radius to navigate the light edge
Supplementary Video 10
Experimental tracking of Euglena exhibiting biased run-and-tumble down the light gradient
Supplementary Video 11
Simulations of Euglena exhibiting light avoidance from a light barrier
Supplementary Video 12
Simulations of Euglena exhibiting polygonal paths of increasing order and navigating the light edge
Supplementary Video 13
Simulations of Euglena exhibiting biased run-and-tumble down the light gradient
Rights and permissions
About this article
Cite this article
Tsang, A.C.H., Lam, A.T. & Riedel-Kruse, I.H. Polygonal motion and adaptable phototaxis via flagellar beat switching in the microswimmer Euglena gracilis. Nature Phys 14, 1216–1222 (2018). https://doi.org/10.1038/s41567-018-0277-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41567-018-0277-7
This article is cited by
-
Light-controlled soft bio-microrobot
Light: Science & Applications (2024)
-
Hydrodynamic pursuit by cognitive self-steering microswimmers
Communications Physics (2023)
-
Active oscillations in microscale navigation
Animal Cognition (2023)
-
Fluidic bacterial diodes rectify magnetotactic cell motility in porous environments
Nature Communications (2021)
-
Microfluidic and mathematical modeling of aquatic microbial communities
Analytical and Bioanalytical Chemistry (2021)